Abstract
Ghrelin has been widely recognized as a key peptide in the cardiovascular system. This study detected the potential of ghrelin in MI management and tried to decode one of the possible underlying mechanisms. H9c2 cells were pretreated with ghrelin and were subjected to hypoxia/reoxygenation (H/R). CCK-8, flow cytometry, Western blot and LDH analysis were conducted to assess the changes in cell survival. LY294002 and Compound C were used to treat H9c2 cells for blocking PI3K/AKT and AMPK pathways, respectively. Ghrelin expression in H9c2 cells was suppressed by siRNA-mediated silencing to see the effects of endogenous ghrelin. We found that, following H/R, H9c2 cells viability was decreased, CyclinD1 and CDK4 were down-regulated, apoptosis was induced, the release of LDH was enhanced, and the expression levels of Cox-2 and iNOS were up-regulated. Ghrelin protected H9c2 cells against H/R induced these alterations. Besides, ghrelin activated PI3K/AKT and AMPK pathways even in H/R-stimulated cells. The protective effects of ghrelin against H/R-induced cell damage were all attenuated by the addition of LY294002 or Compound C. Moreover, endogenous inhibition of ghrelin significantly induced cell death of H9c2 cells. In conclusion, this study demonstrated that ghrelin pretreatment protected H9c2 cells against H/R-induced cell damage, possibly via PI3K/AKT and AMPK pathways.
Introduction
Ghrelin is an endogenous ligand specific for growth hormone secretory receptor (GHSR) which is discovered by Kojima et al. in 1999 [Citation1]. It is a peptide of 28 amino acids mainly produced by X/A-like cells in the stomach, after production, it enters the bloodstream and reaches hypothalamus, pituitary, heart, pancreas, intestine, adipose tissue, gonad, and so forth. In recent years, it has been found that ghrelin is also expressed within the vasculature [Citation2], thyroid gland [Citation3], lung [Citation4], heart [Citation5], and so forth. The discovery of ghrelin expression beyond its traditional tissues suggests its role outside of the stomach. Until now, ghrelin appears to have functions in regulating energy balance and nutritional status [Citation6], circadian and visual cues for the hypothalamic arousal system [Citation7], cardiovascular system [Citation8], and the tumorigenesis of various cancers, like lung cancer [Citation9], colorectal adenocarcinomas [Citation10], and gastrointestinal stromal tumors [Citation11].
With regards to the cardiovascular system, ghrelin has been reported to possess regulatory properties in both heart [Citation12] and vasculature [Citation13]. In patients with heart failure, plasma ghrelin was elevated [Citation14]. Further, in vivo investigations demonstrated that ghrelin was capable of improving the function of left ventricular and preventing muscle wasting in patients with heart failure [Citation15]. Ghrelin is also implicated in protecting vascular endothelial cells and preventing various stimulus-induced apoptosis. Liao et al. reported that ghrelin attenuated high glucose and lipid-induced apoptosis and senescence of human microvascular endothelial cells [Citation16]. Besides, ghrelin may act as a survival factor in cardiomyocytes H9c2 through inhibiting apoptosis and oxidative stress [Citation17,Citation18]. These previous studies demonstrated the significant role of ghrelin in the cardiovascular system. However, the beneficial effect of ghrelin on myocardial infarction (MI) has not been fully studied.
MI is a common heart disease and is one of the leading causes of death worldwide. Cardiomyocyte death due to prolonged ischemia has been recognized as the main event occurred during MI [Citation19]. In this study, H9c2 cells were subjected to hypoxia/reoxygenation (H/R) to mimic a cell model of MI. Effects of ghrelin pretreatment on H/R-simulated H9c2 cells were detected to evaluate the potential of ghrelin in MI management. Besides this, the downstream signaling pathways of ghrelin further revealed to decode the possible underlying mechanisms.
Materials and methods
Cell culture and treatment
H9c2 cells (ATCC, Manassas, VA, USA) were cultured in DMEM (Hyclone, Logan, UT, USA) supplementing with 10% heat-inactivated FBS (Gibco, Grand Island, NY, USA). The cells were routinely maintained in a 75 cm2 flask in a humid atmosphere with 5% CO2 at 37 °C, 5–10 passages of cells were used in the follow-up experiments. To make the H/R model, cells were cultured in the glucose-free medium for 6 h in a hypoxic incubator (1% O2). Following hypoxic injury, the cells were returned to normoxic conditions (5% CO2 in air) for 3 h [Citation20].
Prior to H/R, the cells were pretreated with various concentrations of human ghrelin (RongChuang Technology Co., Ltd., Shanghai, China) for 2 h. LY294002 (Selleck Chemicals, Houston, TX, USA) and Compound C (Selleck Chemicals) were used as the inhibitors of PI3K/AKT and AMPK. The cells were incubated for 24 h with 50 μM of LY294002 or 20 μM of Compound C.
siRNA transfection
The expression of ghrelin was suppressed by siRNA-mediated silencing. siRNA specific for ghrelin and its non-targeting control (si-NC) were obtained from GenePharma (Shanghai, China). Lipofectamine RNAimax (Invitrogen, Carlsbad, CA, USA) was utilized for transfection and lasted for 48 h.
CCK-8 assay
The cells in the exponential phase (5 × 103 cells/well) were treated by ghrelin for 2 h and stimulated by H/R in 96-well plates. Forty-eight hours later, cell viability was determined by CCK-8 solution (Dojindo Molecular Technologies, Kyushu, Japan). OD-value at 450 nm was measured using a Microplate Reader (Bio-Rad, Hercules, CA, USA).
LDH assay
The cytotoxicity of the treatments was evaluated by detecting the release of LDH from H9c2 cells using LDH Cytotoxicity Assay Kit (Beyotime, Shanghai, China). 5 × 103 cells were planted in 96-well plates. The cells were starved by DMEM medium containing 1% FBS overnight and then, the cells were treated by ghrelin and were stimulated by H/R. The 10 μl LDH release reagent was added, the culture supernatant was collected and then LDH activity was detected.
Apoptosis assay
H9c2 cells (5 × 105 cells/well) in 6-well plates were treated by ghrelin and stimulated by H/R. Annexin V-FITC Apoptosis Detection Kit (Beyotime) was utilized for apoptosis assessment. Flow cytometry analysis was done by a FACS can (Beckman Coulter, Fullerton, CA, USA). The data were analyzed by FlowJo software (Treestar, Ashland, OR, USA).
Western blot
Proteins in H9c2 cells were extracted by RIPA lysis buffer (Beyotime). Immunoblotting was carried out by using the following primary antibodies: ghrelin (ab129383), CyclinD1 (ab16663), CDK4 (ab137675), Bax (ab32503), Bcl-2 (ab692), caspase-3 (ab32351), Cox-2 (ab23672), iNOS (ab15323), AMPK (ab32047), p-AMPK (ab131357, Abcam, Cambridge, MA, USA), PI3K (orb395443), p-PI3K (orb106105), AKT (orb29949), p-AKT (orb344403), β-actin (orb69116, Biorbyt, San Francisco, CA, USA). After incubation with the secondary antibodies, the signal was developed by chemiluminescence and autoradiography. Image Lab™ Software (Bio-Rad) was used for calculating relative protein expression.
Statistics
Results represented as mean ± SD. SPSS 19.0 (Chicago, IL, USA) was used for statistical analysis. Difference between groups was tested by ANOVA with Duncan post-hoc procedure. p Values of <.05 was considered to indicate the statistical difference.
Results
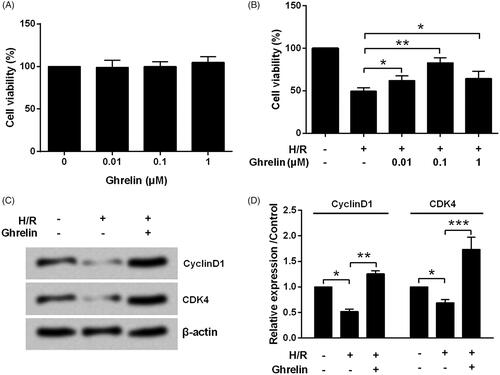
Ghrelin protects H9c2 cells against H/R-induced growth inhibition
By performing the CCK-8 assay, we found that the viability of H9c2 cells treated by 0.01, 0.1 and 1 μM ghrelin was unchanged when compared with the untreated cells (). Next, the effects of ghrelin pre-treatment on H/R-injured H9c2 cells were studied. As a result, the viability in H/R group was increased from 49.54 to 62.18% by 0.01 μM ghrelin (p < .05), to 89.89% by 0.1 μM ghrelin (p < .01), and to 64.50% by 1 μM ghrelin (p < .05, ). For the following investigations, 0.1 μM was selected as a ghrelin-treating concentration. We found that H/R significantly reduced the expression levels of CyclinD1 and CDK4 proteins (both p < .05, ). However, ghrelin pretreatment significantly increased CyclinD1 (p < .01) and CDK4 (p < .001) levels.
Figure 1. Ghrelin protects H9c2 cells against hypoxia/reoxygenation (H/R)-induced growth inhibition. (A) H9c2 cells were treated with various concentrations of ghrelin. (B) H9c2 cells were pre-treated with various concentrations of ghrelin and then subjected to H/R. Cell viability was tested by CCK-8 assay. (C–D) After the treatment of 0.1 μM ghrelin and stimulation with H/R, expression of cell-cycle-related proteins was measured by Western blot. *, ** and *** stand for p < .05, p < .01 and p < .001, respectively.
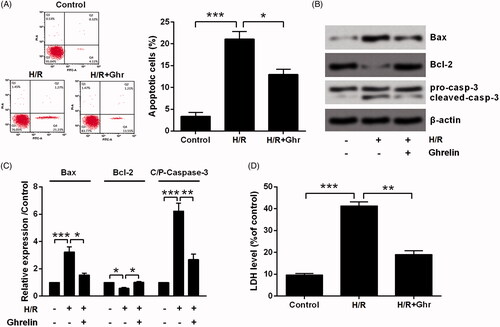
Ghrelin protects H9c2 cells against H/R-induced cell death
Flow cytometry detection results in showed that the apoptosis rate was increased by H/R from 3.39 to 21.10% (p < .001) and was reduced by ghrelin pretreatment to 12.97% (p < .05). Consistently, Bax was up-regulated (p < .001), Bcl-2 was down-regulated (p < .05) and caspase-3 was cleaved (p < .001) by H/R, while the aberrant expression induced by H/R was attenuated by ghrelin pretreatment (p < .05 or p < .01, ). Besides, the release of LDH from H9c2 cells was significantly increased by H/R (p < .001) and was attenuated by ghrelin (p < .01, ).
Figure 2. Ghrelin protects H9c2 cells against hypoxia/reoxygenation (H/R)-induced apoptosis and LDH release. H9c2 cells were pretreated with 0.1 μM ghrelin and were subjected to H/R. (A) Apoptosis rate, (B–C) expression of apoptosis-related proteins, and (D) the release of LDH were respectively assessed by flow cytometry, Western blot, and LDH Cytotoxicity Assay Kit. *, ** and *** stand for p < .05, p < .01 and p < .001, respectively.
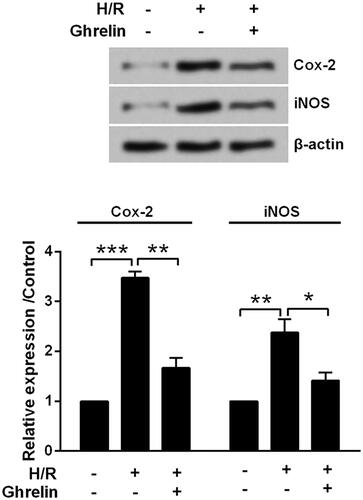
Previous studies have revealed hypoxia triggered a significant cell death by mediating iNOS/Cox-2 pathway [Citation21–23]. Thus, we detected the expression changes of Cox-2 and iNOS in H9c2 cells following ghrelin pre-treatment and H/R. Results from indicated that expression levels of Cox-2 (p < .001) and iNOS proteins (p < .01) were both significantly increased following H/R. The up-regulation of these two induced by H/R were attenuated by ghrelin pretreatment (p < .01 and p < .05).
Figure 3. Ghrelin protects H9c2 cells against hypoxia/reoxygenation (H/R)-induced the expression of Cox-2 and iNOS. H9c2 cells were pretreated with 0.1 μM ghrelin and were subjected to H/R. Expression of Cox-2 and iNOS was measured by Western blot. *, ** and *** stand for p<.05, p<.01 and p<.001, respectively.
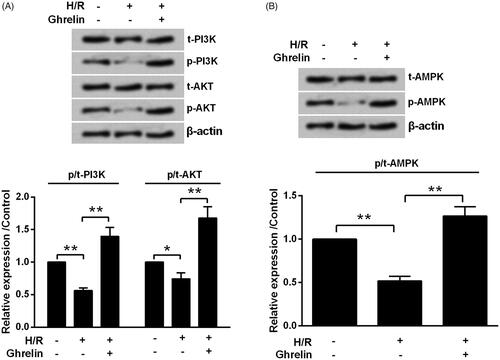
Ghrelin activates PI3K/AKT and AMPK pathways
For further investigation, the activation of PI3K/AKT and AMPK pathways was tested to reveal the possible underlying mechanisms of ghrelin’s protective action. displayed that H/R significantly decreased the phosphorylation levels of PI3K (p < .01), AKT (p < .05) and AMPK (p < .01). However, the phosphorylation levels of those proteins (all p < .01) were increased by ghrelin pretreatment.
Ghrelin protects H9c2 cells against H/R-induced cell damage via PI3K/AKT and AMPK pathways
Next, LY294002 and Compound C were used to treat H9c2 cells for inhibiting PI3K/AKT and AMPK pathways, respectively. We found that LY294002 remarkably reduced the phosphorylation levels of PI3K and AKT and Compound C remarkably reduced the phosphorylation level of AMPK (). Of note, the protective effects of ghrelin on H9c2 cells injured by H/R were all weakened by the addition of LY294002 and Compound C. LY294002 and Compound C treatment significantly decreased H9c2 cells viability (p < .05, ), induced apoptosis (p < .05 or p < .01, ), and increased the release of LDH (p < .05 or p < .01, ). Also, ghrelin reduced the expression of Cox-2 and iNOS was partially eliminated by the addition of LY294002 and Compound C (p < .05 or p < .001, ).
Figure 5. Ghrelin protects H9c2 cells against hypoxia/reoxygenation (H/R)-induced cell loss via PI3K/AKT and AMPK pathways. (A) Expression of proteins in PI3K/AKT and AMPK pathways was measured by Western blot, after the treatment of LY294002 and Compound C. H9c2 cells were pretreated with 0.1 μM ghrelin in the presence or absence of LY294002/Compound C and then subjected to H/R, (B) cell viability, (C) apoptosis rate, (D–E) expression of apoptosis-related proteins, and (F) the release of LDH were assessed by CCK-8 assay, flow cytometry, Western blot, and LDH Cytotoxicity Assay Kit. *, **, and *** stand for p < .05, p < .01 and p < .001, respectively.
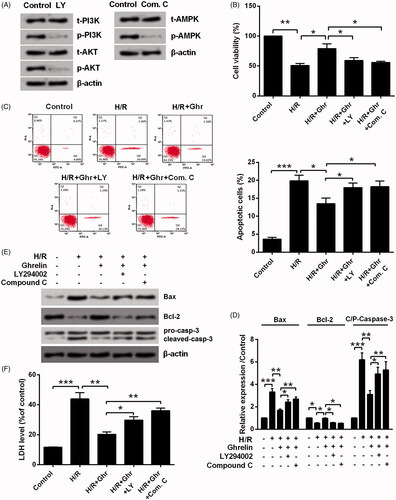
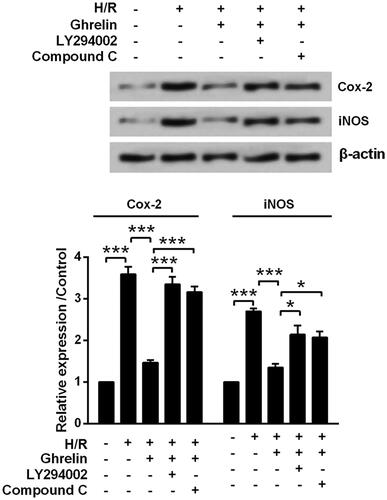
Figure 6. Ghrelin protects H9c2 cells against hypoxia/reoxygenation (H/R)-induced the expression of Cox-2 and iNOS via PI3K/AKT and AMPK pathways. H9c2 cells were pretreated with 0.1 μM ghrelin in the presence or absence of LY294002/Compound C and then were subjected to H/R. Expression of Cox-2 and iNOS was measured by Western blot. * and *** stand for p<.05 and p<.001, respectively.
Silence of ghrelin-induced H9c2 cell death via PI3K/AKT and AMPK pathways
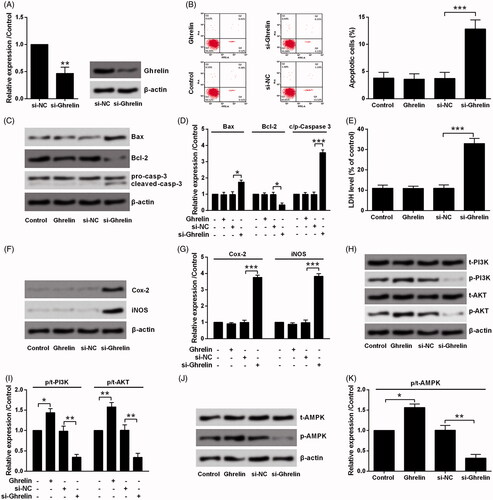
To further confirm the effects of ghrelin, endogenous ghrelin in H9c2 cells was repressed by siRNA-mediated transfection. As results are shown in , mRNA and protein levels of ghrelin were both declined by si-Ghrelin transfection as compared to si-NC transfection. Subsequently, the effects of ghrelin silence on H9c2 cells were studied and exogenous ghrelin was utilized as a positive control. As shown in , ghrelin has no impact on H9c2 cells death, as no significant changes were observed in apoptotic cells rate and LDH release as well as the expression of related proteins. However, the silence of ghrelin could remarkably induce cell death by controlling apoptosis, LDH release, and expression of Cox-2 and iNOS (p < .05 or p < .001). The effects of exogenous and endogenous ghrelin on the activation of PI3K/AKT and AMPK pathways were also tested. showed that exogenous ghrelin significantly increased the phosphorylation levels of PI3K (p < .05), AKT (p < .01) and AMPK (p < .05). As expected, endogenous inhibition of ghrelin decreased the phosphorylation of those proteins (all p < .01).
Figure 7. Silence of ghrelin induced H9c2 cell death via PI3K/AKT and AMPK pathways. (A) Expression of ghrelin in H9c2 cells was measured by qRT-PCR and Western blot, after transfection with the siRNA specific for ghrelin. H9c2 cells were treated with 0.1 μM ghrelin or transfected with ghrelin siRNA. (B) Apoptosis rate, (C–D) expression of apoptosis-related proteins, (E) the release of LDH, (F–G) expression of Cox-2 and iNOS, as well as the expression of proteins in (H–I) PI3K/AKT and (J–K) AMPK pathways were assessed by flow cytometry, Western blot, and LDH Cytotoxicity Assay Kit. *, ** and *** stand for p<.05, p<.01 and p<.001, respectively.
Discussion
In the current study, a cell model of MI was established by stimulating H9c2 cells with H/R to assess the potential functions of ghrelin in MI. We found that H9c2 cells proliferation was repressed by H/R, as cell viability was decreased and cell-cycle-related factors CyclinD1 and CDK4 were down-regulated. Besides, H9c2 cells underwent apoptosis, accompanied by LDH release and the production of Cox-2 and iNOS. Importantly, ghrelin pretreatment protected H9c2 cells against H/R-induced cell damage. Ghrelin conferred its cardiomyocyte protection might be via modulation of PI3K/AKT and AMPK pathways. Besides, endogenous inhibition of ghrelin by using siRNA-mediated silencing significantly induced cell death of H9c2 cells, which further confirmed the cardioprotective function of ghrelin.
A growing number of literature have reported the cardioprotective effects of ghrelin against MI [Citation24–26], suggesting ghrelin as a promising therapeutic agent for MI. During MI, the serum ghrelin levels are decreased and the decreases are correlated with cardiac function [Citation27]. Post-MI in rats, ghrelin prevented apoptosis and oxidative damage, improved cardiac function [Citation24] and led to a decrease in infarct size [Citation28]. Herein, we provided in vitro evidence in H9c2 cells indicating that ghrelin may have cardiomyocyte protective effects against H/R-induced injury. Our findings were consistent with previous studies which are performed in neonatal rat cardiomyocyte [Citation29] and human pulmonary artery endothelial cells [Citation30].
Although the significant role of ghrelin in the cardiovascular system has been widely revealed, the deep reason for its action is not fully understood. Some researchers believed that ghrelin confers its function through modulation of growth hormone level, energy balance, cardiovascular cells’ fate, and autonomic nervous activity [Citation8,Citation31]. Also, current experimental evidence suggests that ghrelin may media various signaling pathways, including PI3K/AKT [Citation30], mTOR [Citation29], ERK1/2 [Citation32], AMPK, NF-κB [Citation33], and MAPK [Citation34] pathways. Among which, PI3K/AKT has been widely accepted as key signaling in the protection of myocardial ischemia-reperfusion injury [Citation35,Citation36]. Also, as an important regulator in the setting of energetic stress, AMPK is essential in controlling glucose uptake and glycolysis and protection of heart from ischemia-induced damage and apoptotic activity [Citation37]. Herein, the activation of PI3K/AKT and AMPK pathways were observed in ghrelin pretreated H9c2 cells. In addition, by using inhibitors of these two signaling (LY294002 and Compound C), the protective effects of ghrelin against H/R-induced cell damage were attenuated. These findings suggested that ghrelin treatment activated these two pathways and thus conferred the protective effects of ghrelin on H/R-stimulated H9c2 cells. Actually, Tong et al. have previously reported the importance of AMPK signaling in ghrelin’s action on CoCl (2)-induced injury in H9c2 cells [Citation17]. Likewise, Yang et al. [Citation30] have mentioned the protective actions of ghrelin on human pulmonary artery endothelial cells against hypoxic and ischemic injury through the PI3K/AKT pathway. However, this study provides the first evidence that ghrelin protected H9c2 cells against H/R-induced injury via these two signaling.
There is continued interest in the development of novel cardioprotective agents for MI. Therapies against ischemic injury, especially cardioprotective agents that are implemented before an ischemic event, have been considered as one of the main strategies for preventing MI. To date, multiple kinds of agents have been mentioned to have cardioprotective effects, such as pioglitazone [Citation38], RRx-001 [Citation39], 17β-Estradiol [Citation40], as well as a natural agent like Tanshinone IIA [Citation41]. Thus, more studies are needed to investigate the superiority and inferiority of ghrelin among these cardioprotective agents. Besides, whether ghrelin can be used inthe clinical management of MI is required to be further studied.
Conclusions
In total, this study demonstrated that ghrelin pretreatment protected H9c2 cells against H/R-induced cell damage, possibly via activation of PI3K/AKT and AMPK signaling pathways. These findings enlarged our understanding of ghrelin and might be helpful for facilitating the clinical use of ghrelin in MI management.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
Authors declare that there is no conflict of interests.
References
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660.
- Lilleness BM, Frishman WH. Ghrelin and the cardiovascular system. Cardiol Rev. 2016;24:288–297.
- Gurgul E, Kasprzak A, Blaszczyk A, et al. Ghrelin and obestatin in thyroid gland – immunohistochemical expression in nodular goiter, papillary and medullary cancer. Folia Histochem Cytobiol. 2015;53:19–25.
- Tian X, Liu Z, Yu T, et al. Ghrelin ameliorates acute lung injury induced by oleic acid via inhibition of endoplasmic reticulum stress. Life Sci. 2018;196:1–8.
- Beiras-Fernandez A, Kreth S, Weis F, et al. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides. 2010;31:2222–2228.
- Gortan Cappellari G, Barazzoni R. Ghrelin forms in the modulation of energy balance and metabolism. Eating and weight disorders. EWD. 2018. doi:10.1007/s40519-018-0599-6.
- Horvath TL, Abizaid A, Dietrich MO, et al. Ghrelin-immunopositive hypothalamic neurons tie the circadian clock and visual system to the lateral hypothalamic arousal center. Mol Metab. 2012;1:79–85.
- Kishimoto I, Tokudome T, Hosoda H, et al. Ghrelin and cardiovascular diseases. J Cardiol. 2012;59:8–13.
- Zhu J, Yao J, Huang R, et al. Ghrelin promotes human non-small cell lung cancer A549 cell proliferation through PI3K/Akt/mTOR/P70S6K and ERK signaling pathways. Biochem Biophys Res Commun. 2018;498:616–620.
- Murphy G, Cross AJ, Dawsey SM, et al. Serum ghrelin is associated with risk of colorectal adenocarcinomas in the ATBC study. Gut 2017;67(9):1646–1651.
- Zhu CZ, Liu D, Kang WM, et al. Ghrelin and gastrointestinal stromal tumors. WJG. 2017;23:1758–1763.
- Wang Q, Lin P, Li P, et al. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017;186:50–58.
- Nagaya N, Miyatake K, Uematsu M, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854–5859.
- Zabarovskaja S, Freda P, Williams JJ, et al. Acylation of ghrelin is increased in heart failure and decreases post heart transplantation. SCJ. 2014;48:343–348.
- Nagaya N, Moriya J, Yasumura Y, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679.
- Liao P, Yang D, Liu D, et al. GLP-1 and Ghrelin attenuate high glucose/high lipid-induced apoptosis and senescence of human microvascular endothelial cells. Cell Physiol Biochem. 2017;44:1842–1855.
- Tong XX, Wu D, Wang X, et al. Ghrelin protects against cobalt chloride-induced hypoxic injury in cardiac H9c2 cells by inhibiting oxidative stress and inducing autophagy. Peptides. 2012;38:217–227.
- Wang X, Yang C, Liu X, et al. Ghrelin alleviates angiotensin II-induced H9c2 apoptosis: impact of the miR-208 family. Med Sci Monit. 2018;24:6707–6716.
- Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875.
- Shen H, Yao Z, Zhao W, et al. miR-21 enhances the protective effect of loperamide on rat cardiomyocytes against hypoxia/reoxygenation, reactive oxygen species production and apoptosis via regulating Akap8 and Bard1 expression. Exp Ther Med. 2019;17:1312–1320.
- Mendes RT, Nguyen D, Stephens D, et al. Hypoxia-induced endothelial cell responses – possible roles during periodontal disease. 2018;4:241–248.
- Bhattacharya I, Dominguez AP, Dragert K, et al. Hypoxia potentiates tumor necrosis factor-alpha induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in white and brown adipocytes. Biochem Biophys Res Commun. 2015;461:287–292.
- Carnieto A, Jr., Dourado PM, Luz PL, et al. Selective cyclooxygenase-2 inhibition protects against myocardial damage in experimental acute ischemia. Clinics (Sao Paulo, Brazil). 2009;64:245–252.
- Eid RA, Alkhateeb MA, Al-Shraim M, et al. Ghrelin prevents cardiac cell apoptosis during cardiac remodelling post experimentally induced myocardial infarction in rats via activation of Raf-MEK1/2-ERK1/2 signalling. Arch Physiol Biochem. 2019; 125:93-103. doi:10.1080/13813455.2018.1437751.
- Eid RA, Zaki MSA, Al-Shraim M, et al. Subacute ghrelin administration inhibits apoptosis and improves ultrastructural abnormalities in remote myocardium post-myocardial infarction. Biomed Pharmacother = Biomedecine & Pharmacotherapie. 2018;101:920–928.
- Eid RA, Alkhateeb MA, Eleawa S, et al. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res Cardiol. 2018;113:13.
- Matsumoto M, Yasuda S, Miyazaki S, et al. Decreased serum ghrelin levels in patients with acute myocardial infarction. Tohoku J Exp Med. 2013;231:235–242.
- Sun N, Wang H, Wang L. Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-kappaB pathway. Mol Med Rep. 2016;14:2764–2770.
- Wang L, Lu Y, Liu X, et al. Ghrelin protected neonatal rat cardiomyocyte against hypoxia/reoxygenation injury by inhibiting apoptosis through Akt-mTOR signal. Mol Biol Rep. 2017;44:219–226.
- Yang D, Liu Z, Zhang H, et al. Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides. 2013;42:112–117.
- Zhang G, Yin X, Qi Y, et al. Ghrelin and cardiovascular diseases. CCR. 2010;6:62–70.
- Ye N, Wang L, Dou Z, et al. Ghrelin accelerates the cartilagic differentiation of rabbit mesenchymal stem cells through the ERK1/2 pathway. Cytotechnology. 2018;70:415–421.
- Zhang M, Wang S, Pan Z, et al. AMPK/NF-kappaB signaling pathway regulated by ghrelin participates in the regulation of HUVEC and THP1 Inflammation. Mol Cell Biochem. 2018;437:45–53.
- Wang X, Wang XL, Chen HL, et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. 2014;88:334–350.
- Tang L, Mo Y, Li Y, et al. Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem Biophys Res Commun. 2017;486:774–780.
- Sulaiman D, Li J, Devarajan A, et al. Paraoxonase 2 protects against acute myocardial ischemia-reperfusion injury by modulating mitochondrial function and oxidative stress via the PI3K/Akt/GSK-3beta RISK pathway. J Mol Cell Cardiol. 2019;129:154–164.
- Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. TEM. 2015;26:422–429.
- Mittal D, Taliyan R, Sharma PL, et al. Effect of pioglitazone on the abrogated cardioprotective effect of ischemic preconditioning in hyperlipidemic rat heart. Indian J Pharmacol. 2016;48:59–63.
- Oronsky B, Ao-Ieong ES, Yalcin O, et al. Cardioprotective effect of phase 3 clinical anticancer agent, RRx-001, in doxorubicin-induced acute cardiotoxicity in mice. Mol Pharm. 2019. doi:10.1021/acs.molpharmaceut.9b00150.
- Angeloni C, Teti G, Barbalace MC, et al. 17Beta-estradiol enhances sulforaphane cardioprotection against oxidative stress. J Nutr Biochem. 2017;42:26–36.
- Gu Y, Liang Z, Wang H, et al. Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Exp Ther Med. 2016;12:1147–1152.