Abstract
Objective
We carried out a meta-analysis of case-control studies to determine whether epoxide hydrolase 1 (EPHX1) gene polymorphism rs1051740 was related to the risk of ovarian cancer.
Methods
Electronic databases were searched for relevant articles published in English or Chinese language. We calculated crude odds ratios (ORs) with their 95% confidence intervals (95% CIs) to assess the relationship of EPHX1 polymorphism rs1051740 with ovarian cancer risk. In addition, subgroup analyses were also conducted based on ethnicity and control source. Between-study heterogeneity was inspected with Q test and I2 statistic.
Results
Five eligible studies with a total of 1919 ovarian cancer patients and 1829 controls were ultimately included in the present meta-analysis. Overall results demonstrated that the association between EPHX1 polymorphism rs1051740 and ovarian cancer risk had no statistical significance either in total analysis or in subgroup analyses by ethnicity and source of control.
Conclusion
EPHX1 polymorphism rs1051740 may have no independent effect on ovarian cancer susceptibility.
Introduction
Ovarian cancer is one of the most common malignancies in female reproductive system and a leading cause of deaths related to gynecological cancers [Citation1,Citation2]. It is estimated that every year, around 225,500 patients are diagnosed with this malignancy, which leads to 140,200 deaths throughout the world [Citation3]. In early stage, ovarian cancer is asymptomatic or has non-specific symptoms, so the patients are usually diagnosed at advanced stages [Citation4]. In addition, there is no effective screening measure for this cancer, which also contributes to extremely low 5-year survival rate [Citation5]. With a complex process, ovarian cancer is affected by multiple aspects, such as obesity, hormonal exposure, family history, reproductive status, age of menarche and number of ovulatory cycles [Citation6,Citation7]. Apart from these elements, genetic factors have also been demonstrated to play a critical role in the development of this malignancy [Citation8,Citation9].
Epoxide hydrolase 1 (EPHX1) is an important phase II biotransformation enzyme in vivo, and plays a significant role in the activation and detoxification of toxins [Citation10]. Generally, epoxides are regarded as the most toxicologically active form of drugs or environmental chemicals [Citation11], while EPHX1 can promote the hydrolysis of epoxides into trans-dihydrodiols [Citation12]. EPHX1 gene, coding for this enzyme, is located at chromosome 1q42.1 with 20271 base-pairs, and is composed of 9 exons and 8 introns [Citation13]. In this gene, more than 10 single nucleotide polymorphisms (SNPs) have been identified. Among them, the polymorphism rs1051740 leading to the substitution of tyrosine to histidine at exon 3 has been reported to reduce the enzyme activity by more than 40% in vitro [Citation14]. Considering the role of EPHX1 in response to exogenous toxins, this polymorphism of the gene EPHX1 has been proposed to be related to the risk of multiple cancers, including ovarian cancer [Citation15], hepatocellular carcinoma [Citation13] and colorectal cancer [Citation14].
Only few studies discussed the association between EPHX1 polymorphism rs1051740 and the susceptibility to ovarian cancer, but their findings were still inconsistent or even contradictory. To further understand such correlation, we performed this meta-analysis based on previously published articles involving the topic.
Materials and methods
Search strategy
In order to identify eligible publications, we conducted a comprehensive search in PubMed, EMBASE, ISI Web of Science, Wanfang and CNKI (China National Knowledge Infrastructure) databases. Studies on the correlation between EPHX1 polymorphism rs1051740 and ovarian cancer susceptibility were retrieved using the combinations of the following keywords: “epoxide hydrolase 1 or EPHX1 or mEH” and “polymorphism or mutation or variant” and “ovarian cancer”. Additional articles were searched by checking the references of relevant studies.
Selection criteria
Included studies must meet the following criteria: (1) focusing on the association of EPHX1 polymorphism rs1051740 with ovarian cancer risk; (2) case-control studies; and (3) providing sufficient information on genotype frequencies in case and control groups for calculating crude odds ratios (ORs) with their 95% confidence intervals (95% CIs). Major reasons for exclusion were: (1) case-only studies; (2) based on duplicated data; and (3) review papers. If more than one study reported the same group of study objects, the one providing most information or latest published was selected.
Data extraction
Two reviewers abstracted essential data from all included studies using standardized form, and recorded data contained the following aspects: the first author’s name, year of publication, original country, ethnicity, control source, genotyping method, sample size (cases/controls), genotype distributions in cases and controls, and p values for Hardy–Weinberg equilibrium (HWE) in controls. All disagreements over extracted data were resolved through discussion between the two reviewers to reach consensus.
Statistical analysis
STATA 12.0 software (Stata Corp, College Station, TX, USA) was applied to complete all statistical analyses in this study. We calculated pooled ORs with the corresponding 95% CIs to evaluate the relationship between EPHX1 polymorphism rs1051740 and ovarian cancer risk under five genetic models of CC vs. TT, CC + TC vs. TT, CC vs. TT + TC, allele C vs. allele T and TC vs. TT. The conformity of genotype frequencies of EPHX1 polymorphism rs1051740 to HWE in controls was tested through chi-square test. We also performed subgroup analyses based on ethnicity and control source to further explore specific association. Cochran’s Q-statistic and I2 test were used to evaluate between-study heterogeneity [Citation16]. If p < .05 and I2>50%, indicating significant heterogeneity, random-effect model was selected for calculating pooled ORs; otherwise fixed-effects model was employed. Sensitivity analysis was performed to assess the influence of each individual dataset on overall results.
Results
Study characteristics
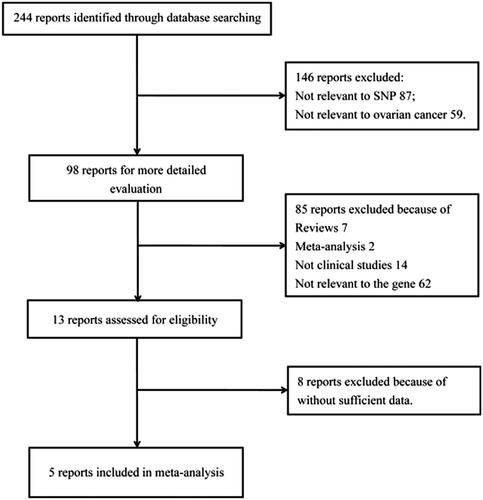
According to the search strategy, 244 articles were initially identified. We scanned the titles and abstracts of all articles, and thus excluded 146 reports not involving our studied polymorphisms (87) or ovarian cancer (59). Then, 85 articles were further excluded during full-text examination because of reviews (7), meta-analyses (2), non-clinical studies (14) and not relevant to EPHX1 gene (62). Next, 8 additional publications were removed for lacking essential data. Finally, 5 case-control studies, including 1919 ovarian cancer patients and 1829 controls, were encompassed in this study [Citation15,Citation17–20]. describes selection process of eligible articles, and main characteristics of the included studies are presented in . Except the study by Kang and colleagues [Citation19], genotype distribution in all included studies conformed to HWE in this meta-analysis (p > .05).
Table 1. Principal characteristics of the studies included in the Meta-analysis.
Meta-analysis results
As shown in , EPHX1 polymorphism rs1051740 was not significantly associated with the risk of ovarian cancer (), nor was it after stratified analyses by ethnicity and control source.
Figure 2. Forest plot for ovarian cancer risk associated with EPHX1 polymorphism rs1051740 under CC vs. TT model stratified by ethnicity. The squares and horizontal lines correspond to study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). Diamond represents the summary OR and 95% CI.
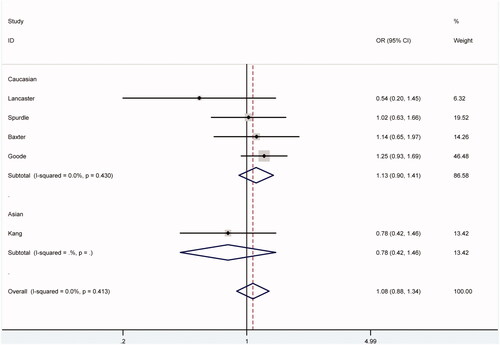
Table 2. EPHX1 Polymorphism rs1051740 and ovarian cancer risk.
There was no significant heterogeneity among eligible studies under all comparison models (p > .05). Thus, we adopted the fixed-effects model to calculate pooled ORs in this meta-analysis.
Sensitivity analysis
Sensitivity analysis was carried out to detect the effect of single study on pooled ORs, through sequential omission of included studies one at a time. And such operation did not alter overall estimates qualitatively (data not shown), indicating the robustness and reliability of our results.
Discussion
Ovarian cancer is one of the most malignant tumours in female reproductive system. Without any distinct symptoms at early stage, more than 70% of the patients have already entered in advanced stage when they are first diagnosed. Five-year survival rate of ovarian cancer patients sees no marked increase despite significant improvements in diagnosis and treatment during the past two decades. Although exact pathogenesis of ovarian cancer remains unclear, the role of genetic factors has attracted increasing attentions in studies on this malignancy.
EPHX1, an important phase II biotransformation enzyme, takes part in the hydrolysis of endogenous and exogenous epoxides as well as intermediate products during phase I metabolism, and thus eliminates these epoxides from the body after transforming them into dihydrodiols, playing critical roles in maintaining internal environment and normal functions of cells [Citation21,Citation22]. Expressed in many tissues in the body, EPHX1 acts on a variety of substrates, including various exogenous polycyclic aromatics [Citation23], and may participate in the metabolism of steroids [Citation24]. Besides, documents show that polymorphisms in the gene EPHX1 can change functional activity of the enzyme through affecting the stability of its protein [Citation25]. Over the past years, in the field of gynaecological reproduction and endocrinology, EPHX1 polymorphisms have been widely discussed in researches on preeclampsia [Citation26], spontaneous abortion [Citation27] and ovarian cancer.
The polymorphism rs1051740 at exon 3 of the gene EPHX1 can decrease the activity of the enzyme by approximately 50%, and has been related to individual susceptibility to ovarian cancer in some studies. For example, Goode and colleagues revealed an increasing effect of this polymorphism on the risk of invasive ovarian cancer, especially for serous subtype (OR = 1.17, 95% CI = 1.04–1.32), in their study among white non-Hispanic Caucasians [Citation18]. Additionally, among an Australian population, Spurdle and colleagues found that the TT genotype of the polymorphism decreased the risk of invasive ovarian cancer of the endometrioid subtype (age-adjusted OR = 0.38, 95% CI = 0.17–0.87), though the distribution of genotypes did not differ significantly between total ovarian cancer patients and unaffected controls [Citation20]. Interestingly, Lancaster and colleagues in their study demonstrated that for EPHX1 polymorphism rs1051740 in Americans, and that the frequency of the homozygous high-activity genotype (TT) was 41% in controls and 64% in patients with ovarian cancer, with an OR for the cancer of 2.6 (95% CI = 1.3–5.0) [Citation15]. However, other two studies by Baxter et al. and Kang et al., respectively, manifested no significant difference in the frequencies of genotypes or alleles of EPHX1 polymorphism rs1051740 between ovarian cancer cases and controls [Citation17,Citation19]. These discrepancies between findings from previous studies might be attributed to multiple aspects, such as diverse genetic backgrounds and living surrounds, differences in age distribution of study participants, uneven sample sizes and inadequate adjustment for confounding factors.
To abstract a more reliable conclusion on the relationship between EPHX1 polymorphism rs1051740 and ovarian cancer risk, we performed this meta-analysis to combine their inconsistent findings. After strict data syntheses, we detected no significant influence for the polymorphism on the susceptibility to ovarian cancer in total analysis; nor did we in subgroup analyses stratified by ethnicity and control source. All these results indicated that this polymorphism might not independently affect the risk of ovarian cancer. And our findings were similar to those from an earlier meta-analysis by Zhong and colleagues [Citation28]. In their study, no stratification analysis on ethnicity or control source was performed, and such omission has been supplemented in our work.
The current study had some limitations that should be acknowledged. Firstly, only five articles were included in this meta-analysis and the sample size was small, so the comprehensiveness of our results might be limited. Secondly, due to few published studies about this topic, publication bias was not detected. Thirdly, further subgroup analyses based on age, family history or other relevant factors were not performed. Fourthly, interactions between genetic and environmental factors were not considered in the study.
Conclusion
The results of our meta-analysis demonstrated that EPHX1 polymorphism rs1051740 might be not related to the risk of ovarian cancer. However, due to the limitations mentioned above, more investigations with larger sample sizes are warranted to verify final results in future.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sehouli J, Alvarez AM, Manouchehrpour S, et al. A phase II trial of pemetrexed in combination with carboplatin in patients with recurrent ovarian or primary peritoneal cancer. Gynecol Oncol. 2012;124:205–209.
- Schuler S, Lattrich C, Skrzypczak M, et al. Icb-1 gene polymorphism rs1467465 is associated with susceptibility to ovarian cancer. J Ovarian Res. 2014;7:42.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Chen LP, Cai PS, Liang HB. Association of the genetic polymorphisms of NFKB1 with susceptibility to ovarian cancer. Genet Mol Res. 2015;14:8273–8282. GMR.
- Koensgen D, Bruennert D, Ungureanu S, et al. Polymorphism of the IL-8 gene and the risk of ovarian cancer. Cytokine 2015;71:334–338.
- Romero I, Bast RC. Jr., Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–1602.
- Qin X, Ma L, Yang S, et al. The Asn680Ser polymorphism of the follicle stimulating hormone receptor gene and ovarian cancer risk: a meta-analysis. J Assist Reprod Genet. 2014;31:683–688.
- Yuan C, Liu X, Yan S, et al. Analyzing association of the XRCC3 gene polymorphism with ovarian cancer risk. BioMed Res Int. 2014;2014:1.
- Lu ZH, Gu XJ, Shi KZ, et al. Association between genetic polymorphisms of inflammatory response genes and the risk of ovarian cancer. J Formosan Med Assoc. 2015.
- Jang JH, Cotterchio M, Borgida A, et al. Genetic variants in carcinogen-metabolizing enzymes, cigarette smoking and pancreatic cancer risk. Carcinogenesis. 2012;33:818–827.
- Liu F, Yuan D, Wei Y, et al. Systematic review and meta-analysis of the relationship between EPHX1 polymorphisms and colorectal cancer risk. PloS One. 2012;7:e43821.
- Li QT, Kang W, Wang M, et al. Association between esophageal cancer risk and EPHX1 polymorphisms: a meta-analysis. World J Gastroenterol. 2014;20:5124–5130.
- Duan CY, Liu MY, Li SB, et al. Lack of association of EPHX1 gene polymorphisms with risk of hepatocellular carcinoma: a meta-analysis. Tumor Biol. 2014;35:659–666.
- Tan X, He WW, Wang YY, et al. EPHX1 Tyr113His and His139Arg polymorphisms in esophageal cancer risk: a meta-analysis. Genet Mol Res. 2014;13:649–659.
- Lancaster JM, Brownlee HA, Bell DA, et al. Microsomal epoxide hydrolase polymorphism as a risk factor for ovarian cancer. Mol Carcinog. 1996;17:160–162.
- Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3673.
- Baxter SW, Choong DY, Campbell IG. Microsomal epoxide hydrolase polymorphism and susceptibility to ovarian cancer. Cancer Lett. 2002;177:75–81.
- Goode EL, White KL, Vierkant RA, et al. Xenobiotic-Metabolizing gene polymorphisms and ovarian cancer risk. Mol Carcinog. 2011;50:397–402.
- Kang S, Duan LH, Li Y, et al. Relation Between microsomal epoxide hydrolase polymorphism and susceptibility to ovarian epithelial cancer. Zhonghua Fu Chan Ke Za Zhi. 2004;39:556–557.
- Spurdle AB, Purdie DM, Webb PM, et al. The microsomal epoxide hydrolase Tyr113His polymorphism: association with risk of ovarian cancer. Mol Carcinog. 2001;30:71–78.
- Vaclavikova R, Hughes DJ, Soucek P. Microsomal epoxide hydrolase 1 (EPHX1): gene, structure, function, and role in human disease. Gene. 2015;571:8.
- Nithipatikom K, Endsley MP, Pfeiffer AW, et al. A novel activity of microsomal epoxide hydrolase: metabolism of the endocannabinoid 2-arachidonoylglycerol. J Lipid Res. 2014;55:2093–2102.
- El-Sherbeni AA, El-Kadi AO. The role of epoxide hydrolases in health and disease. Arch Toxicol. 2014;88:2013–2032.
- Tumer TB, Sahin G, Arinc E. Association between polymorphisms of EPHX1 and XRCC1 genes and the risk of childhood acute lymphoblastic leukemia. Arch Toxicol. 2012;86:431–439.
- Li H, Fu WP, Hong ZH. Microsomal epoxide hydrolase gene polymorphisms and risk of chronic obstructive pulmonary disease: a comprehensive meta-analysis. Oncol Lett. 2013;5:1022–1030.
- Groten T, Schleussner E, Lehmann T, et al. eNOSI4 and EPHX1 polymorphisms affect maternal susceptibility to preeclampsia: analysis of five polymorphisms predisposing to cardiovascular disease in 279 Caucasian and 241 African women. Arch Gynecol Obstet. 2014;289:581–593.
- Morisseau C, Bernay M, Escaich A, et al. Development of fluorescent substrates for microsomal epoxide hydrolase and application to inhibition studies. Anal Biochem. 2011;414:154–162.
- Zhong JH, Zhang ZM, Li LQ. mEH Tyr113His polymorphism and the risk of ovarian cancer development. J Ovarian Res. 2013;6:40.