?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the effects of miR-143-3p and MAPK7 on the proliferation, migration, and invasion of U2OS human osteosarcoma (OS) cells.
Methods
The expression of miR-143-3p and MAPK7 in U2OS cells were detected by qRT-PCR, and the protein level of MAPK7 was measured by western blot assay. The targeting relationship between miR-143-3p and MAPK7 was predicted by TargetScan and verified by dual luciferase reporter assay. MTT and Transwell assays were used to detect cell viability, migrated cells and invaded cells of U2OS cells.
Results
Compared with hFOB1.19 cells, the expression of miR-143-3p was down-regulated and MAPK7 was up-regulated in U2OS cells. Cell viability, migration and invasion ability significantly decreased induced by miR-143-3p overexpression or MAPK7 knockdown in U2OS cells. The results of dual luciferase reporter assay indicated that miR-143-3p interacted with MAPK7. Furthermore, overexpression of MAPK7 could reverse the inhibitory effects on cell proliferation, migration and invasion in U2OS cells induced by miR-143-3p mimics.
Conclusion
miR-143-3p could inhibit proliferation, migration and invasion of U2OS cells by targeting MAPK7.
Introduction
Osteosarcoma (OS) is a highly malignant primary bone tumour, which often occurs in the femur, humerus and tibia of children and adolescents. The incidence of OS is about 4.4/100,000, ranking second in primary bone tumours [Citation1]. OS is usually diagnosed by tissue biopsy, radionuclide bone scan, CT and other methods. The clinical treatment is mainly based on surgical treatment such as tumour resection and amputation, supplemented by chemotherapy, which can effectively prolong the survival of patients. The 5-year survival rate of patients has reached 70–80%. However, about 20% of patients have distant metastasis at the time of diagnosis, and the 5-year survival rate of OS patients with metastasis is only 30% [Citation2,Citation3]. In recent years, due to its small side effects and strong pertinence, new therapeutic methods such as immunotherapy and molecular targeted therapy have been increasingly researched in the field of tumours, and it has received extensive attention in related research of OS [Citation4,Citation5].
MicroRNA (miRNA) plays an important role in the development of tumours. It is reported that a variety of miRNA molecules are abnormally expressed in tumour tissues, and their expression levels are related to tumour growth, disease classification and patient prognosis [Citation6]. Moreover, miRNAs such as miR-214 and miR-138 are involved in the regulation of OS cell growth, phenotype, exosome function and tumour growth microenvironment, etc. The mechanism of miRNA is helpful for the diagnosis and treatment of OS. [Citation7]. Actually, a previous study reported that miR-143-3p was decreased in OS tissues and cells, and its expression level could affect cell proliferation, migration and invasion [Citation8]. In this study, we found that miR-143-3p could regulate cell proliferation, migration and invasion in OS by targeting MAPK1. Thus, this novel pathway may provide a new target for the diagnosis and treatment for OS patients.
Materials and methods
Materials
U2OS cell line and human normal osteoblasts hFOB1.19 cell line was purchased from American Type Culture Collection (ATCC). Fetal bovine serum (FBS), DMEM medium and trypsin were obtained from Sigma Company (Sigma, Kawasaki, Japan), Lipofectamine 2000 transfection reagent was bought from Invitrogen Company (Invitrogen, Waltham, MA). MTT, Trizol reagent, PVDF film, ECL luminescent solution, RIPA cracking solution, dimethyl sulfoxide (DMSO), mitomycin C were purchased from Beijing Solarbio Company (Beijing, China). Rabbit anti-MAPK7 and GAPDH polyclonal antibodies and goat anti-rabbit IgG labeled with horseradish peroxidase (HRP) were obtained from Abcam Company, USA, and Transwell Chamber was purchased from Corning Company (New York, NY). TaKaRa reverse transcription kit and real-time fluorescence quantitative kit were purchased from Biotechnology Co., Ltd. (Dalian, China); The dual luciferase reporter assay system was purchased from Promega (USA); Control siRNA, MAPK7 si-RNA was designed and synthesized by Shandong vigene Bioscience Co., Ltd. (Shandong, China) Control inhibitors, miR-143-3p inhibitors, control mimics and miR-143-3p mimics were designed and synthesized by Shanghai Gene Pharma Co., Ltd. (Shanghai, China). The pcDNA-MAPK7 overexpression vector was constructed by Jinruisi Biotechnology Co., Ltd.; MCO-18AC type CO2 incubator was purchased from SANYO Company of Japan; CFX96 Touch TM real-time PCR detection system and ChemiDoc TM MP gel imaging system were purchased from Bio-Rad Company of USA; Nanodrop ND-2000 ultra-micronucleic acid protein analyzer was purchased from Shanghai Chuangmeng Biotechnology Co., Ltd. (Shanghai, China); HBS-1096B microplate reader was purchased from Nanjing Detie Laboratory Equipment Company (Nanjiang, China).
Cell culture and transfection
The frozen U2OS and hFOB1.19 cells were removed, thawed in a 37 °C water bath, and the cell suspension was transferred to a sterile centrifuge tube and washed in serum-free DMEM medium. U2OS and hFOB1.19 cells were cultured with DMEM containing 10% FBS at 37 °C and 5% CO2 saturated humidity. The cells were passaged when the cell confluence was about 80%. U2OS cells in logarithmic growth phase were collected and re-suspended after trypsin digestion. Cell suspension concentration was adjusted to 3 × 104/mL and inoculated into 96-well plate. After the cells were grown to a confluence of ∼45%, according to the instructions of LipofectamineTM2000 transfection reagent, the control mimics, miR-143-3p mimics, control inhibitors, miR-143-3p inhibitors, control siRNA, MAPK7 siRNA, mir-143-3p MICs and pcDNA 3.1 empty vectors, miR-143-3p MICs and pDNA 3.1-MAPK7 overexpression vectors were transfected into U2OS cells, which were labelled as miR-NC group, miR-143-3p group, anti-miR-NC group, anti-miR-143-3p group, si-NC group, si-MAPK7 group, miR-143-3p + pcDNA1.3 vector and miR-143-3p + pcDNA1.3-MAPK7 group, each group of 6 duplicate wells, the experiment was repeated 3 times.
qRT-PCR
the U2OS and hFOB1.19 cells in a logarithmic growth phase, the miR-NC group and the miR-143-3p group were collected and ground adequately. Total RNA was extracted by Trizol, and the purity and concentration of RNA were detected by micronucleic acid analyzer. The RNA sample was reverse transcribed into cDNA using the TaKaRa reverse transcription kit. The quantitative PCR was performed on a real-time fluorescence quantitative analyzer. Each sample was repeated 3 times with GAPDH as an internal reference, F = 2−ΔΔCt method was used to calculate the relative expression level of the target gene. The primers used are shown in .
Table 1. Primers and sequences of qRT-PCR.
Western blot
U2OS and hFOB1.19 cells in a logarithmic growth phase, as well as microRNA-NC group, microRNA-143-3p group, anti-microRNA-NC group, anti-microRNA-143-3p group, si-NC group and si-MAPK7 group were collected. RIPA lysate was added to the cells in each group and placed on ice to lyse cells, centrifuged at 12,000 g for 15 min at 4 °C, and the protein supernatant was collected. The protein sample was separated by SDS-PAGE. After electrophoresis, the protein was transferred to PVDF membrane and blocked in 5% skim milk powder at room temperature for 1 h. Rabbit anti-MAPK7 (1:1000) and anti-GAPDH (1:1000) primary polyclonal antibodies were added to incubate overnight at 4 °C, and TBST was washed 3 times for 10 min each time. The membrane with HRP-labeled goat anti-rabbit IgG (1:5000) was added and incubated for 2 h at room temperature, then washed with TBST; ECL luminescence was utilized to detect the chemiluminescence. Each protein sample was repeated 3 times.
MTT assay for cell viability
Cells were cultured in the miR-NC group, miR-143-3p group, si-NC group, si-MAPK7 group, miR-143-3p + pcDNA group and miR-143-3p + pcDNA-MAPK7 group for 24, 48 and 72 h, respectively. 20 μL of MTT at a concentration of 5 mg/mL was added and incubation for 4 h. The medium was discarded and reacted with 150 μL of DMSO for 10 min. The absorbance (OD) at 490 nm was measured by a microplate reader.
Transwell detection of cell migration and invasion
Cells in miR-NC group, miR-143-3p group, si-NC group, si-MAPK7 group, miR-143-3p + pcDNA 3.1 group and miR-143-3p + pcDNA-MAPK7 group were treated with 10 μg/mL mitomycin C for 2 h. The cells were re-suspended in serum-free DMEM medium, and the cell suspension concentration was adjusted to 1 × 104 cells/mL. 200 μL was inoculated into the Transwell upper chamber of coated or uncoated matrix glue, and 600 μL DMEM medium containing 10% FBS was added into the lower chamber, with 6 replicate wells in each group. After 24 h of routine culture, the unperforated cells in the upper chamber were gently wiped off with cotton swabs, fixed with 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet for 10 min, washed with double steaming water until the water was cleared. After natural air-drying, the cells were observed under a microscope and randomly selected five visual fields to take photographs, and then the number of perforating cells was counted.
Dual luciferase reporter assay
U2OS cells in logarithmic growth phase were co-transfected with MAPK7 3'UTR wild-type plasmid or mutant plasmid and miR-143-3p mimics or its control mimics according to Lipofectamine 2000 transfection reagent instructions. The cells were cultured at 37 °C and 5% CO2 for 48 h. After lysed the cells by lysis, the supernatant was collected and the luciferase activity was detected at 4 °C and centrifuged at 12,000 rpm for 10 min.
Statistical analysis
The experimental data were expressed as ± s. SPSS 22.0 statistical software was used for data analysis and GraphPad Prism 7 software was used for drawing. T-test was used to compare the correlation between the two groups, and one-way ANOVA was used to compare the correlation among more than three groups, p < .05 was considered statistically significant.
Results
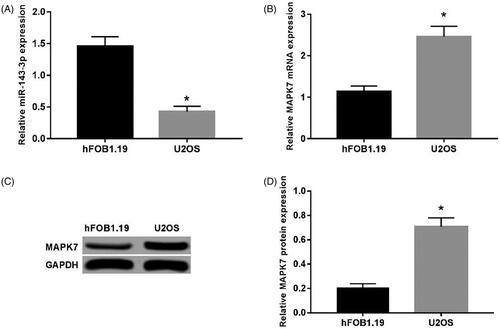
Expression of MAPK7 and miR-143-3p in U2OS cells
The results of qRT-PCR () showed that the expression of miR-143-3p in U2OS cells was significantly reduced than that in hFOB1.19 cells (p < .05). While the qRT-PCR and western blot assay presented that the mRNA and protein level of MAPK7 were both strikingly up-regulated in U2OS cells related to that in hFOB10.19 (p < .05). ()
Figure 1. Expression of MAPK7 and miR-143-3p in U2OS cells. (A) The expression of miR-143-3p mRNA in U2OS and hFOB1.19 cells; (B) the expression of MAPK7 mRNA in U2OS and hFOB1.19 cells; (C) the detection of MAPK7 protein expression; (D) the MAPK7 protein in U2OS and expression levels in hFOB 1.19 cells. Compared with hFOB1.19 cells, *p < .05.
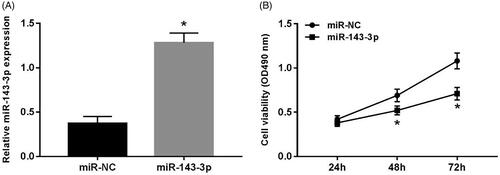
Overexpression of miR-143-3p inhibits U2OS cell proliferation
After transfecting miR-143-3p mimics into U2OS cells, the expression level of miR-143-3p was notably increased (p < .05) (), and the cell viability at 48 and 72 h was dramatically decreased than that of miR-NC group (p < .05) ().
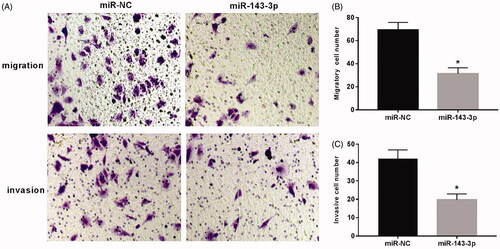
Overexpression of miR-143-3p inhibits U2OS cell migration and invasion
Transwell assay was used to detect cell migration and invasion. As shown in , the number of migration and invasion cells in the visual field was apparently decreased in U2OS cells transfected with miR-143-3p mimics compared to that in the miR-NC group (p < .05).
Figure 3. Effect of overexpression of miR-143-3p on migration and invasion of U2OS cells. (A) The cell migration and invasion assay; (B) the migration of U2OS cells after overexpression of miR-143-3p; (C) the invasion of U2OS cells after overexpression of miR-143-3p mimics. Compared with the miR-NC group, *p < .05.
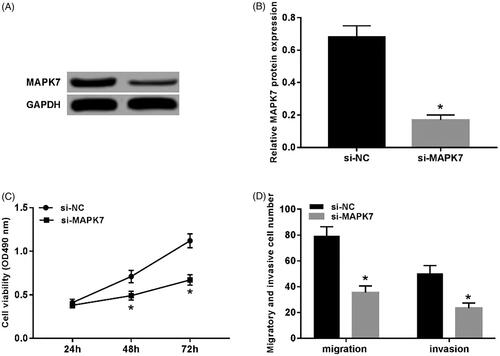
Knockdown of MAPK7 inhibits U2OS cell proliferation, migration and invasion
The transfection of si-MAPK7 resulted in the significantly decreased protein level of MAPK7 in U2OS cells (p < .05) (). Compared with the si-NC group, the cell viability of the si-MAPK7 group remarkably decreased (p < .05) (), and the number of migrating and invading cells also decreased (p < .05) ().
Figure 4. Knockdown of MAPK7 inhibits U2OS cell proliferation, migration and invasion. (A) The detection of MAPK7 protein expression; (B) the expression level of MAPK7 protein; (C) the detection of U2OS cell viability by knockdown MAPK7; (D) the migration and invasion detection of U2OS cells after knockdown of MAPK7. Compared with the si-NC group, *p < .05.
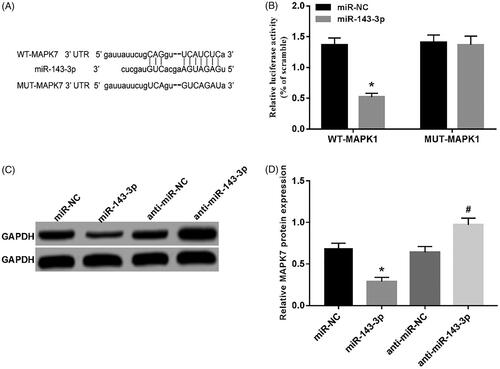
MiR-143-3p can target and regulate MAPK7 expression
According to the online prediction of TargetScan, there were complementary binding sites between miRNA-143-3p and MAPK7 3'UTR (). Further dual luciferase reporter assay showed that the activity of luciferase in WT-MAPK7 group was evidently decreased by microRNA-143-3p mimics (p < .05) without affecting the activity of luciferase in MUT-MAPK7 group (). Moreover, MAPK7 protein expression was down-regulated in miR-143-3p group (p < .05), while inhibition of miR-143-3p expression up-regulated MAPK7 protein expression (p < .05) ()
Figure 5. MiR-143-3p can target MAPK7 protein expression. (A) The complement of MAPK7 3′UTR and miR-143-3p; (B) the detection of luciferase activity; (C) the detection of MAPK7 protein expression; (D) the expression of MAPK7 protein by miR-143-3p. Compared with the miR-NC group, *p < .05; compared with the anti-miR-NC group, #p < .05.
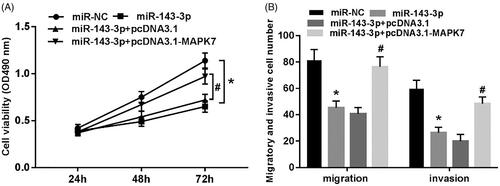
Overexpression of MAPK7 reverses the inhibitory effect of miR-143-3p on proliferation, migration and invasion of U2OS cells
It can be seen from that the viability, migrating and invading cells of U2OS cells were all significantly decreased (p < .05) after the transfection of miR-143-3p mimics into U2OS cells. However, the co-transfection of miR-143-3p mimics and pcDNA 3.1-MAPK7 into U2OS cells reversed the decreasing trend on cell viability, migration and invasion ability (p < .05).
Figure 6. Overexpression of MAPK7 reverses the inhibitory effect of miR-143-3p on proliferation, migration and invasion of U2OS cells. (A) The cell viability test of each group; (B) the cell migration and invasion test of each group. Compared with miR-NC group, *p < .05; compared with miR-143-3p + pcDNA3.1 group, #p < .05.
Discussion
MiRNA is a type of endogenous non-coding single-stranded RNA molecule of 18–22 nt in length that can degrade or inhibit translation by specific binding to the 3'UTR region of the target mRNA, and it also plays an important regulatory role in the biological processes of cell growth, differentiation, embryonic development and immune response [Citation9]. According to previous reports [Citation10–12], 2588 mature miRNAs have been identified in the human body, and each miRNA can widely participate in various life activities of the human body, and regulate the occurrence and development of diseases and cancers. A number of studies [Citation13,Citation14] showed that miR-203, miR-148b and other miRNAs were abnormally expressed in OS tissues, and their expression levels were closely associated with cell sensitivity, tumour stage, lung metastasis and prognosis.
MiR-143 is located on human chromosome 5 and is down-regulated in the bladder and ovarian cancer. Its expression level is significantly correlated with clinical stage, disease grade and lymph node metastasis. Overexpression of miR-143 inhibits tumour development by inhibiting cell proliferation, migration and invasion and enhancing cell chemosensitivity [Citation15,Citation16]; Moreover, the low expression of microRNA-143 can inhibit apoptosis by regulating Bcl-2, thereby promoting the development of OS [Citation17]. The stem-loop structure of miR-143 precursor can form miR-143-3p and miR-143-5p depending on the site where the cleavage occurs. The expression of miR-143-3p has been confirmed to be down-regulated in hepatocellular carcinoma and triple-negative breast cancer. Regulating the expression of miR-143-3p can inhibit or promote cell proliferation, migration and invasion [Citation18,Citation19]. In OS tissues and their cell lines, the expression level of miR-143-3p was significantly decreased, and overexpression of miR-143-3p inhibited cell proliferation and metastasis and induced apoptosis [Citation8]. In this study, qRT-PCR assay showed that the expression of miR-143-3p was low expressed in U2OS cells, and the viability, migration and invasion of U2OS cells after transfecting with miR-143-3p mimics were significantly reduced, which was consistent with the above conclusion [Citation8]. In addition, TargetScan’s online prediction showed that there was a binding site of miR-143-3p on MAPK7 3'UTR. Further dual luciferase reporter assay and western blot assay showed that miR-143-3p could target and regulate the expression of MAPK7.
Mitogen-activated protein kinase (MAPK) is a highly conserved serine/threonine protein kinase that is activated by the epidermal growth factor, inflammatory cytokine and growth factor. It can be used as a signal transmitter in cells to regulate various physiological processes such as stress response, inflammatory reaction, cell growth and development [Citation20]. It has been reported that MAPK signal transduction pathway plays an important regulatory role in tumourigenesis and development. Different MAPK signaling pathways can regulate each other and mediate a variety of cellular biological reactions, regulating the proliferation, differentiation and apoptosis of cancer cells [Citation21]. MAPK7, one of its family members, is also known as extracellular signal-regulated kinase 5 (ERK5) and is expressed in a variety of tissues. Some studies [Citation22] showed that MAPK7 depletion alleviated DNA damage of thymocytes in mice, and also inhibited the growth of thymic lymphoma in mice. Gavine et al. [Citation23] found that MAPK7 was highly expressed in squamous cell carcinoma and esophageal cancer tissues. Cell proliferation decreased when MAPK7 was knocked down. In addition, emerging evidence [Citation24] confirmed that MAPK7 silencing can effectively inhibit the proliferation, migration and invasion of OS cells, and enhance the chemosensitivity of cells, thereby exerting tumour suppressive effects. In the above experiments, we found that the expression of MAPK7 protein can be regulated by miR-143-3p. It is suspected that the role of miR-143-3p in regulating the proliferation, migration and invasion of U2OS cells may be related to the expression of MAPK7. To verify this hypothesis, RNAi technology was used to silence the expression of MAPK7 in U2OS cells. It was found that the proliferation, migration and invasion of U2OS cells were significantly inhibited, which was consistent with the results in the literature [Citation24]. However, up-regulation of MAPK7 expression by pcDNA3.1-MAPK7 can reverse the inhibitory effect of miR-143-3p on the proliferation, migration and invasion of U2OS cells.
In conclusion, miR-143-3p is lowly expressed and MAPK7 is highly expressed in OS cells. MiR-143-3p inhibited cell proliferation, migration and invasion by targeting MAPK7 expression. This study is helpful to study the mechanism of miRNA in OS, and may provide an experimental basis for improving the clinical treatment of OS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. 2017;4:25–43.
- Faisham WI, Mat Saad AZ, Alsaigh LN, et al. Prognostic factors and survival rate of OS: a single-institution study. Asia-Pac J Clin Oncol. 2017;13:e104–e110.
- Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292.
- Lettieri CK, Appel N, Labban N, et al. Progress and opportunities for immune therapeutics in osteosarcoma. Immunotherapy 2016;8:1233–1244.
- Angulo P, Kaushik G, Subramaniam D, et al. Natural compounds targeting major cell signaling pathways: a novel paradigm for OS therapy. J Hematol Oncol. 2017;10:1–13.
- Kumar RR, Boro A, Fuchs B. Involvement and clinical aspects of microRNA in osteosarcoma. Int J Mol Sci. 2016;17:1–8.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222.
- Sun X, Dai G, Yu L, et al. miR-143-3p inhibits the proliferation, migration and invasion in OS by targeting FOSL2. Sci Rep. 2018;8:1–10.
- Kim D, Chang HR, Baek D. Rules for functional microRNA targeting. BMB Rep. 2017;50:554–559.
- Lai X, Wolkenhauer O, Vera J. Understanding microRNA-mediated gene regulatory networks through mathematical modelling. Nucleic Acids Res. 2016;44:6019–6035.
- Wojciechowska A, Osiak A. Katarzyna K-K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 2017;26:865–874.
- Markopoulos GS, Roupakia E, Tokamani M, et al. A step-by-step microRNA guide to cancer development and metastasis. Cell Oncol (Dordr). 2017;40:303–339.
- Chen X, Chen XG, Hu X, et al. MiR-34a and miR-203 inhibit Survivin expression to control cell proliferation and survival in human osteosarcoma cells. J Cancer. 2016;7:1057–1065.
- Lin W, Lin W, Wang L, et al. Analysis of miR-148b expression differences in stage-I and II parosteal osteosarcoma. Oncol Lett. 2018;16:998–1002.
- Wang H, Li Q, Niu X, et al. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett. 2017;13:435–440.
- Wang L, He J, Xu H, et al. MiR-143 targets CTGF and exerts tumour-suppressing functions in epithelial ovarian cancer. Am J Transl Res. 2016;8:2716–2726.
- Zhang H, Wang G, Ding C, et al. Increased circular RNA UBAP2 acts as a sponge of miR-143 to promote osteosarcoma progression. Oncotarget 2017;8:61687–61697.
- Chen L, Yao H, Wang K, et al. Long non-coding RNA MALAT1 regulates ZEB1 expression by sponging miR-143-3p and promotes hepatocellular carcinoma progression. J Cell Biochem. 2017;118:4836–4843.
- Li D, Hu J, Song H, et al. miR-143-3p targeting LIM domain kinase 1 suppresses the progression of triple-negative breast cancer cells. Am J Transl Res. 2017;9:2276–2285.
- Dreas A, Mikulski M, Milik M, et al. Mitogen-activated protein kinase (MAPK) interacting kinases 1 and 2 (MNK1 and MNK2) as targets for cancer therapy: recent progress in the development of MNK inhibitors. Curr Med Chem. 2017;24:3025–3053.
- Acosta AM, Kadkol S. Mitogen-activated protein kinase signaling pathway in cutaneous melanoma: an updated review. Arch Pathol Lab Med. 2016;140:1290–1296.
- Granados-Jaén A, Angulo-Ibáñez M, Rovira-Clavé X, et al. Absence of ERK5/MAPK7 delays tumourigenesis in Atm−/− mice. Oncotarget 2016;7:74435–74447.
- Gavine PR, Wang M, Yu D, et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer 2015;15:454.
- Tesser-Gamba F, Lopes LJ, Petrilli AS, et al. MAPK7 gene controls proliferation, migration and cell invasion in OS. Mol Carcinog. 2016;55:1700–1713.