Abstract
In this study, latex of Azadirachta indica was used for the synthesis of silver nanoparticles (AgNP). UV-visible spectroscopy revealed the formation of AgNPs and the absorption band optimized at 442 nm. Fourier transform infrared (FTIR) spectroscopy shows different functional groups (carboxyl, amine and hydroxyl) of biomolecule which are responsible for reduction and capping process. X-ray diffraction (XRD) analysis confirms the nanoparticles are crystalline silver and cubic (AgCl) with face-centered cubic (Ag) types. Electron microscopics (SEM and TEM) were used to characterize the shape and size of the nanoparticles. The anticandidal and antibiofilm activity of AgNPs was using Fluconazole resistant clinical isolate of Candida tropicalis. The new approach of plant-mediated AgNPs synthesis appears to be cost-effective, eco-friendly and easy methods. The synthesized AgNPs considered as a novel and alternative agent to prevent C. tropicalis biofilms.
Introduction
Nanotechnology is the most attractive field of research in modern material science [Citation1]. Nanoparticles (NPs) are zerovalent metals, metal oxides and salts with an emphasis on recent developments of greener route of nanotechnology [Citation2]. Nanoscale materials are usually ranging from 1 to ≤100 nanometers (nm) [Citation3]. Nanoparticles are classified into two major types, they are organic and inorganic NPs [Citation4]. Carbon NPs (i.e. dendrimers, liposome, micelles and carbon nanotubes) are called organic NPs and the remains of magnetic (iron, nickel and cobalt), noble metal (platinum, gold and silver), and semiconductor NPs (titanium dioxide and zinc oxide) are inorganic NPs [Citation1]. The noble metal NPs are well known to have important characteristic applications in the fields of physical, chemical, electronic, electrical, mechanical, magnetic, thermal, dielectric, optical, photography, optoelectronic, biological labeling, catalytic and information storage [Citation5–9].
Silver nanoparticle (SNP or AgNP) is one of the most significant constituents of the noble metal nanoparticle. The SNPs have a variety of morphological characters (i.e. spherical, triangular decahedral, quasi-spherical, ellipsoidal, hexagonal, nanospheres, nanotriangles, nanorods and polygonal prisms) are synthesized by physical, chemical, and biological methods [Citation10]. The main drawback of physical and chemical methods for NPs synthesis is the utilization of high energy and generation of hazardous chemicals [Citation11]. The biological methods are an alternative tool for chemical and physical methods of synthesis of SNPs. The biological organisms of plant materials (i.e. leaves, flowers, bark, root, seed, fruit, fruit peels and latex) and microorganisms (bacteria, fungi and algae) potentially synthesize the inorganic NPs of gold, silver, calcium, silicon, titanium, iron, gypsum and lead [Citation10]. Consequently, the plant extracts are eco-friendly, non-toxic and cost-effective for the synthesis of large scale NPs [Citation12]. These biogenic SNPs are extensively used in antibacterial, antifungal, antiparasitic, larvacidal and anticancer activities [Citation13–17].
Biofilms are both cell-cell and self-produced matrix of extracellular polymeric substances (EPS). Candida tropicalis is one among the potential fungal pathogen and is the second most virulent Candida sp. after Candida albicans [Citation18]. C. tropicalis is one of the common opportunistic diseases causing agent in tropical countries, especially found on skin, respiratory, gastrointestinal and urogenital tract [Citation19–22]. Candida tropicalis is most virulent due to its ability to produce biofilms, secrete lytic enzymes and adhere to epithelial and endothelial cells [Citation18,Citation23]. Candida tropicalis fungemia is common in patients with leukemia [Citation24,Citation25] and more virulent in neutropenic patient with hematological malignancy [Citation26]. The fungal organisms in the biofilms are highly resistant toantifungal drugs [Citation27]. Recently, many reports have been published on the nanoparticles are effective anti-biofilm agents against for both bacteria and fungi [Citation28–30].
The present study was aimed to investigate the biosynthesis of AgNPs using latex from neem, Azadirachta indica, to characterize the synthesized AgNPs and to evaluate the anti-candidal biofilm inhibition activity of AgNPs against fluconazole-resistant clinical isolate of C. tropicalis. To our knowledge, the use of A. indica latex for the synthesis of AgNPs has not been reported earlier.
Materials and methods
Materials
The rare incidence of milk-like fluid was seeping out from the neem tree, A. indica, the leaked latex was collected in a sterile container. Then, the latex solution was filtered through a muslin cloth and filtered latex was stored at −20 °C for further use. Silver nitrate (AgNO3) analytical grade was procured from Sigma–Aldrich (St. Louis, MO, USA)
Synthesis of silver nanoparticles
The crude latex (1.0 ml) was diluted into de-ionized water (100 ml) to make it 1% latex solution. About 1.0 ml of the diluted latex solution was treated with 10 ml of 1.0 mM silver nitrate (AgNO3) solution in an Erlenmeyer flask, incubated for 30 min at room temperature. Then, the bioreduced (colour changed) aqueous component was used to measure UV-Vis spectra.
Optimization of silver nanoparticles
After the preliminary confirmation of synthesis of AgNP, the present study optimized the biosynthesis of AgNP. The stoichiometric ratio which gave better nanoparticle synthesis to the AgNO3 and latex solution was used for the synthesis of silver nanoparticles. Keeping the amount of 1.0 mM AgNO3 solution as constant (10.0 ml), the amount of latex added varied (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.25 and 1.5 ml). Tubes were incubated at room temperature in 30 min.
Characterization of nanoparticles
The progress of AgNPs synthesis was measured by UV–visible spectrophotometer at 300–900 nm. FTIR performed the bioreduction of AgNO3, the latex which was exposed before and after the addition of silver nitrate solution. The dried latex and bioreduced AgNO3 were ground with KBr pellets used for FT-IR measurements. The FT-IR spectra were recorded in the wavenumber range of 4000 and 400 cm−1. The formation and quality of nanoparticles were measured by X-ray diffraction (XRD) spectra. Finally, the morphological analysis of the nanoparticle was done by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM).
Antibiogram
The six non-repetitive C. tropicalis isolates were obtained from Research Laboratories, Department of Biology, College of Science, Majmaah University, Al Zulfi, Kingdom of Saudi Arabia. The isolates were maintained in the Sabouraud Dextrose Agar (SDA, Mumbai, Himedia, India) medium. The subsequent antibiotics (amphotericin-B (AP) 0.002–32 mcg/mL, anidulafungin (AND) 0.002–32 mcg/mL, caspofungin (CAS) 0.002–32 mcg/mL, fluconazole (FLC) 0.016–256 mcg/mL, flucytosine (FLU) 0.002–32 mcg/mL, intraconazole (ITR) 0.002–32 mcg/mL and voriconazole (VRC) 0.002–32 mcg/mL) were tested by disk diffusion method. The isolates were inoculated on SDA medium and the antibiotic disks were placed on the agar surfaces. The petri plates were incubated at 37 °C for 48 h. Sensitivity and resistance of the antibiotics were interpreted after 48 h incubation according to the guidelines of Clinical and Laboratory Standards Institute (CLSI) (). Fluconazole-sensitive and resistant isolates of C. tropicalis were stored at –20 °C until further use.
Table 1. Antibiogram of clinical isolates of Candida tropicalis.
Preparation of standard fungal cell suspension
In the present work, fluconazole sensitive (C. tropicalis isolate-2) and resistant (C. tropicalis isolate-6) isolates were used for biofilm formation and antifungal activity. Both the strains were grown on SDA medium at 37 °C for 24 h and then the colonies are enriched into Yeast Nitrogen Base (YNB, Mumbai, Himedia, India) medium with 50 mM dextrose and incubated at 37 °C for 24 h. After that, fungal colonies were transferred into sterile phosphate buffer saline (PBS; pH 7.2). The turbidity of suspension was adjusted to an optical density of 1.0 at 610 nm, equivalent to 0.5 MacFarland standards (1 × 106 cells/mL1) for the estimation of cell density.
Biofilm formation
The culture containing medium was enriched by 100 μL of YNB medium with 50 mM dextrose, then 10 μL of C. tropicalis cells were inoculated into each well of 24-well plates. Candida tropicalis were permitted to adhere into the bottom of the plates at 37 °C for 90 min. Subsequently, each well was added 50 mM dextrose containing 200 μL YNB medium and incubated at 37 °C for 48 h. Finally, the well contained YNB medium was discarded, then the wells were washed through PBS. The different concentration of AgNPs (2–10 μg/mL) and fluconazole (2–10 μg/mL) added into each well, then the final volume of wells was made into 100 μL with Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher, Waltham, USA). Culture without AgNP and fluconazole served as a negative control.
XTT assay
XTT assay was used to evaluate the antifungal effect of the AgNPs. XTT cell viability assay was performed by CyQUANT XTT Cell Viability assay kit (Thermo Fisher) according to the manufacturer’s protocol. Optical absorbance was measured at the wavelength of 490 nm by an ELISA reader (BioTek-800TS, Winooski, VT, USA).
ATPase assay
After the biofilm formation and treatment of the cells by AgNPs, ATP cell viability assay was performed by ATP Bioluminescence Assay Kit CLS II (Sigma-Aldrich, St. Louis, Missouri, USA ) according to the manufacturer’s instructions. Then, the optical density was measured by illuminometer at 560 nm (Berthold-Centro LB 960, Bad Wildbad, German).
Statistical analysis
Data analysis was performed by t-test method with SPSS Statistics version 16, USA. The level of statistical significance was set at p < .05.
Results
Synthesis of silver nanoparticles
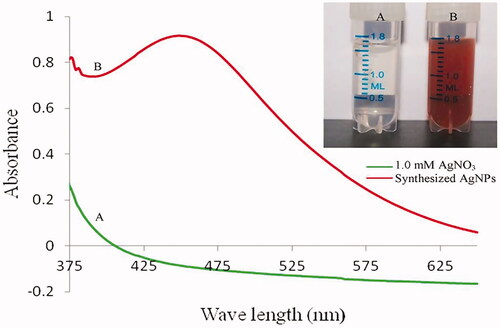
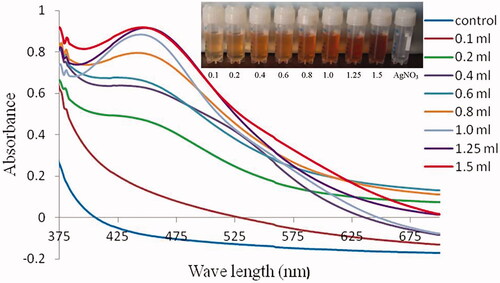
Rapid biosynthesis and bioreduction reactions started within a few minutes after the addition of neem latex in 1.0 mM AgNO3. The colourless clear AgNO3 solution was converted into brownish yellow. In this same condition, no colour change was noted without neem latex in AgNO3 ().
Characterization of AgNPs
The UV-visible spectroscopy absorption band optimized at 442 nm clearly indicating the formation of silver nanoparticles (). The stoichiometric optimization of the nanoparticle synthesis revealed 1.25 ml of neem latex that optimally reduced the 10.0 ml of 1.0 mM AgNO3 (.
Figure 2. Optimization of AgNPs synthesis; constant of 1.0 mM AgNO3 (5.0 ml) with different volume (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.25 and 1.5 ml) of diluted neem latex.
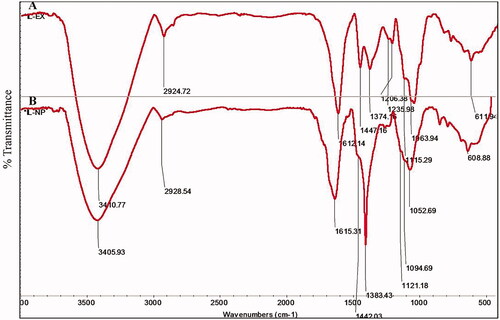
The IR spectrum of neem latex alone showed a distinct peak in the range of 3410.77, 2924.72, 1612.14, 1447.16, 1374.16, 1235.98, 1206.38, 1115.29, 1063.94 and 611.94 cm−1 (). The spectrum of IR peak 3410.77 cm−1 may be due to the strong stretching vibration of (–NH3) primary amine group possibly arising from the protein. A peak around 2924.72 cm−1 represents from (–OH) polyphenols. The strong peak absorption located at 1612.14 cm−1 was identified as the alkene (–C = C) group. The band appeared 1447.16 cm−1 assigned to –C–N stretching vibration of aliphatic amines. The weak band appeared 1235.98 cm−1 that can be assigned to –C–O amide III bending vibration. The weak band 1374.16 cm−1 and medium band 1206.38 cm−1 assigned due to –C–H stretching of the methyl (–CH3) and phenolic (–OH) stretching, respectively. The medium to strong bands 1115.29 cm−1 and 1063 cm−1 can be assigned due to –C–O stretching of primary and secondary alcohols, respectively. The weak band appears at 611.94 cm−1 to be assigned due to –C–C alkanes are representing in neem latex. FTIR spectrum of bio-reduced silver nanoparticles showed a distinct peak in the range of 3405.93, 2928.54, 1615.31, 1442.03, 1383.43, 1121.18, 1094.69, 1052.69 and 608.88 cm−1 ().
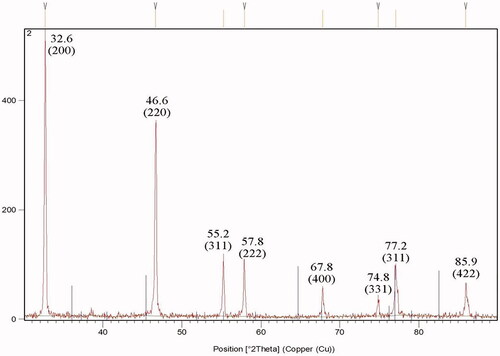
The X-ray diffraction pattern of AgNPs produced by neem latex is shown in , the signals exist at 32.6°, 46.6°, 55.2°, 57.8°, 67.8°, 74.8°, 77.2° and 85.9° and that corresponds to the (200), (220), (311), (222), (400), (331), (311) and (422) khl planes. The AgNPs intensity of the (200) and (220) diffractions were much stronger than those of (222), (400), (331), (311) and (422) khl planes. The mean size of AgNPs was found to be 23.25 nm, which was calculated by Debye–Scherrer’s equation.
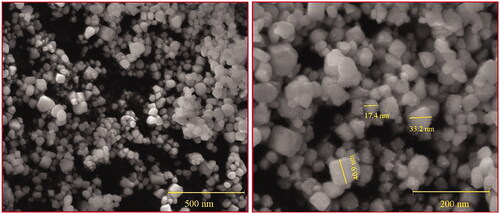
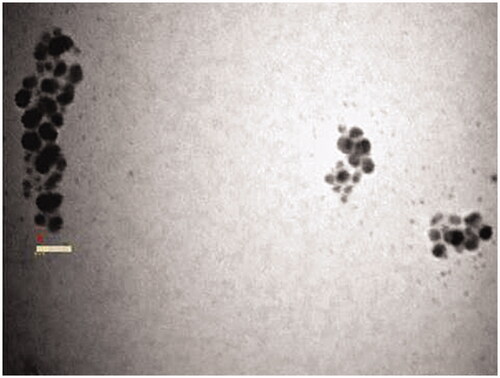
SEM analyses of the synthesized AgNPs were triangles, tetragons, pentagons and hexagons in structure and their size ranged between 17.4 and 40.9 nm in diameter (). In addition, the small AgNPs with a size range of 5–10 nm were attached on the surface of large AgNPs, they are compatible with dendrite structures (. Similarly, TEM analyses of the synthesized AgNPs were spherical in shape and their size ranged from 12.27 to 27.20 nm in diameter, agglomerated particles were seen ().
Antifungal/antibiofilm activity
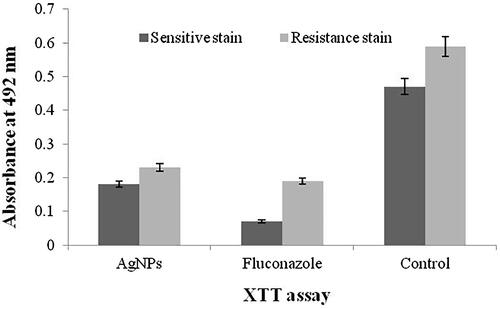
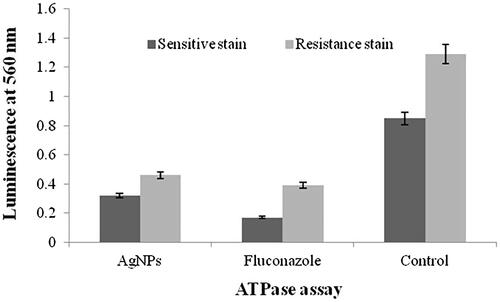
and shows the XTT and ATPase assays of antimicrobial and biofilm inhibition activities of fluconazole-susceptible and fluconazole-resistant isolates of C. tropicalis. The least concentration of 3.25 and 4.12 μg/mL of AgNPs inhibited the biofilms of fluconazole-susceptible and fluconazole-resistant C. tropicalis, respectively. Fluconazole at the concentration of 3 and 9 μg/mL inhibited the biofilms of susceptible and resistant C. tropicalis, respectively. The comparison results of XTT and ATPase methods difference was more than the control.
Discussion
A detailed study on the rapid green synthesis of eco-friendly, cost-effective and potential silver nanoparticles by A. indica latex and the anticandidal and antibiofilm activity was reported from the present research. Several approaches have been reported to obtain a better synthesis of silver nanoparticles such as physical, chemical and biological methods. Earlier, silver nanoparticles have been synthesized from plant latex of Jatropha curcas [Citation31,Citation32], Jatropha gossypifolia [Citation33], Lannea grandis [Citation32], Calotropis procera [Citation34] and Pedilanthus tithymaloides [Citation31].
The intrinsic properties of silver nanoparticles are determined by its size, shape and crystalline structure. In green synthesis, the major factors are responsible for the bioreduction of silver ions (Ag+) into silver nanoparticles (Ago), the unchanged Ago metal particles gave colour change in aqueous solution [Citation35]. In the present study, rapid colour change clearly indicates the synthesis of AgNPs. The UV-visible spectra absorption band of AgNPs was optimized from 420 to 450 nm, which is clearly indicating the formation of silver nanoparticles in the aqueous solution [Citation36]. Similar observation was also found in the present study that showed an absorption band of AgNPs optimized at 442 nm, which also indicated the formation of AgNPs.
In FTIR analysis, a large shift in the absorbance peak with decreased band intensity was observed from 3410.77 to 3405.93 cm−1, 1612.14 to 1615 cm−1, 1447.16 to 1442.03 cm−1 and 1235.98 to 1121.18 cm−1, which represent primary amine (I), amide (I), aliphatic amine and amide (III) bands of protein and saponin that capped and stabilized the nanoparticles [Citation37,Citation38]. In addition, FTIR band at 2924.72 cm−1 and 1206.38 is assigned to polyphenol and phenol, respectively. The high polyphenol content of neem latex act as chelating, reducing and capping agents for nanoparticle synthesis [Citation2]. FTIR signals clearly revealed the presence on polyphenol, protein, carbohydrate and saponin in neem latex which acts as a reducing and capping agents for the synthesis of silver nanoparticles. The FTIR spectra study confirmed that the neem latex has the ability to perform the dual function of reduction and stabilization of silver nanoparticles. The eco-friendly biological organisms of plant materials contain glycosides, proteins, enzymes, phenols, flavonoids, alkaloids, terpenoids and cofactors which act as both reducing and capping agents forming stable and shape-controlled AgNPs [Citation39].
XRD pattern show typically peaks at 32.6°, 46.6°, 55.2°, 57.8°, 67.8°, 74.8°, and 85.9°, corresponding to the (200), (220), (311), (222), (400), (331) and (422) diffractions for cubic phase of AgCl phases (JCPDS file: 31–1238), that coexists with the face-centered cubic (fcc) silver 77.2° and that corresponds to the (311) hkl plane (JCPS 04–0783). XRD pattern clearly shows that the silver/silver chloride (Ag/AgCl) nanoparticles formed in the present synthesis are crystalline in nature.
Electron microscopy analyses revealed the morphology, shape and size of the synthesized nanoparticles, the present study clearly shows that the triangles, tetragons, pentagons and hexagonal structure with a size range of 35–60 nm. The present study of SEM analyses exhibits dendrite-like structures, which is similar with those described by Cai et al. (2017) [Citation40] who demonstrated that the small silver nanoparticles were attached on the surface of large silver chloride (AgCl) nanoparticles. The SEM analysis shows the dendrite-like silver nanoparticles on the surface of the silver chloride nanoparticles, suggesting successful silver nanoparticles synthesis using neem latex. The data obtained from the SEM correlated with that of TEM results.
Biofilm inhibitions have been reported in several nanoparticles (i.e. gold, silver, ZnO and TiO) [Citation41,Citation42]. The present study showed that the AgNPs inhibits the growth of C. tropicalis with minimal concentrations and XTT assay clearly exhibited the antifungal activity of the AgNPs. Elimination of Candida biofilms by AgNPs was achieved inthe concentration of 3.25 μg/mL for fluconazole-susceptible stain and 4.12 μg/mL for fluconazole-resistant strain. The present result revealed that the viability of C. tropicalis biofilms declined with the increasing of AgNPs. In this finding, we observed appropriate results in comparison to fluconazole as a potent drug.
Conclusion
Silver nanoparticles were synthesized by a simple chemical reduction method using neem latex without the intervention of any manmade chemicals. The amount of plant material was found to play an important role in bioreduction and stabilization of nanoparticles. The present study suggests that neem latex can be used as both a reducing and stabilizing agent for the synthesis of AgNPs. The XTT and ATPase assays can be used for anti-biofilm effects of nanoparticles, although ATPase assay provides more reliable results with time-consuming method. The AgNPs inhibited the adherence and biofilm growth of C. tropicalis on the catheter surface. Due to the important suggestion of Candida spp. in human pathogenesis, especially in prosthetic devices related to infections and antifungal resistance, using the new coated shell based on AgNPs to inhibit fungal adherence to the prosthetic device could be of great interest for the biomedical field.
Acknowledgements
I gratefully acknowledge the Department of biology, College of Science, Majmaah University, Kingdom of Saudi Arabia for providing all the resources and platform to complete this research.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Manivasagan P, Kim SK. Biosynthesis of nanoparticles using marine algae: a review. In: Kwon Kim S, Chojnacka K, editors. Marine algae extracts: processes, products, and applications; UK: Wily publishers, 2015. p. 295–304.
- Kharissova OV, Dias HR, Kharisov BI, et al. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–248.
- Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35:583–592.
- Núñez C, Estévez SV, del Pilar Chantada M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J Biol Inorg Chem. 2018;23:331–345.
- Pareek V, Bhargava A, Gupta R, et al. Synthesis and applications of noble metal nanoparticles: a review. Adv Sci Engng Med. 2017;9:527–544.
- Mickymaray S, Alturaiki W. Antifungal efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules. 2018; 23:3032.
- Okuda M, Kobayashi Y, Suzuki K, et al. Self-organized inorganic nanoparticle arrays on protein lattices. Nano Lett. 2005;5:991–993.
- Dai J, Bruening ML. Catalytic nanoparticles formed by reduction of metal ions in multilayered polyelectrolyte films. Nano Lett. 2002;2:497–501.
- Murray RW. Nanoelectrochemistry: metal nanoparticles, nanoelectrodes, and nanopores. Chem Rev. 2008;108:2688–2720.
- Prabakar K, Sivalingam P, Rabeek SIM, et al. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf B Biointerfaces. 2013;104:282–288.
- Li X, Xu H, Chen ZS, et al. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:1.
- Kotakadi VS, Rao YS, Gaddam SA, et al. Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus. Linn. G. Donn and its antimicrobial activity. Colloids Surfaces B Biointerfaces. 2013;105:194–198.
- Mickymaray S, Vinodhini R, Moorthy K, et al. Incidence and virulence traits of Candida dubliniensis isolated from clinically suspected patients. Asian J Pharm Clin Res. 2016;9:77–81.
- Mallmann EJJ, Cunha FA, Castro BN, et al. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop S Paulo. 2015;57:165–167.
- Suresh M, Rath PK, Panneerselvam A, et al. Antifungal activity of selected Indian medicinal plant salts. J Glob Pharma Technol. 2010;2:71–74.
- Sutthanont N, Attrapadung S, Nuchprayoon S. Larvicidal activity of synthesized silver nanoparticles from Curcuma zedoaria essential oil against Culex quinquefasciatus. Insects. 2019;10:27.
- Buttacavoli M, Albanese NN, Di Cara G, et al. Anticancer activity of biogenerated silver nanoparticles: an integrated proteomic investigation. Oncotarget. 2018;9:9685.
- Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An update on Candida tropicalis based on basic and clinical approaches. Front Microbiol. 2017;8:1927.
- Jacobs PH and Nall L, editors. Antifungal drug therapy: a complete guide for the practitioner. New York: Marcel Dekker; 1990. p. 32–45.
- Koh AY. Gastrointestinal colonization of fungi. Curr Fungal Infect Rep. 2013;7:144–151.
- Pendleton KM, Huffnagle GB, Dickson RP. The significance of Candida in the human respiratory tract: our evolving understanding. Pathogens Dis. 2017;75:ftx029.
- Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–273.
- Richardson JP, Ho J, Naglik JR. Candida–epithelial interactions. J Fungi (Basel). 2018;4:22.
- Martino P, Girmenia C, Micozzi A, et al. Fungemia in patients with leukemia. Am J Med Sci. 1993;306:225–232.
- Tang HJ, Liu WL, Lin HL, et al. Epidemiology and prognostic factors of Candidemia in cancer patients. PLoS One. 2014;9:e99103.
- Mohammadi R, Foroughifar E. Candida infections among neutropenic patients. Caspian J Int Med. 2016;7:71.
- Silva S, Rodrigues CF, Araújo D, et al. Candida species biofilms’ antifungal resistance. JOF. 2017;3:8.
- Hamid S, Zainab S, Faryal R, et al. Inhibition of secreted aspartyl proteinase activity in biofilms of Candida species by mycogenic silver nanoparticles. Artif Cells Nanomed Biotechnol. 2018;46:551–557.
- Vinoth J, Murugan S, Stalin C, et al. Candida tropicalis biofilm inhibition by ZnO nanoparticles and EDTA. Arch Oral Biol. 2017;73:21–24.
- de Alteriis E, Maselli V, Falanga A, et al. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. IDR. 2018;11:915.
- Patil SV, Borase HP, Patil CD, et al. Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol. 2012;167:776–790.
- Kumar S, Halder D, Mitra A. Characterization of silver nanoparticles synthesized using latex of Jatropha curcas and Lannea grandis. JSST. 2017;32:115–120.
- Patil CD, Borase HP, Patil SV, et al. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol Res. 2012;111:555–562.
- Mohamed NH, Ismail MA, Abdel-Mageed WM, et al. Antimicrobial activity of latex silver nanoparticles using Calotropis procera. Asian Pac J Trop Biomed. 2014;4:876–883.
- Iravani S, Korbekandi H, Mirmohammadi SV, et al. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9:385.
- Raja K, Saravanakumar A, Vijayakumar R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochimica Acta A. 2012;97:490–494.
- Philip D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochimica Acta A. 2009;73:374–381.
- Ramezani F, Jebali A, Kazemi B. A green approach for synthesis of gold and silver nanoparticles by Leishmania sp. Appl Biochem Biotechnol. 2012;168:1549–1555.
- Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interf Sci. 2009;145:83–96.
- Cai WF, Pu KB, Ma Q, et al. Insight into the fabrication and perspective of dendritic Ag nanostructures. J Exp Nanosci. 2017;12:319–337.
- Yu Q, Li J, Zhang Y, et al. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep. 2016;6:26667.
- Maurer-Jones MA, Gunsolus IL, Meyer BM, et al. Impact of TiO2 nanoparticles on growth, biofilm formation, and flavin secretion in Shewanella oneidensis. Anal Chem. 2013;85:5810–5818.