Abstract
Obstructive sleep apnoea-hypopnoea syndrome (OSAHS) is a condition causing apnea and hypopnea. LncRNA-ROR revealed properties in regulating hypoxia response. Our study explored the roles of ROR in CoCl2-induced hypoxia injury in HK-2 cells. HK-2 cells were treated with CoCl2 to induce hypoxia injury. Cell viability, cell apoptosis and apoptotic proteins were detected using CCK-8, flow cytometry and western blot, respectively. The alter expression of ROR and miR-145 was achieved through transfection. Moreover, the expressions of HIF-α and ERK and MAPK related factors were examined using western blot. We found that CoCl2 decreased cell viability and increased apoptosis as well as increased the expression of ROR. ROR overexpression increased cell viability, decreased cell apoptosis. ROR overexpression upregulated anti-apoptotic proteins Bcl-2 and decreased p53, Bax and cleaved-Caspase-3. ROR overexpression also increased the expression of HIF-α. On the opposite, ROR silence led to the opposite results as relative to ROR overexpression. ROR overexpression decreased expression of miR-145. Co-transfection with ROR overexpression and miR-145 impaired the promoting effects of ROR in CoCl2 treated cells. ROR increased phosphorylation of ERK while decreased phosphorylation of MAPK. In conclusion, lncRNA ROR alleviated CoCl2-induced hypoxia injury through regulation of miR-145 as well as modulating ERK and MAPK signalling.
CoCl2 induces ROR upregulation;
Overexpression of ROR reduces CoCl2-induced HK-2 cell injury;
Silence of ROR promotes CoCl2-induced HK-2 cell injury;
Overexpression of ROR decreases miR-145 expression;
ROR overexpression modulates ERK and MAPK signalling pathways through regulation of miR-145.
Highlights
Introduction
Obstructive sleep apnoea-hypopnoea syndrome (OSAHS) is a condition in which the pharyngeal airway collapses and blocks during sleep, causing apnea and hypopnea [Citation1,Citation2]. It is usually associated with snoring, sleep disorders, frequent oxygen desaturation during sleep, daytime sleepiness and inattention [Citation3]. Current studies have shown that recurrent apnea during sleep and/or shallow breathing, as well as recurrent hypoxemia, hypercapnia and sleep disorders at night can lead to impaired renal function, further researches pointed out that long time OSAHS could induce serious diseases such as hypertension, myocardial infarction and stroke [Citation2,Citation3]. In the past years, treatment with OSAHS has not received enough attention due to wrong idea about this disease. Therefore, except continuous positive airway pressure approach [Citation4], even some researches also point out to use mandibular advancement devices [Citation5]. However, no breakthrough was made in the treatment of OSAHS. Therefore, novel and effective treatment was urgently needed and deeper understanding about OSAHS is also requisite.
Nowadays, using molecular approach, such as lncRNAs to resolve some diseases are becoming popular [Citation6]. Scientists started to search for the disease-related lncRNAs to looking for novel approach for the understating or even treatment of respiratory diseases [Citation7]. For example, lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) silence promoted cell apoptosis in neonatal respiratory distress syndrome [Citation8]. In these lncRNAs, regulator of reprogramming (ROR) is prominently found in malignant cancers, in which lncRNA-ROR mainly exerts as a key competing endogamous miRNA sponge promoting to cancer development [Citation9,Citation10]. In addition, lncRNA ROR was also known as a suppressor for p53 expression in response to DNA damage and ROR could also regulate cell apoptosis [Citation11,Citation12]. Combined with the above information, we aimed to explore whether lncRNA was connected with OSAHS. Moreover, in the disease of OSAHS, activation of inflammatory pathways and hypoxia injury was involved [Citation13,Citation14]. Therefore, in our study, we used cobalt chloride (CoCl2) to stimulate HK-2 cells to establish an OSAHS cell model in vitro.
In addition, lncRNAs are often found to cooperate with miRNAs to regulate biological progression in transcription level [Citation15]. miR-145 is often used in various inflammatory progressions in Asthma as well as in chronic obstructive pulmonary disease (COPD) [Citation16]. Is there possible that miR-145 was also involved in OSAHS? Or even whether miR-145 could cooperate with lncRNA ROR? This is what our study explored. In our study, we explored the roles of ROR in CoCl2 to stimulate HK-2 cells and the underlying mechanism by which ROR achieved its functions.
Material and methods
Reagents and cell culture
Reagents: Roswell Park Memorial Institute (RPMI)-1640 (Gibo, Grand Island, Newyork, USA), fetal bovine serum (FBS; Life Science, UT, USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Sigma-Aldrich, St. Louis, USA).
Cell culture: HK-2 cells were provided by Riken cell bank (Ibaraki, Japan). HK-2 cells were cultured in the medium of RPMI-1640 with 10% heat-FBS, and penicillin and streptomycin. Then, HK-2 cells were maintained in a humidified environment with 95% air and 5% CO2 and warm place at around 37 °C. When cells reached 60–70% confluence, cells were administrated with CoCl2 (200 μM) and maintained in different time durations to induce inflammatory injury.
Cell viability assay
HK-2 cells in logarithmic growth phase were collected and subcultured in 96-well plate at the density of 10,000 cells in each well. After one night time, then the culture medium was changed. 10 μl cell counting kit-8 (CCK-8) solution was added to each well at 37 °C for 1 h. In the end, absorption values were obtained using a Microplate Reader (Bio-Rad, Hercules, CA) at 450 nm.
Cell apoptosis assay
HK-2 cells were digested routinely and centrifuged at 100 × g for 5 min. Then, cells were washed with phosphate-buffered saline (PBS) for two times, and then slowly added with 500 μl binding buffer. Cells were gently shaken and resuspended. Fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI) 5 μl staining were added to the cell suspension. After that, the apoptotic cell's rate was determined with flow cytometer (Beckman Coulter, USA) following the manufacturer's instruction.
Western blot
HK-2 cells were collected and using RIPA lysis buffer (Article No:R0010, Solarbio, Beijing, China) for cell lysis. The BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA) was used for detecting protein quantification. Bio-Rad Bis-Tris Gel system was applied for constructing western blot system. Primary antibodies (Abcam, Cambridge, UK) were maintained in the dark at 4 °C overnight and the membrane was washed the next day. Secondary antibody incubates with horseradish peroxidase (HRP) conjugated and the bands were quantified by Image Lab™ Software (Bio-Rad, Shanghai, China).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) and was reverse transcribed into cDNA using a Reverse Transcription Kit (Takara, Dalian, China) according to the manufacturer’s instructions. The expression of ROR was conducted by qRT-PCR using the SYBR Green Master Mix (Takara) according to the manufacturer’s instruction. The mRNA of GAPDH level was used as an internal control and relative expression changes were calculated using the 2-ΔΔCt method.
The TaqMan MicroRNA Assay was employed to determine the expression of miR-145 (Applied Biosystems, Foster City, CA, USA). The expression of miR-145 was normalized to U6 snRNA.
Cell transfection
In order to alter the expression of genes, miR-145 mimic and their corresponding negative control (NC); another group pcROR and si-ROR (GenePharma Co., Shanghai, China) were transfected into HK-2 cells. Cells were transfected with miR-145 mimic using Lipofectamine 2000 reagent (Invitrogen).
Statistical analysis
All the results were shown by mean + standard deviation (SD). Data analysis was achieved by Graphpad Prism 5 software (GraphPad, San Diego, CA, USA). p Values were counted using a one-way analysis of variance (ANOVA). *(p < .05), **(p < .01) and ***(p < .001) stand for significant difference.
Results
CoCl2 induced HK-2 cells hypoxia injury
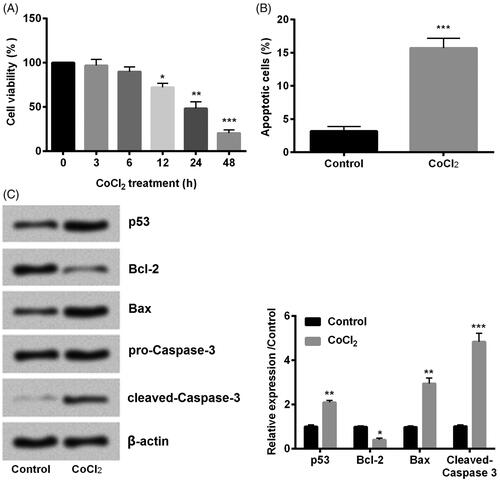
Cell viability and apoptosis are two important aspects to detect cell growth. As presented in , cell viability was significantly decreased with the increasing of time duration especially when treatment time was longer than 12 h (p < .05, p < .01 or p < .001). 24 h was used in the following experiment as the treatment time. On the other hand, we found that cell apoptosis was significantly higher than CoCl2 compared with control (p < .001, ). Moreover, we examined the expression of apoptotic proteins. Results showed that CoCl2 induced the expression of p53 (p < .01), Bax (p < .01) and cleaved-aspase-3 (p < .001) while inhibited Bcl-2 (p < .05) expression compared with control (). Taken together, we found that CoCl2 induced hypoxia injury successfully.
Figure 1. Cobalt chloride (CoCl2) induced hypoxia injury. (A) Cell viability, (B) cell apoptosis, (C) cell apoptotic proteins p53, Bcl-2, Bax and cleaved-Caspase-3 were detected using cell counting kit-8 assay, flow cytometry and western blot, respectively. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05, **p < .01 and ***p < .001 were all significant difference.
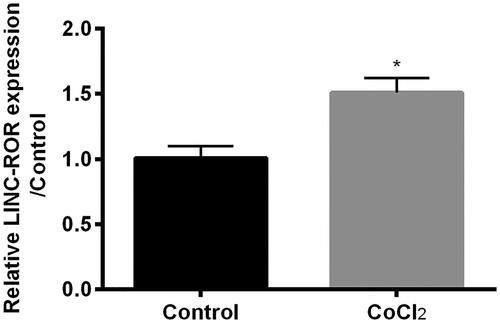
Cocl2 induced ROR upregulation
ROR was involved in p53 expression and regulated cell apoptosis [Citation11,Citation12], which might play vital roles in response to hypoxia injury. Therefore, we detected the expression of ROR in HK-2 cells. Result showed that the expression of ROR was upregulated by CoCl2 treatment as relative to control (p < .05, ), which indicated that ROR might join the regulation network in hypoxia injury of HK-2 cells.
Overexpression of ROR reduced CoCl2-induced HK-2 cell injury
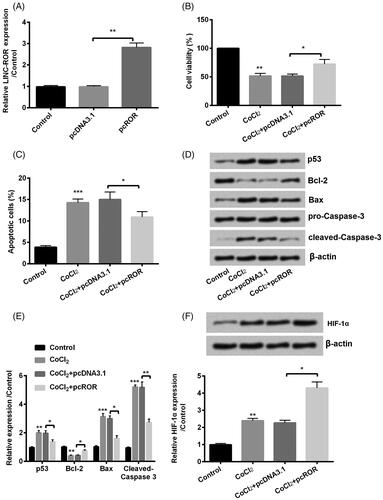
In order to validate the roles of ROR, cells were transfected with pcROR to upregulated expression of ROR. The upregulation of ROR by transfection with pcROR indicated high transfection efficiency as shown in (p < .01). Then we detected ROR overexpression on cell growth factors. Interestingly, cell viability was enhanced (p < .05, ), and apoptosis was inhibited (p < .05, ) by ROR overexpression compared with NC. In addition, several apoptotic factors were analyzed using western blot. Results showed that p53 (p < .05), Bax (p < .05) and cleaved-Caspase-3 (p < .01) were downregulated while Bcl-2 (p < .05) was upregulated by ROR overexpression (). In the end, we determined the expression of hypoxia-inducible factor (HIF)-α and found that ROR overexpression increased the expression of HIF-α both in RNA and protein levels (). Taken together, we found that overexpression of ROR decreased CoCl2-induced cell injury.
Figure 3. Overexpression of regulator of reprogramming (ROR) promoted cobalt chloride (CoCl2) treated HK-2 cell growth. (A) The alter expression of ROR was via transfection and expression of ROR was determined using qRT-PCR. (B) Cell viability, (C) cell apoptosis, (D,E) cell apoptosis-related proteins and (F) HIF-α expression were analyzed using cell counting kit-8 assay, flow cytometry and western blot, respectively. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05, **p < .01 and ***p < .001 were all significant difference.
Silence of ROR promoted CoCl2-induced cell injury
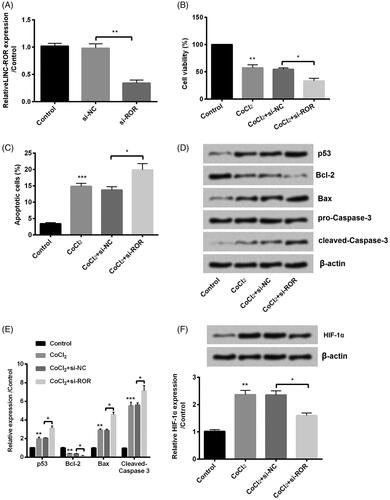
Similarly, we detected the silence of ROR. First, ROR was silenced by transfection with si-ROR (p < .01, ). Then, the same series of experiments were performed in our study. Of the contrary, opposite results were induced by silence of ROR as evidence by inhibiting cell viability (p < .05, ), enhancing cell apoptosis (p < .05, ). In addition, the apoptotic protein p53, Bax, cleaved-Caspase-3 and Bcl-2 were altered by ROR silence (). In the end, we found that silence of ROR decreased the expression of HIF-α (). Above all, silence of ROR not only decreased the CoCl2-induced injury but also promoted the injury.
Figure 4. Silence of regulator of reprogramming (ROR) inhibited cobalt chloride (CoCl2) treated HK-2 cell growth. (A) The alter expression of ROR was via transfection and expression of ROR was determined using qRT-PCR. (B) Cell viability, (C) cell apoptosis, (D,E) cell apoptosis-related proteins and (F) hypoxia-inducible factor (HIF)-α expression were analyzed using cell counting kit-8 assay, flow cytometry and western blot, respectively. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05, **p < .01 and ***p < .001 were all significant difference.
Overexpression of ROR decreased miR-145 expression
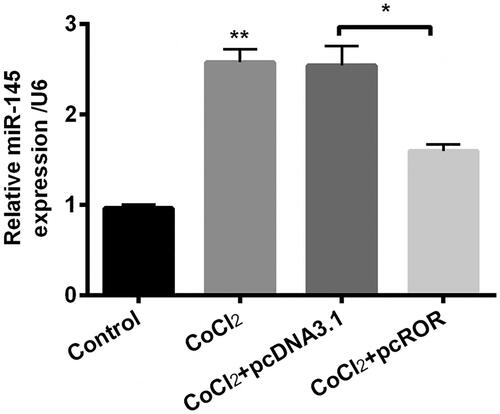
Previous study pointed out that lncRNA ROR functions via targeting miR-145 in hepatocellular cells [Citation17]. Therefore, we detected the expression of miR-145 in CoCl2-induced injury in HK-2 cells. Interestingly, we found that CoCl2 induced expression of miR-145 (p < .01) while ROR overexpression downregulated expression of miR-145 (p < .05, ), which indicated that miR-145 might be involved in the regulation network of ROR on CoCl2-induced hypoxia injury.
Figure 5. Regulator of reprogramming (ROR) decreased the expression of miR-145 in cobalt chloride (CoCl2) treated cells. The expression of miR-145 was detected using qRT-PCR. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05 and **p < .01 were both significant difference.
ROR overexpression decreased CoCl2-induced injury via downregulation of miR-145
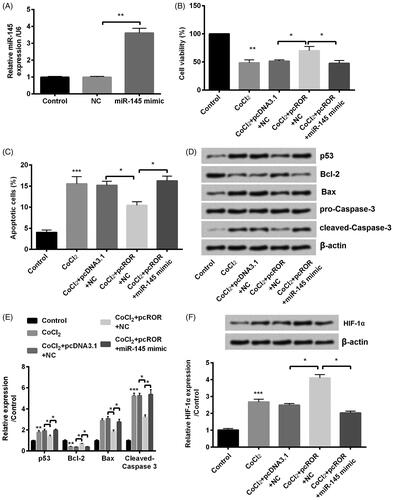
To verify the roles of miR-145 in the network of ROR on cell injury, miR-145 mimic was transfected into cells to dysregulate expression of miR-145. The upregulation of miR-145 by transfection with miR-145 mimic (p < .01) suggested high transfection efficiency (). Then, further experiments were carried out to verify whether regulation work of ROR was related with miR-145. As expected, co-transfection with pcROR and miR-145 mimic decreased cell viability (p < .05, ) and enhanced cell apoptosis (p < .05, ) compared with NC in CoCl2 treated HK-2 cells. In addition, miR-145 overexpression totally changed the expression of apoptotic proteins p53 (p < .05), Bcl-2 (p < .05), Bax (p < .05) and cleaved-Caspase-3 (p < .05, ). Furthermore, miR-145 overexpression also decreased expression of HIF-α (p < .05, ). From the results above, we can infer that the effects of ROR might through regulation of miR-145.
Figure 6. Overexpression of regulator of reprogramming (ROR) promoted cobalt chloride (CoCl2) treated HK-2 cell growth through downregulation of miR-145. (A) The alter expression of miR-145 was via transfection and expression of miR-145 was determined using qRT-PCR. (B) Cell viability, (C) cell apoptosis, (D,E) cell apoptosis-related proteins and (F) hypoxia-inducible factor (HIF)-α expression were analyzed using cell counting kit-8 assay, flow cytometry and western blot, respectively. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05, **p < .01 and ***p < .001 were all significant difference.
ROR overexpression modulated extracellular regulated protein kinases (ERK) and mitogen-activated protein kinase (MAPK) signalling pathways through regulation of miR-145
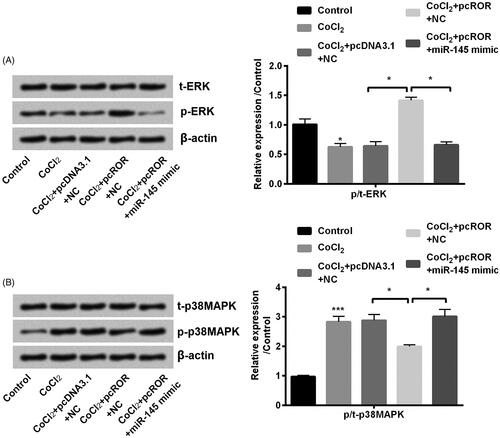
ERK and MAPK are signalling reported to be in hypoxia injury and also participated in OSAHS [Citation18,Citation19]. In our study, we found that CoCl2 decreased the phosphorylation of ERK (p < .05, ) and increased the phosphorylation of p38MAPK (p < .001, ) in HK-2 cells. ROR overexpression led to the opposite result led by CoCl2 (both p < .05, ). Interestingly, another reversed trend was induced by co-transfection with pcROR and miR-145 mimic which indicated that miR-145 overexpression impaired the effects of ROR overexpression (both p < .05, ). Taken together, we concluded that ROR could affect CoCl2-induced injury via ERK and MAPK signalling pathways.
Figure 7. Regulator of reprogramming (ROR) activated ERK and inactivated MAPK pathway. (A) The phosphorylation of ERK and (B) MAPK was determined through western blot. All data demonstrated as mean + standard deviation (SD) of three replicates. *p < .05 and **p < .01 were both significant difference.
Discussion
Clinical data pointed out that OSAHS, as one kind of common disease which influence the quality of life for the patients [Citation20]. The current study was designed to explore further about the mechanism of OSAHS in order to search better treatment approaches for this disease. Our study investigated the roles of lncRNA ROR in CoCl2-induced HK-2 cell injury. Furthermore, we also explored the network ROR did with the regulation of miR-145.
As aforementioned, OSAHS is a complex biological disease with multiple cellular and molecular regulation process. One important changed happened in this disease is hypoxia inflammatory injury [Citation13,Citation14]. In our study, we used CoCl2 to stimulate HK-2 cells. Results with decreasing cell viability and increasing cell apoptosis even with altering the expression of apoptotic proteins indicated that CoCl2 induced hypoxia injury in HK-2 cells. Similar with this treatment, CoCl2 was often used to induce hypoxia injury [Citation21].
Except the functions on cell viability and apoptosis, we also detected the effects of CoCl2 on lncRNA ROR expression. Previous study has proved that the expression of ROR was enhanced in hypoxia treatment in hepatocellular cells [Citation17]. Accordingly, our results showed that CoCl2 increased the expression of ROR in HK-2 cells, which suggested that ROR might also work in HK-2 cells with hypoxia injury.
A series of experiments were performed to determine the function of ROR in HK-2 cells with hypoxia injury. Interestingly, our results demonstrated that overexpression of ROR promoted CoCl2-stimulated HK-2 cells growth as evidenced by increasing cell viability and decreasing cell apoptosis. However, in H/R treated H9c2 cells, ROR overexpression blocked cell viability and promoted apoptosis [Citation22]. Consider the hypoxia treatment situations are variants and also the cells are different, we could not make a conclusion about the roles of ROR just from the limited information we obtained.
Furthermore, we also detected the effects of ROR on apoptotic factors. As we have mentioned before, ROR was reported to functions in p53 expression [Citation23]. Previous study was done in cancers but our study was HK-2 cells. Interestingly, our study showed that ROR overexpression decreased the accumulated level of p53, Bax and cleaved-Caspase-3 while upregulated expression of Bcl-2, which validated ROR inhibited cell apoptosis. Even though not so many previous studies were found in researching ROR on HK-2 cells, however, still several studies reported about the roles of ROR in cancers. For example, ROR enhanced proliferation, migration and chemoresistance of nasopharyngeal carcinoma [Citation24]. Cited this reference, combined with our study, we inferred that ROR could regulate cell biological activities.
On the one hand, HIF-α which consist of an oxygen-sensitive α-subunit is an oxygen-dependent transcriptional activator closely connected with mammalian development [Citation25]. In our study, ROR overexpression promoted HIF-α expression indicated that ROR overexpression initiated its protective effects through HIF-α activation, similar like its roles in kidney injury [Citation26]. On the other hand, silence of ROR underwent similar approaches from these series of experiments. Opposite was led by ROR silence as related to ROR overexpression, suggesting the same conclusion that ROR promoted HK-2 cells growth and development.
As we have mentioned data from former study that ROR has been functioning through miR-145 in hepatocellular cells [Citation17]. From our study, we saw downregulation of miR-145 by ROR overexpression. Further studies demonstrated that miR-145 overexpression impaired the role of ROR overexpression in CoCl2 stimulated HK-2 cells, suggesting that ROR overexpression achieved its functions through downregulation of miR-145. miR-145 was normally reported as a tumour suppressor inhibiting cell proliferation, migration, invasions in various kinds of cancers, such as human lung adenocarcinoma [Citation27], prostate cancer [Citation28], bladder cancer [Citation29] and gastric cancer [Citation30]. In addition, miR-145 was also popularly used in multiple injuries induced by different stimuli. For example, miR-145 alleviated lipopolysaccharides-induced inflammatory injury in human fibroblast-like synoviocyte MH7A cells [Citation31]. By contrary, miR-145 was reported to promoted hypoxia-induced injury in H9c2 cells [Citation32]. Combined previous studies and our own result, the roles of miR-145 various maybe because of different cell type and different treatments. However, to our best knowledge, this is the first time that the roles of ROR and miR-145 were investigated.
ERK and MAPK are two important signal pathways involved in hypoxia injury and importantly, these two pathways are also involved in OSAHS [Citation18,Citation19]. In our study, we found that CoCl2 inactivated ERK while activated MAPK pathways while ROR overexpression reversed this trend shown by activation of ERK and inactivation of MAPK. Our study was consistent with the previous study that lncRNA ROR activated ERK pathway through in breast cancer [Citation33]. Our study went further and unveiled one possible underlying in which miR-145 was involved in.
In conclusion, our study demonstrated that lncRNA ROR mitigated CoCl2-induced hypoxia injury in HK-2 cells as evidenced by increasing cell viability, decreasing cell apoptosis and HIF-α expression. Moreover, our study explored further and found that the network of ROR in which miR-145 was involved in. ERK and MAPK were also connected in ROR response to hypoxia injury in HK-2 cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agha B, Johal A. Facial phenotype in obstructive sleep apnea-hypopnea syndrome: a systematic review and meta-analysis. J Sleep Res. 2017;26:122–131.
- De Backer W. Obstructive sleep apnea/hypopnea syndrome. Panminerva Med. 2013;55:191–195.
- McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13:iii–iv, xi–xiv, 1–119, 143–274.
- Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33:907–914.
- Basyuni S, Barabas M, Quinnell T. An update on mandibular advancement devices for the treatment of obstructive sleep apnoea hypopnoea syndrome. J Thorac Dis. 2018;10:S48–S56.
- Yao Q, Wu L, Li J, et al. Global prioritizing disease candidate lncRNAs via a multi-level composite network. Sci. Rep. 2017;7:39516.
- Zhang J, Zhu Y, Wang R. Long noncoding RNAs in respiratory diseases. Histol Histopathol. 2018;33:747–756.
- Juan C, Wang Q, Mao Y, et al. Knockdown of LncRNA MALAT1 contributes to cell apoptosis via regulating NF-κB/CD80 axis in neonatal respiratory distress syndrome. Int J Biochem Cell Biol. 2018;104:138–148.
- Yang S, Chen J, Yu Y, et al. Long noncoding RNA ROR as a novel biomarker for progress and prognosis outcome in human cancer: a meta-analysis in the Asian population. CMAR. 2018;10:4641–4652.
- Weidle UH, Birzele F, Kollmorgen G, et al. Long non-coding RNAs and their role in metastasis. CGP. 2017;14:143–160.
- Bauderlique-Le Roy H, Vennin C, Brocqueville G, et al. Enrichment of human stem-like prostate cells with s-SHIP promoter activity uncovers a role in stemness for the long noncoding RNA H19. Stem Cells Dev. 2015;24:1252–1262.
- Yang Z, Tang Y, Lu H, et al. Long non-coding RNA reprogramming (lncRNA-ROR) regulates cell apoptosis and autophagy in chondrocytes. J Cell Biochem. 2018;119:8432–8440.
- Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–1205.
- Passali D, Corallo G, Yaremchuk S, et al. Oxidative stress in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Italic. 2015;35:420–425.
- Liu Y, Zhang R, Ying K. Long noncoding RNAs: novel links in respiratory diseases (review). Mol Med Rep. 2015;11:4025–4031.
- Lacedonia D, Scioscia G, Pia Palladino G, et al. MicroRNA expression profile during different conditions of hypoxia. Oncotarget. 2018;9:35114–35122.
- Takahashi K, Yan IK, Haga H, et al. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594.
- Ding W, Chen X, Li W, et al. Genistein protects genioglossus myoblast against hypoxia-induced injury through PI3K-Akt and ERK MAPK pathways. Sci Rep. 2017;7:5085.
- Retraction: genistein protects genioglossus myocyte against hypoxia-induced injury through PI3K-Akt and ERK MAPK pathways. J Cell Biochem. 2012;113:1809.
- Chen Q, Lin RJ, Hong X, et al. Treatment and prevention of inflammatory responses and oxidative stress in patients with obstructive sleep apnea hypopnea syndrome using Chinese herbal medicines. Exp Ther Med. 2016;12:1572–1578.
- Chu CY, Jin YT, Zhang W, et al. CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer. Int J Oncol. 2016;48:271–280.
- Zhang W, Li Y, Wang P. Long non-coding RNA-ROR aggravates myocardial ischemia/reperfusion injury. Braz J Med Biol Res. 2018;51:e6555.
- Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261.
- Li L, Gu M, You B, et al. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016;107:1215–1222.
- Lee JW, Bae SH, Jeong JW, et al. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12.
- Zhang J, Liu A, Hou R, et al. Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol. 2009;607:6–14.
- Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011;8:125–131.
- Fuse M, Nohata N, Kojima S, et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38:1093–1101.
- Chiyomaru T, Enokida H, Tatarano S, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–891.
- Qiu T, Zhou X, Wang J, et al. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177.
- Zhong F, Xu J, Yang X, et al. miR-145 eliminates lipopolysaccharides-induced inflammatory injury in human fibroblast-like synoviocyte MH7A cells. J Cell Biochem. 2018;119:10059–10066.
- Wang X, Zhang Y, Wang H, et al. MicroRNA-145 aggravates hypoxia-induced injury by targeting Rac1 in H9c2 cells. Cell Physiol Biochem. 2017;43:1974–1986.
- Peng WX, Huang JG, Yang L, et al. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16:161.