Abstract
Swainsonine (SW) is an indolizidine alkaloid first discovered in Swainsona canescens. This study explored the effects of SW on mouse embryo fibroblast NIH-3T3 cell proliferation and collagen synthesis, as well as potential molecule mechanisms. We discovered that SW exposure lowered the viability and proliferation of NIH-3T3 cells. The collagen synthesis was reduced after SW exposure, as evidenced by declines of the mRNA and protein levels of collagen I (CoI I), collagen III (CoI III) and α-smooth muscle actin (α-SMA) in NIH-3T3 cells, as well as reduction of collagen concentration in the culture supernatant of NIH-3T3 cells. Mechanically, transforming growth factor β1 (TGF-β1) stimulation elevated the microRNA-21 (miR-21) expression in NIH-3T3 cells. SW reversed the TGF-β1-caused elevation of miR-21. Up-regulation of miR-21 attenuated the inhibitory influences of SW on NIH-3T3 cell viability, proliferation and collagen synthesis. Silence of miR-21 had converse influence. Besides, SW inactivated PI3K/AKT and NF-κB pathways via declining miR-21. Altogether, SW inhibited the proliferation and collagen synthesis of fibroblast NIH-3T3 might be through declining miR-21 and then suppressing PI3K/AKT and NF-κB pathways. SW may be an effective therapeutic medicine for scar hyperplasia.
Introduction
Scar is a pathological change of skin tissue caused by various traumas [Citation1]. It is an inevitable product in the process of human wound repair [Citation2]. When the scar grows beyond a certain boundary, a number of complications, such as damage of the shape and functional activity disorder, may happen, which brings great physical and mental pain to the patients [Citation3]. Hyper-proliferation and collagen synthesis of fibroblasts have been considered as the major pathological features of scar hyperplasia [Citation4,Citation5]. Therefore, searching drugs that can inhibit fibroblast hyper-proliferation and collagen synthesis is of great significance for the clinical treatment of scar hyperplasia.
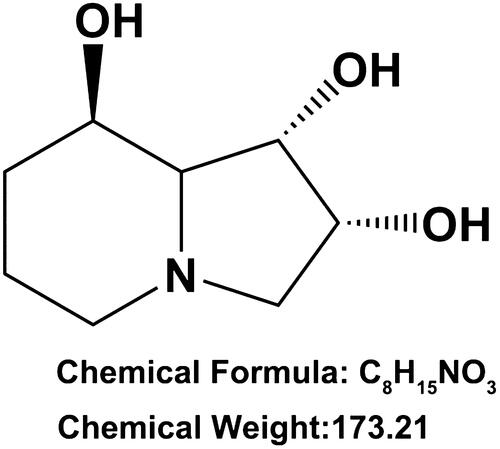
Swainsonine (SW, CAS number: 72741–87-8, ) is an indolizidine alkaloid first discovered in Swainsona canescens and widely disturbed in a number of plants worldwide [Citation6]. Currently, there are three pathways for SW production: isolation from infected plants, synthesis using chemical methods and fermentation from fungi [Citation7]. Previous studies proved that SW could serve as a special inhibitor of α-mannosidase II in the Golgi complex to influence the synthesis of a number of carbohydrates, glycoproteins and glycolipids in cells, as well as reduce the concentration of oligosaccharides at the cell surface [Citation7,Citation8]. Chotai et al. reported that SW could uptake into normal human fibroblasts in culture [Citation9]. Cenci et al. indicated that SW could induce the accumulation of mannose-rich oligosaccharides in human fibroblasts [Citation10]. However, until now, no any literature is available concerning the effects of SW on fibroblasts proliferation and collagen synthesis.
MicroRNAs (miRNAs) are small and non-coding RNAs with critical regulatory activities in multiple biological processes [Citation11]. It is proved that miRNAs were associated with the modulation of many genes by transcriptional cleavage or translation repression [Citation12]. Moreover, miRNAs also can be modulated by a number of compounds, including alkaloid isolated from the plants [Citation13,Citation14]. miRNA-21 (miR-21) has been discovered to possess fibro-proliferative activity in hypertrophic scars derived fibroblasts [Citation15,Citation16]. Li et al. reported that transforming growth factor β1 (TGF-β1) promoted scar fibroblasts proliferation and trans-differentiation through enhancing miR-21 expression [Citation17].
Herein, we tested the influences of SW on fibroblasts proliferation and collagen synthesis. TGF-β1 was used as the stimulator of collagen synthesis [Citation18]. Mechanically, we analyzed the regulatory activity of SW on miR-21 expression, as well as PI3K/AKT and NF-κB pathways in fibroblasts. We think that the outcomes of our research will afford experimental evidence for comprehension of the beneficial influences of SW on scar hyperplasia.
Materials and methods
Cell culture and treatment
Mouse embryo fibroblast NIH-3T3 cells (National Infrastructure of Cell Line Resource, Beijing, China) were grown in DMEM (11960–044, Invitrogen, Carlsbad, CA, USA) including with 10% (v/v) FBS (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd, Hangzhou, China ), 1% (v/v) non-essential amino acid (100X, 11140–050, Invitrogen) and 1% (v/v) sodium pyruvate solution (100 nM, 11360–070, Invitrogen) in flask at 37 °C with 5% CO2.
SW powder was received from Sigma-Aldrich (purity >98%, St Louis, MO, USA), dissolved in distilled water to 1 mg/ml, sterilized by ultrafiltration (Millipore, Bedford, MA, USA) and stored at −20 °C until use. NIH-3T3 cells were treated by 10, 20, 30, 40 or 50 μM SW for 24 h.
To stimulate collagen generation, NIH-3T3 cells were stimulated by 5 ng/ml TGF-β1 (100–21, Peprotech, Hamburg, Germany) for 24 h as described previously [Citation19].
miRNA transfection
miR-21 mimic, miR-21 inhibitor, NC mimic and inhibitor NC were received from GenePharma Corporation (Shanghai, China). The sequences of miR-21 mimic were: 5′-UAGCUUAUCAGACUGAUGUUGA-3′ (sense) and 5′-AACAUCAGUCUGAUAAGCUAUU-3′ (antisense). The sequence of miR-21 inhibitor was: 5′-UCAACAUCAGUCUGAUAAUCUA -3′. miR-21 mimic, miR-21 inhibitor, NC mimic or inhibitor NC was transfected into NIH-3T3 cells with the help of LipofectamineTM 3000 Transfection Reagent (L3000-008, Invitrogen). Transfection efficiency was tested by miR-21 expression in HIH-3T3 cells.
qRT-PCR
The collagen I (CoI I), collagen III (CoI III), α-smooth muscle actin (α-SMA) and miR-21 expressions in NIH-3T3 cells were measured using qRT-PCR. Briefly, after different stimulation, total RNAs in NIH-3T3 cells were collected with the help of RNA isolation Plus kit (9109, Takara Biomedical Technology, Beijing, China) supplemented with Recombinant DNase I (RNase-free) solution (2270 A, Takara Biomedical Technology, Beijing, China). Then, the CoI I, CoI III and α-SMA expressions were measured with the help of PrimeScriptTM RT-PCR kit (RR014(A × 4), Takara Biomedical Technology) and normalized to β-actin. The miR-21 expression was measured with the help of Mir-XTM miRNA qRT-PCR TB GreenTM kit (638316, Takara Biomedical Technology) and normalized to U6 snRNA. Primers for CoI I were: 5′-CCCCCTCCCCAGCCACAAAG-3′ (F) and 5′-TCTTGGTCGGTGGGTGACTCT-3′ (R). Primers for CoI III were: 5′-CCAAACTCTATCTGAA-3′ (F) and 5′-GGACTCATAGAATACA-3′ (R). Primers for α-SMA were: 5′-AATGAGATGGCCACTGCCGC-3′ (F) and 5′-CAGAGTATTTGCGCTCCGGA-3′ (R). Primers for β-actin were: 5′-TGCTTGCTGATCCACATCTG-3′ (F) and 5′-TGCTTGCTGATCCACATCTG-3′ (R). Primers for miR-21 were: 5′-GCCCGCTAGCTTATCAGACTGATC-3′ (F) and 5′-GTGCAGGGTCCGAGGT-3′ (R). Primers for U6 snRNA were: 5′-CTCGCTTCGGCAGCACA-3′ (F) and 5′-AACGCTTCACGAATTTGCGT-3′ (R). Data were calculated by 2-△△Ct method [Citation20].
Cell viability assay
Cell counting kit-8 (CCK-8) assay (HY-K0301, MedChemExpress, New Jersey, NJ, USA) offers a method to test cell viability. Briefly, NIH-3T3 cells were seeded into 96-well plate with 1 × 104 cells/well and exposed to 10, 20, 30, 40 or 50 μM SW for 24 h. Subsequently, 10 μl CCK-8 kit solution was mixed into the culture medium of each well for 1 h at 37 °C. After that, the absorbance was measured. Data were presented as the percentage of control.
Collagen generation assay
NIH-3T3 cells were cultivated into 24-well plate with 3 × 104 cells/well and exposed to 5 ng/ml TGF-β1 and/or 30 μM SW for 24 h. Then, the culture supernatant was collected in line with the experiment design and the total soluble collagen concentration in the culture supernatant was tested using Sircol Collagen Assay kit (S1000, Biocolor Ltd. Antrim, United Kingdom).
Western blotting
Total proteins in NIH-3T3 cells were separated using RIPA lysis and extraction buffer (Thermo Scientific Fisher, Waltham, MA, USA) including protease inhibitors (58927–1001, Roche, Basel, Switzerland). BCA Protein Assay kit (P0011, Beyotime Biotechnology, Shanghai, China) was used for measuring the concentration of total proteins. Western blotting was performed as previously described [Citation21]. Following antibodies were used: anti-vascular endothelial growth factor (VEGF) antibody (ab11939), anti-Cyclin D1 antibody (ab40754), anti-p53 antibody (ab131442), anti-CoI I antibody (ab34710), anti-CoI III antibody (ab7778), anti-α-SMA antibody (ab5694), anti-t-PI3K antibody (ab86714), anti-p-PI3K antibody (ab182651), anti-t-AKT antibody (ab8805), anti-p-AKT antibody (ab38449), anti-t-p65 antibody (ab16502), anti-p-p65 antibody (ab131100), anti-t-IκBα antibody (ab7217), anti-p-IκBα antibody (ab24783), anti-β-actin antibody (ab8226), goat anti-rabbit IgG (H + L) HRP (ab205718) and goat anti-mouse IgG (H + L) HRP (ab205719, Abcam Biotechnology, Cambridge, MA, USA). Bio-Rad ChemiDocTM XRS system (Bio-Rad Laboratories, Hercules, CA, USA) offer a method to capture protein signals. Data were calculated with the help of Image LabTM software (Bio-Rad Laboratories).
Statistical analysis
Data were presented as the mean ± standard deviation (SD) from at least three repeated experiments. Graphpad 6.0 software (Graphpad, San Diego, CA, USA) was carried out for statistical analysis. p Values were calculated using one-way analysis of variance (ANOVA). A significant difference was set at p ˂ .05.
Results
SW inhibited NIH-3T3 cell proliferation and collagen synthesis
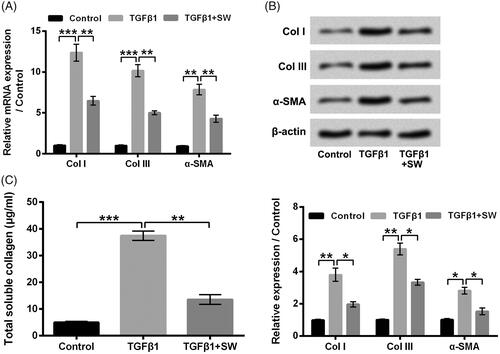
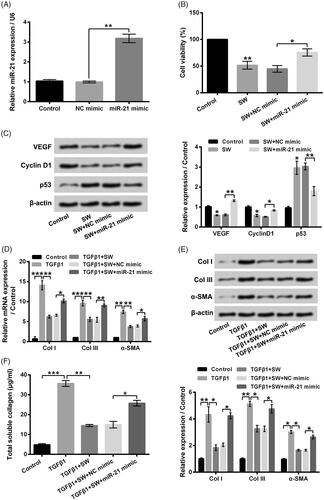
showed that 10–50 μM SW exposure suppressed NIH-3T3 cell viability with a concentration-dependent pathway (p < .05, p < .01 or p < .001). 30 μM SW exposure lowered the NIH-3T3 cell viability to 50.32 ± 2.59%, which was chosen for use in further experiments. The VEGF and Cyclin D1 expressions were both decreased, while the p53 level was increased in NIH-3T3 cells after 30 μM SW exposure (p < .05 or p < .01), indicating that SW inhibited the proliferation of NIH-3T3 cells. Moreover, displayed that 5 ng/ml TGF-β1 stimulation remarkably up-regulated the mRNA and protein levels of CoI I, CoI III and α-SMA in NIH-3T3 cells (p < .05, p < .01 or p < .001). 30 μM SW exposure notably alleviated the increases of CoI I, CoI III and α-SMA expressions in NIH-3T3 cells caused by 5 ng/ml TGF-β1 stimulation (p < .05 or p < .01). Besides, 30 μM SW exposure also relieved the 5 ng/ml TGF-β1 stimulation-caused enhancement of collagen generation in the culture supernatant of NIH-3T3 cells (, p < .01). These outcomes suggested that SW could inhibit NIH-3T3 cell viability, proliferation and collagen synthesis.
Figure 2. SW inhibited NIH-3T3 cell viability and proliferation. (A) Viability of NIH-3T3 cells after 10, 20, 30, 40 or 50 μM SW exposure was tested. (B) The VEGF, Cyclin D1 and p53 protein levels in NIH-3T3 cells after 30 μM SW exposure were assessed. N = 3. Data were showed as mean ± SD. p-Values were tested by ANOVA. *p ˂ .05; **p ˂ .01; ***p ˂ .001.
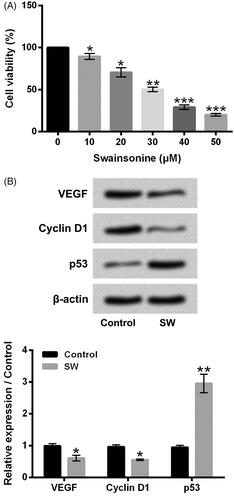
Figure 3. SW suppressed collagen synthesis in NIH-3T3 cells. (A and B) The CoI I, CoI III and α-SMA mRNA and protein levels in NIH-3T3 cells after 5 ng/ml TGF-β1 stimulation and/or 30 μM SW exposure were assessed respectively. (B) Followed by 5 ng/ml TGF-β1 stimulation and/or 30 μM SW exposure, the concentration of collagen in culture supernatant of NIH-3T3 cells was measured. N = 3. Data were shown as mean ± SD. p-Values were tested by ANOVA. *p ˂ .05; **p ˂ .01; ***p ˂ .001.
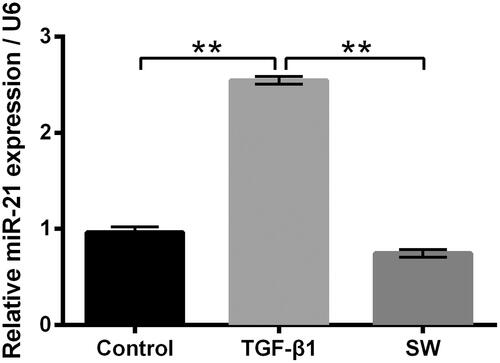
SW declined the miR-21 expression in TGF-β1-treated NIH-3T3 cells
Then, the miR-21 expression in NIH-3T3 cells after 5 ng/ml TGF-β1 and/or 30 μM SW exposure were tested. As shown in 5 ng/ml TGF-β1 stimulation significantly elevated the miR-21 expression in NIH-3T3 cells (p < .01). 30 μM SW exposure notably reversed the TGF-β1 stimulation-caused elevation of miR-21 in NIH-3T3 cells (p < .01). These outcomes hinted that miR-21 might be associated with the inhibitory effects of SW on NIH-3T3 cell viability, proliferation and collagen synthesis.
Overexpression of miR-21 attenuated the influences of SW on NIH-3T3 cells
To elevate miR-21 expression in NIH-3T3 cells, miR-21 mimic was transfected into NIH-3T3 cells ( < .01). displayed that miR-21 mimic transfection dramatically mitigated the SW exposure-caused NIH-3T3 cell viability reduction (p < .05). The VEGF and Cyclin D1 expressions in NIH-3T3 cells were both increased, while the p53 level was decreased followed by miR-21 mimic transfection ( < .05 or p < .01). Moreover, relative to TGF-β1 + SW + NC mimic group, the CoI I, CoI III and α-SMA expressions in NIH-3T3 cells were all elevated in TGF-β1 + SW + miR-21 mimic group (, p < .05 or p < .01). pointed out that miR-21 mimic transfection also alleviated the inhibitory effect of SW on collagen synthesis in TGF-β1-stimulated NIH-3T3 cells (, p < .05). These outcomes illustrated that SW inhibited NIH-3T3 cell viability, proliferation and collagen synthesis might be via declining miR-21.
Figure 5. Overexpression of miR-21 attenuated the influences of SW on NIH-3T3 cells. (A) The miR-21 expression in NIH-3T3 cells was measured after relevant transfection. Followed by 30 μM SW exposure and/or miR-21 mimic (NC mimic) transfection, (B) NIH-3T3 cell viability and (C) the VEGF, Cyclin D1 and p53 protein levels in NIH-3T3 cells were assessed. Followed by 5 ng/ml TGF-β1 stimulation and/or 30 μM SW exposure or miR-21 mimic (NC mimic) transfection, (D and E) the mRNA and protein levels of CoI I, CoI III and α-SMA in NIH-3T3 cells were assessed and (F) the concentration of collagen in culture supernatant of NIH-3T3 cells was measured. N = 3. Data were shown as mean ± SD. p-Values were tested by ANOVA. *p ˂ .05; **p ˂ .01; ***p ˂ .001.
Silence of miR-21 promoted the effects of SW on NIH-3T3 cells
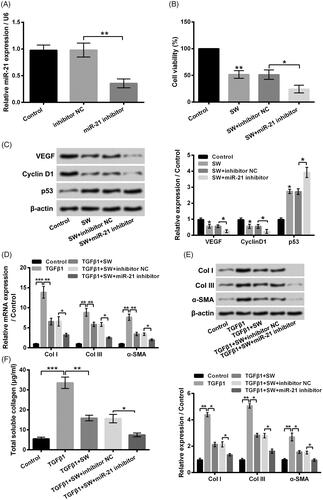
In order to further analyze the influence of miR-21 on SW-caused NIH-3T3 cell viability decrease, proliferation suppression and collagen synthesis inhibition, miR-21 inhibitor was also transfected into NIH-3T3 cells. Data in pointed out that miR-21 expression was lowered in NIH-3T3 cells followed by miR-21 inhibitor transfection (p < .01). illustrated that miR-21 inhibitor transfection notably promoted the SW-caused NIH-3T3 cell viability decrease (p < .05). Relative to SW + inhibitor NC group, the VEGF, Cyclin D1 protein levels were reduced, and the p53 level was enhanced in SW + miR-21 inhibitor group (, p < .05). Moreover, the CoI I, CoI III and α-SMA expressions in NIH-3T3 cells were all declined in TGF-β1 + SW + miR-21 inhibitor group, relative to TGF-β1 + SW + inhibitor NC group (, p < .05). Besides, miR-21 inhibitor also promoted the inhibitory effect of SW on collagen synthesis in TGF-β1-stimulated NIH-3T3 cells (, p < .05). These outcomes further confirmed that SW inhibited NIH-3T3 cell viability, proliferation and collagen synthesis via down-regulating miR-21.
Figure 6. Silence of miR-21 promoted the influences of SW on NIH-3T3 cells. (A) Followed by different transfection, the miR-21 expression in NIH-3T3 cells was tested. Followed by 30 μM SW exposure and/or miR-21 inhibitor (inhibitor NC) transfection, (B) the NIH-3T3 cell viability and (C) the VEGF, Cyclin D1 and p53 protein levels in NIH-3T3 cells were assessed, respectively. Followed by 5 ng/ml TGF-β1 stimulation and/or 30 μM SW exposure or miR-21 inhibitor (inhibitor NC) transfection, (D and E) the mRNA and protein levels of CoI I, CoI III and α-SMA in NIH-3T3 cells, as well as (F) the concentration of collagen in culture supernatant of NIH-3T3 cells were tested, respectively. N = 3. Data were shown as mean ± SD. p-Values were tested by ANOVA. *p˂.05; **p˂.01; ***p˂.001.
SW suppressed PI3K/AKT and NF-κB pathways in NIH-3T3 cells via declining miR-21
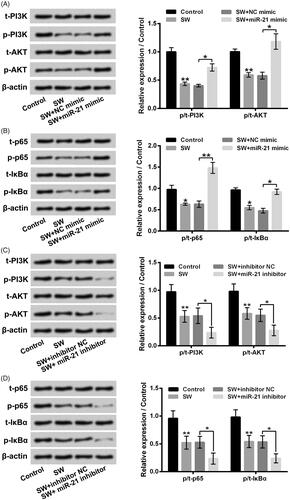
showed that SW exposure suppressed PI3K/AKT and NF-κB pathways in NIH-3T3 cells by decreasing p/t-PI3K, p/t-AKT, p/t-p65 and p/t-IκBα expression rates (p < .05 or p < .01). miR-21 mimic transfection notably reversed the effects of SW on PI3K/AKT and NF-κB pathways in NIH-3T3 cells (p < .05 or p < .01). miR-21 inhibitor transfection had opposite influence (), p < .05), which further aggravated the SW-caused inactivation of PI3K/AKT and NF-κB pathways. These outcomes illustrated that SW could suppress PI3K/AKT and NF-κB pathways in NIH-3T3 cells via declining miR-21.
Figure 7. SW suppressed PI3K/AKT and NF-κB pathways in NIH-3T3 cells via declining miR-21. (A–D) Followed by 30 μM SW exposure and/or miR-21 mimic (NC mimic) or miR-21 inhibitor (inhibitor NC) transfection, the t-PI3K, p-PI3K, t-AKT, p-AKT, t-p65, p-p65, t-IκBα and p-IκBα expressions in NIH-3T3 cells were assessed. N = 3. Data were shown as mean ± SD. p-Values were tested by ANOVA. *p˂.05; **p˂.01.
Discussion
Scar hyperplasia brings great physical and mental pain to the patients [Citation3]. Thus, considerable efforts have been devoted to the clinical management and exposure of scar hyperplasia [Citation22,Citation23]. Herein, we revealed that SW, an indolizidine alkaloid [Citation6], could inhibit the proliferation and collagen synthesis of fibroblast NIH-3T3. Mechanically, we discovered that SW reduced the miR-21 expression in TGF-β1-treated NIH-3T3 cells. Reduction of miR-21 was closely related to the inhibitory effects of SW on NIH-3T3 cell proliferation and collagen synthesis. Besides, SW could suppress PI3K/AKT and NF-κB pathways in NIH-3T3 cells via declining miR-21.
Dynamic biological changes in fibroblasts have been found to be related to the formation and progression of hypertrophic scar [Citation4,Citation24]. The hyper-expression of TGF-β1 and VEGF in fibroblasts both contributes to the progression of hypertrophic scar [Citation4]. TGF-β1 can promote the synthesis of CoI I and CoI III [Citation25]. VEFG can promote the angiogenesis in hypertrophic scar [Citation26]. As an inhibitor of α-mannosidase II, SW can influence the synthesis of many carbohydrates, glycoproteins and glycolipids in cells and oligosaccharides at the cell surface [Citation8]. In this research, we found that SW inhibited NIH-3T3 cell viability with a dose-dependent pathway. Cyclin D1, which played key roles in promoting cell proliferation [Citation27], was decreased in NIH-3T3 cells. p53, which played key roles in inhibiting cell proliferation [Citation28], was increased in NIH-3T3 cells. Moreover, the VEFG level was also reduced in NIH-3T3 cells. TGF-β1 was used as the stimulator of collagen synthesis in NIH-3T3 cells. We discovered that SW alleviated the TGF-β1 stimulation-caused enhancement of CoI I, CoI III and α-SMA in NIH-3T3 cells, as well as collagen generation in the culture supernatant of NIH-3T3 cells. These above outcomes indicated that SW could exert inhibitory function on scar hyperplasia via suppressing fibroblasts cell viability, proliferation and collagen synthesis.
miRNAs are located throughout the genome [Citation11]. The biological importance of miRNAs in cell function regulation has been widely demonstrated [Citation29,Citation30]. miRNAs have also been discovered to be related to the development of many human diseases, including fibroblasts proliferation and collagen synthesis in scar hyperplasia [Citation15,Citation31,Citation32]. Previous study indicated that TGF-β1 could elevate the expression level of miR-21 in fibroblasts [Citation17]. We revealed that TGF-β1 elevated the miR-21 expression in NIH-3T3 cells. Besides, SW reversed the TGF-β1-caused elevation of miR-21 in NIH-3T3 cells. More importantly, overexpression of miR-21 attenuated the influences of SW on NIH-3T3 cells, while silence of miR-21 promoted the influences of SW on NIH-3T3 cells. These findings suggested that SW exerted inhibitory function on fibroblasts viability, proliferation and collagen synthesis, at least partially via declining miR-21.
PI3K/AKT and NF-κB pathways are two important pro-proliferative and pro-survival signal transduction pathway in cells, which both take part in the modulation of collagen synthesis in fibroblasts [Citation33–35]. Zhang et al. reported that increased periostin expression affected the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxia condition by regulating the PI3K/AKT pathway [Citation36]. Poligone et al. proved that increased NF-κB activity led to the hyperproliferation and dysplasia of mouse epidermis [Citation37]. In the present research, we discovered that SW suppressed the PI3K/AKT and NF-κB pathways in NIH-3T3 cells. Overexpression of miR-21 reversed the suppression of PI3K/AKT and NF-κB pathways in NIH-3T3 cells caused by SW. Silence of miR-21 had the opposite influence. These findings implied that SW inhibited fibroblasts viability, proliferation and collagen synthesis might be achieved by down-regulating miR-21 and then suppressing PI3K/AKT and NF-κB pathways.
In total, we verified the inhibitory influences of SW on fibroblasts proliferation and collagen synthesis. SW inhibited the proliferation and collagen synthesis of fibroblast NIH-3T3 at least be achieved by declining miR-21 and then suppressing PI3K/AKT and NF-κB pathways. We propose that SW may be an effective therapeutic medicine for scar hyperplasia, although further in vivo study and safety evaluation are still needed.
Disclosure statement
The authors declare no conflict of interest.
References
- Kerwin LY, El Tal AK, Stiff MA. Scar prevention and remodeling: a review of the medical, surgical, topical and light treatment approaches. Int J Dermatol. 2014;53:922–936.
- Zhu Z, Ding J, Ma Z, et al. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Rep Reg. 2016;24:644–656.
- Guo J, Lin Q, Shao Y, et al. BMP7 suppresses excessive scar formation by activating the BMP7/Smad1/5/8 signaling pathway. Mol Med Rep. 2017;16:1957–1963.
- Chun Q, ZhiYong W, Fei S, et al. Dynamic biological changes in fibroblasts during hypertrophic scar formation and regression. Int Wound J. 2016;13:257–262.
- Nong Q, Li S, Wu Y, et al. LncRNA COL1A2-AS1 inhibits the scar fibroblasts proliferation via regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun. 2018;495:319–324.
- Cook D, Gardner DR, Pfister JA. Swainsonine-containing plants and their relationship to endophytic fungi. J Agric Food Chem. 2014;62:7326–7334.
- Ren Z, Song R, Wang S, et al. The biosynthesis pathway of Swainsonine, a new anticancer drug from three endophytic fungi. J Microbiol Biotechnol. 2017;27:1897–1906.
- Wu C, Han T, Lu H, et al. The toxicology mechanism of endophytic fungus and swainsonine in locoweed. Environ Toxicol Pharmacol. 2016;47:38–46.
- Chotai K, Jennings C, Winchester B, et al. The uptake of swainsonine, a specific inhibitor of alpha-D-mannosidase, into normal human fibroblasts in culture. J Cell Biochem. 1983;21:107–117.
- Tulsiani DR, Broquist HP, James LF, et al. Production of hybrid glycoproteins and accumulation of oligosaccharides in the brain of sheep and pigs administered swainsonine or locoweed. Arch Biochem Biophys. 1988;264:607–617.
- Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14.
- Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17(10):pii: E1712.
- Ayati SH, Fazeli B, Momtazi-Borojeni AA, et al. Regulatory effects of berberine on microRNome in cancer and other conditions. Crit Rev Oncol Hematol. 2017;116:147–158.
- Yang Q, Zhang D, Li Y, et al. Paclitaxel alleviated liver injury of septic mice by alleviating inflammatory response via microRNA-27a/TAB3/NF-kappaB signaling pathway. Biomed Pharmacother = Biomedecine & Pharmacotherapie. 2018;97:1424–1433.
- Zhou R, Zhang Q, Zhang Y, et al. Aberrant miR-21 and miR-200b expression and its pro-fibrotic potential in hypertrophic scars. Exp Cell Res. 2015;339:360–366.
- Li G, Zhou R, Zhang Q, et al. Fibroproliferative effect of microRNA-21 in hypertrophic scar derived fibroblasts. Exp Cell Res. 2016;345:93–99.
- Liu Y, Li Y, Li N, et al. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating microRNA-21. Sci Rep. 2016;6:32231.
- Li Y, Liu H, Liang Y, et al. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-beta1/Smad signaling pathway. Biomed Pharmacother = Biomedecine & Pharmacotherapie. 2017;91:174–180.
- Grande J, Melder D, Zinsmeister A, et al. Transforming growth factor-beta 1 induces collagen IV gene expression in NIH-3T3 cells. Lab Invest. 1993;69:387–395.
- Ish-Shalom S, Lichter A. Analysis of fungal gene expression by real time quantitative PCR. Methods Mol Biol. 2010;638:103–114.
- Chu Y, Fang Y, Chi J, et al. Astragalus polysaccharides decrease proliferation, migration, and invasion but increase apoptosis of human osteosarcoma cells by up-regulation of microRNA-133a. Braz J Med Biol Res = Revista Brasileira de Pesquisas Medicas e Biologicas 2018;51:e7665.
- Krakowski AC, Totri CR, Donelan MB, et al. Scar management in the pediatric and adolescent populations. Pediatrics. 2016;137:e20142065.
- Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics (Sao Paulo, Brazil). 2014;69:565–573.
- Wang X, Cao P, Liu J, et al. 5-Aminolaevulinic acid-based photodynamic therapy restrains pathological hyperplasia of fibroblasts. Med Sci Monit. 2017;23:46–56.
- Hsieh SC, Wu CC, Hsu SL, et al. Gallic acid attenuates TGF-beta1-stimulated collagen gel contraction via suppression of RhoA/Rho-kinase pathway in hypertrophic scar fibroblasts. Life Sci. 2016;161:19–26.
- Mi L, Li YY, Lin WH, et al. The effect of integrin-linked kinase on VEGF expression in fibroblasts from human hypertrophic scar. Zhonghua Zheng Xing Wai ke za Zhi = Zhonghua Zhengxing Waike Zazhi = Chin J Plastic Surg. 2011;27:289–293.
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development (Cambridge, England). 2013;140:3079–3093.
- Sullivan KD, Galbraith MD. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018;25(1):133–143.
- Borralho PM, Rodrigues CMP, Steer CJ. Mitochondrial microRNAs and their potential role in cell function. Curr Pathobiol Rep. 2014;2:123–132.
- Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by microRNAs. Stem Cell Rev and Rep. 2018;14:309–322.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol (Clifton, NJ). 2017;1509:1–10.
- Wang BB, Xie H, Wu T, et al. Controlled-release mitomycin C-polylactic acid film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs. Eur Rev Med Pharmacol Sci. 2017;21:2526–2537.
- Ren X, Ge M, Qin X, et al. S100a8/NF-kappaB signal pathway is involved in the 800-nm diode laser-induced skin collagen remodeling. Lasers Med Sci. 2016;31:673–678.
- Chen HN, Wang DJ, Ren MY, et al. TWEAK/Fn14 promotes the proliferation and collagen synthesis of rat cardiac fibroblasts via the NF-small ka, CyrillicB pathway. Mol Biol Rep. 2012;39:8231–8241.
- Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060.
- Zhang Z, Nie F, Kang C, et al. Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med. 2014;34:253–261.
- Poligone B, Hayden MS, Chen L, et al. A role for NF-kappaB activity in skin hyperplasia and the development of keratoacanthomata in mice. PloS One. 2013;8:e71887.