Abstract
Oral leukoplakia is one of the most common oral potentially malignant disorders (OPMDs) and its malignant transformation to oral cancer is highly associated with chronic inflammation. Extracellular vesicles (EVs) or exosome-delivered microRNAs modulate inflammatory responses and alleviate irritations that predisposes to cancer. We previously reported that microRNA-185 (miR-185) was significantly decreased in the buccal tissue of patients with oral cancer. In this study, we utilized genetically modified mesenchymal stem cells (MSCs) derived EVs with high expression of miR-185 to pasted MSC-EV-miR-185 on buccal lesions in dimethylbenzanthracene (DMBA) induced OPMD model. We found that treatment with MSC-EV-miR-185 remarkably attenuated inflammation severity and significantly decreased the incidence and the number of dysplasia in the OPMD tissue. Immunohistochemistry showed significantly decreased expression of proliferation marker PCNA and angiogenic marker CD31 in the lesion treated with MSC-EV-miR-185. Furthermore, miR-185 specifically targeted Akt genes by promoting activation of the apoptotic pathway, confirmed by the increased levels of activated caspase-3 and 9. In conclusion, genetically modified MSC-derived EVs enriched with miR-185 alleviate inflammatory response, inhibit cell proliferation and angiogenesis, and induce cell apoptosis, suggesting that their potential role as a novel therapeutic option for OPMD.
Introduction
Cancer of the oral cavity is one of the most common types of malignant neoplasms. Over 270,000 new cases are diagnosed annually. Oral cancer has a higher rate of morbidity and mortality in developing countries [Citation1] due to various unhealthy habits such as the consumption of tobacco, alcohol, areca nut, etc. More than 90% of oral cancers are identified as oral squamous cell carcinomas (OSCCs) [Citation2]. In most cases, OSCC is preceded by asymptomatic clinical lesions referred to as oral potentially malignant disorders (OPMDs) which include oral leukoplakia, erythroplakia and oral submucous fibrosis.
Emerging evidence suggests that the tumour microenvironment is an indispensable participant in fostering proliferation, survival, invasiveness and migration of cancer cells [Citation3]. Recent studies report that inflammation and cancer share intrinsic and extrinsic pathways, and that these pathways are driven by transcription factors, cytokines and chemokines [Citation4]. For instance, IL-6 is known to act as a crucial player during chronic inflammation, resulting in immune escape and acceleration of tumour progression [Citation5]. IL-6 aids in the progression of inflammation to carcinogenesis through its ability to activate the JAK1-STAT3, RAS-MAPK and PI3K-AKT signalling pathways in various tumours [Citation6,Citation7]. It has been shown that IL-6 can promote oral tumourigenesis by DNA hypomethylation. IL-1β is another pleiotropic cancer-inflammation-linked cytokine which has been reported to be upregulated in human breast, colon, lung, head and neck cancers [Citation8,Citation9]. As one of the most highly interconnected nodes in both epithelial and fibroblast networks, IL-1β interconnects with a subnetwork of genes that are associated with cancer progression, such as COX2, TGFβ, CCL2, MMP9 and MMP13. IL-1β has been shown to promote malignant transformation and tumour aggressiveness in oral cancer [Citation10].
Determining how microRNA (miRNA) levels are altered during inflammation, may provide important insights in discovering the molecular pathways involved during the transition from inflammation to cancer. It has been reported that many miRNAs, such as miR-21, miR-146a, miR-155 are involved in inflammation through their regulation of molecular targets including IL-6, IL-1β, IL-23, NF-κB/STAT3, PI3K, IL-10, and are important in the onset and progression of diseases such as colorectal cancer, breast cancer and hepatocellular cancer [Citation11–13]. More recently, it has been found that microRNA-185 (miR-185) expression is inversely associated with cancer and also acts as a tumour suppressor. A study found that miR-185-5p had low expression levels in OSCC tissue. Additionally, PDIA3P has been demonstrated to negatively regulate miR-185-5p and facilitate cell proliferation in OSCC cells [Citation14]. While much has been elucidated about the actions of miR-185 in cancer, as of yet, the functional properties of miR-185 during pre-OSCC inflammation have not been investigated.
Extracellular vehicles (EVs) are 30–150 nm membranous vesicles, which contain a broad spectrum of miRNAs, mRNAs and proteins that are protected from extracellular degradation and which reflect the phenotypic status of their respective producer cells [Citation15]. As miRNA-carriers, they offer increased stability for cell-free miRNAs. These EV-transferred miRNAs are capable of invoking transcriptional or translational modifications in the recipient cells that cause them to undergo phenotypic and functional changes [Citation16]. Emerging evidence suggests that mesenchymal stem cells (MSCs)-derived EVs are the ideal candidate for miRNA delivery, which can be used in clinical applications for cancer therapy [Citation17,Citation18].
Our previous in vitro study identified a novel mechanism of OSCC regulation in which miR-185 was shuttled to and taken up by recipient OSCC cells by EVs. Upon release of content, including miR-185, miR-185 then directly targeted the 3′UTR of Akt, resulting in OPMD signalling modulation. On the basis of these observations, in the present study, we explored the effects of genetically modified MSC-derived EVs (miR-185-EVs) on a well-established dimethylbenzanthracene (DMBA)-induced hamster OPMD model in vivo. We first analysed the role of miR-185-EVs during progression of OPMD from inflammation to cancer, followed by further analysis of the effect of miR-185-EVs on proliferation, angiogenesis and apoptosis. Here, we identified and explored the role of miR-185-EVs in anti-inflammation, anti-angiogenesis, anti-proliferation and promoting apoptosis. Our findings suggest that increasing miR-185 levels in MSC-EVs may be an important development in utilizing MSC-derived EVs as a therapeutic tool against oral cancer.
Materials and methods
Isolation and identification of BM-MSCs
Male C57BL/6 mice (6–8 weeks of age) were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and housed under pathogen-free conditions in the animal facility of Capital Medical University (Beijing, China). Murine bone marrow-derived MSCs (BM-MSCs) were harvested and grown in D-MEM/F12/Glutamax-I™ (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) in T-75 flasks. The trilineage (osteogenic, adipogenic and chondrogenic) differentiation potential of these cells was previously confirmed.
Transfection of BM-MSCs with miR-185 mimic and MSC-EV isolation
BM-MSCs were transfected with 100 nM human miR-185 mimic (Qiagen, Redwood City, CA) by mixing with Lipofectamine 2000 (Thermo Fisher, Branchburg, NJ) and opti-MEM. The transfected BM-MSCs were cultured in D-MEM/F12/Glutamax-I™ (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% exosome depleted FBS (provided by Genexosome Technologies Inc., Freehold, NJ) for 48 h, at which time EVs were isolated from the BM-MSC-conditioned media using GETTM Exosome Isolation Kit (for stem cells) according to the manufacturer’s instructions (Genexosome Technologies Inc., Freehold, NJ). EVs were stored at 4 °C and used within 72 h or were frozen at –80 °C.
Characterization of bone marrow MSC-EVs
Purified miR-185 transfected MSC-EVs were evaluated for size and concentration by nanoparticle tracking analysis (NTA, PMX ZETAVIEW I, Meerbusch, Germany), and the concentration of EVs was adjusted. MSC-EVs were probed with primary antibodies against CD81 (1:400 dilution, ProSci, Collins, CO), CD9 (1:300 dilution, Lifespan Bioscience Inc., Seattle, WA) and flotillin-1 (1:500 dilution, Abcam, Cambridge, MA) by Western blotting. EV RNAs were isolated, reverse transcribed, and qPCR was performed. Characterization was done using transmission electron microscopy (TEM). Slides were prepared for TEM according to the usual protocols. These miR-185 transfected MSC-EV were then used for the in vivo study.
DMBA-induced OPMD and related inflammation in hamsters
All animal care and protocols were approved by the Animal Ethics Committee of Beijing Stomatological Hospital, Capital Medical University (permit number: KQYY-201704-001). Syrian golden hamsters (male, ages 7 weeks, average weight 115 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Sixty hamsters were randomly divided into three groups: healthy control group (Ctrl, n = 20), DMBA-scramble control group (n = 20) and miR-185 EVs group (n = 20). The DMBA-induced OPMD animal model was established as the follows: the left cheek pouch of the hamster was exposed and received 100 μL of 0.5% DMBA painting on the mucosa surface three times per week. After administration, the cheek pouch was kept open for 1 min to allow maximal absorption. Since the third week, a cohort of animals received miR-185 EVs solution painted on the same pouch lesion three times per week. Each animal received 100 μL solution of miR-185-EVs with concentration of 2 × 1011 EV particles/kg body weight for each treatment, and the DMBA-scramble group received same amount of normal saline as control. Hamsters were sacrificed at the end of the third, fourth, fifth and sixth weeks. After sacrifice for all animals, serum and individual buccal tissue were obtained.
OSCC cell culture and EV binding assays
Human OSCC cells (Cal27) were obtained from the Institute of Beijing Stomatological Hospital (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM, high glucose, Hyclone, Logan, UT) supplemented with 10% FBS was used to culture cell lines at 37 °C with 5% CO2. Five microlitres PKH26-labelled miR-185-EVs were added to each well of a six-well plate followed by a 12-h incubation. After incubation, cells were dyed with 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher, Branchburg, NJ). Cellular uptake of labelled MSC-derived EVs was observed at 590/540 nm using fluorescence microscopy (OLYMPUS, IX71, Tokyo, Japan).
Histology
The snap-frozen buccal tissue in liquid nitrogen was embedded in OCT (Sakura Finetek, Torrance, CA) and cut into 10 μm-thickness frozen slices. The slices were dyed with DAPI and the occupation and distribution of PKH26-labelled EVs in tissue were observed by fluorescence microscopy (OLYMPUS, BX61, Tokyo, Japan) or fresh tissue were fixed directly in 10% neutral formalin and embedded in liquid paraffin. Five micrometre-thickness paraffin sections were cut and stained with haematoxylin and eosin (H&E). Inflammatory cells, epithelial hyperplasia and dysplasia were diagnosed and counted by three pathologists who were blinded to the experimental groups.
Immunohistochemistry
Antigen retrieval by microwave in citrate buffer solution was performed on buccal sections followed by blocking with goat serum. Sections were then incubated with primary antibodies against PCNA and CD31 (1:30,000 and 1:200 dilution, Abcam, Cambridge, MA) overnight at 4 °C, followed by incubation with a secondary antibody. The presence of PCNA and CD31 was then detected using DAB (Maixin, Fuzhou, China) which was counter stained with H&E.
ALT, AST, Scr and BUN assay
Alanine transaminase (ALT), aspartate transaminase (AST), serum creatinine (Scr) and blood urea nitrogen (BUN) were analysed in serum using biochemical kits (Intec PRODUCTS, Inc., Xiamen, China) according to the manufacturer’s instructions.
ELISA
Cytokine levels (IL-6, IL-1β and IL-10) were quantified in serum obtained from the hamsters using ELISA Kits (MyBioSource, San Diego, CA) according to the manufacturer’s instructions. The O.D. for each well was read at 450 nm using an ELISA reader.
Mouse cytokine microarray
A mouse cytokine proteome profilerTM array (R&D, Minneapolis, MN) was used to detect 40 different cytokines or chemokines in buccal mucosa extracts. Each array was incubated with total protein obtained from buccal tissue (n = 5 hamster per group). According to the manufacturers’ instructions, arrays were analysed by chemiluminescence and the mean pixel density for each pair of duplicate component spots was quantified.
Western blotting
The proteins extracted from buccal tissue were separated by SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were incubated with 5% non-fat milk, and then probed with primary antibodies against Akt, phospho-Akt, NF-κB, phospho-NF-κB, IL-6, IL-1β, procaspase-3, cleaved caspase-3, GAPDH (1:1000 dilution, Abclonal, Wuhan, China), PCNA, procaspase-9, cleaved caspase-9 and VEGFA (1:1000 dilution, Abcam, Cambridge, MA), overnight at 4 °C. After washing, the membranes were incubated with an HRP-conjugated secondary antibody (1:2000 dilution, APPLYGEN, Beijing, China). Finally, membranes were exposed with X-ray film for anywhere from 10 s up to 5 min.
Terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL)
Tissue sections were analysed for apoptosis by TUNEL staining using a one-step TUNEL apoptosis assay kit (Beyotime, Xiamen, China) according to the manufacturer’s protocol. The number of TUNEL-positive cells was counted.
Statistical analysis
All experiments were performed at least three times with triplicate measurements with the data presented as mean ± standard deviation (SD). Hyperplasia and dysplasia counts were expressed as the median (interquartile range). Fluorescence images were scanned and quantified using Image J software (NIH, Bethesda, MD). Statistical significance was determined using Student’s t-test, one-way ANOVA and the Kruskal–Wallis by SPSS 20.0 (SPSS, Chicago, IL). p Values less than .05 were considered statistically significant.
Results
Characterization of EVs from MSCs
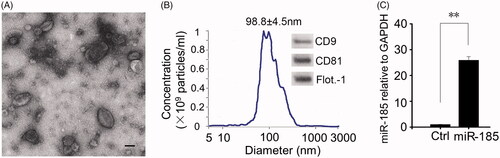
As assessed by TEM, the isolated miR-185-EVs were approximately 100 nm (consistent with NTA measurements), appeared as translucent and spherical particles (). The original concentration detected by NTA was 2 × 1013 particles/mL. Western blotting demonstrated that these particles were positive for well-characterized mouse markers of EVs, including CD9, CD81 and flotillin-1 (). q-PCR results showed that miR-185 levels in EVs had a 25-fold increase when obtained from MSCs that had been transfected with miR-185 as compared to non-miR-185-transfected MSCs-derived EVs (). As we have previously demonstrated that the EVs derived from MSC transfected with miR-185, but not from original MSC, are capable of downregulating the expression of Akt in OSCC (supplemental data), this in vivo study will only focus on the effect of miR-185 transfected MSC-derived EVs.
Figure 1. Characterization of miR-185 enriched MSC-EVs. (A) Isolated miR-185 MSC-EVs were characterized by TEM. Representative image is shown (scale bar = 50 nm). (B) NTA was performed on the EVs to determine their concentration and size. EV diameter was measured and represented as mean ± SD, n = 3 independent experiments performed in triplicate. Western blotting (insets) for EV markers CD9, CD81 and flotillin-1. (C) qPCR analysis for expression of miR-185 in EVs. n = 5 independent experiments performed in triplicate (**p < .01 versus Ctrl).
Binding abilities of miR-185-EVs into buccal mucosa and OSCC cells
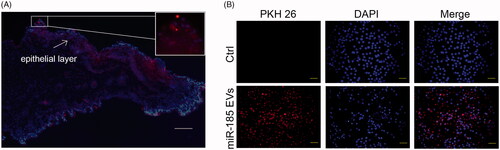
To determine cellular targets of miR-185-EVs in the buccal mucosa, miR-185-EVs were labelled with PKH26, and then administered to the buccal mucosa of hamsters and OSCC (Cal27) cells. To identify the trail of EVs in vivo, we administered PKH26-labelled EVs on the hamster mucosa and traced the EVs for 12 h. Fluorescent images showed that EVs could spread from the surface to the deeper layer of mucosa with strong binding ability for epithelial cells (). Furthermore, Cal27 cells incubated with PKH26 labelled EVs for 12 h had a scattered punctate distribution throughout the cell cytoplasm demonstrating a high binding efficiency ().
Figure 2. EVs binding abilities. (A) Binding of miR-185 MSC-EVs to buccal mucosa. miR-185 enriched MSC-EVs were prelabelled with PKH26 red dye and pasted on mucosa surface of lesion in hamsters. Images were captured at 12 h after the treatment and tissue distribution of PKH26 stained EVs was evaluated by fluorescent microscopy (blue indicates DAPI, red indicates PKH26-stained EVs, scale bar = 50 μm). Most fluorescent red staining was localized in the epithelial layer of the mucosa (insets). (B) Intercellular internalization of miR-185 MSC-EVs to OSCC cells (Cal27). Cal27 cells were incubated with PKH26 labelled miR-185 MSC-EVs for 12 h and cellular localization of PKH26 fluorescence was evaluated by fluorescent microscopy. Cal27 cells were incubated with unstained EVs as a negative control (blue indicates DAPI, red indicates PKH26-stained EVs). Images shown are representative of five independent experiments (scale bar = 20 μm).
MiR-185-EVs delay the progression of OPMD
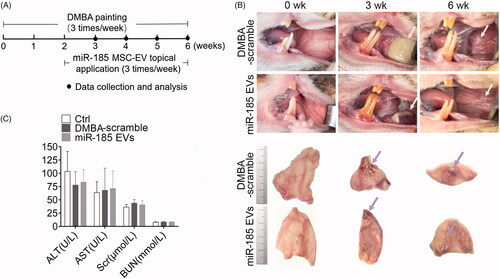
The intact buccal mucosa was pink, smooth, thin and submucosal vessels were clearly visible. After exposure to DMBA for 1–2 weeks, there was marked acute inflammation response represented by the presence of hyperaemia, oedema and yellow liquid-like inflammatory exudation. After 5–6 weeks of DMBA exposure, the mucosa showed increased keratinization, roughness, thickening and decreased elasticity, with visible white patches. Strikingly, miR-185-EVs treatment remarkedly reduced the inflammatory response as represented by diminished inflammatory exudation during the acute stage, and reduced white patch size during the late stage of inflammation (). We further investigated the systemic effects of topical application of miR-185-EVs and found no significant changes of the levels of ALT, AST, Scr and BUN in the animal serum (), suggesting that topical application of the inflammation “modulator” miR-185-EVs has no alteration on liver and renal function.
Figure 3. MiR-185 MSC-EVs delay the progression of OPMD in DMBA-induced hamsters. (A) Schematic diagram of miR-185 MSC-EVs treatment in a DMBA induced OPMD hamster model. (B) Representative gross images of the hamster buccal mucosa on weeks 0, 3, and 6 of DMBA exposure (upper, white arrows indicate inflammatory exudation (middle) and white patches (right)). Representative gross images of in vitro buccal tissue (lower). (C) Serum from blood collected at the time of sacrifice was assayed for the level of ALT, AST, Scr and BUN.
MiR-185-EVs dampen DMBA-induced inflammation
To investigate the effects of miR-185-EVs on the suppression of DMBA-induced inflammation, we first pasted 0.5% DMBA on the buccal mucosa of hamsters to cause acute inflammation. Our results showed that an inflammation was triggered one week after DMBA exposure, which resulted in abundant aggregation of inflammatory cells in tissue by H&E staining. However, in the miR-185-EVs treated hamsters, there was significant suppression of the inflammatory cell infiltration in tissue (). In addition, this attenuated histological inflammatory response was accompanied by an obvious reduction of proinflammatory cytokines and chemokines (IL-1β, IL-16, etc.) in the harvested tissue pieces as assessed by protein array analysis, with some of them returned to the baseline levels as shown in . Furthermore, hamsters administered with miR-185-EVs demonstrated significantly decreased circulating levels of the proinflammatory cytokines IL-6 and IL-1β and significantly increased levels of anti-inflammatory cytokine IL-10 at week 3 (). The expression levels of IL-6 and IL-1β in the buccal tissue were further verified by Western blotting ().
Figure 4. MiR-185 MSC-EVs suppress inflammation. (A) Inflammatory cells in buccal sections were stained with H&E (left, dotted squares indicate the area with dense inflammatory cells of buccal mucosa painted with DMBA, 10 sections per animal, n = 5 in each group, scale bar = 50 μm) and then quantified (right, miR-185 EVs versus DMBA-scramble, *p < .05). (B) Protein array analysis showing altered expression of cytokines and chemokines in buccal tissue (left) and quantification (right). Numbers above the X axis indicate partial pro-inflammatory cytokines and chemokines, and numbers below the X axis indicate partial anti-inflammatory cytokines and chemokines. (C) Serum concentrations of IL-6, IL-1β and IL-10 were assessed by ELISA (n = 5 in each group, miR-185 EVs versus DMBA-scramble, *p < .05, **p < .01). (D) The protein levels of IL-6 and IL-1β, and pathway-related protein of Akt, p-Akt, NF-κB and p-NF-κB in buccal tissue were determined by Western blotting (left) and quantified (right, n = 5 in each group, miR-185 EVs versus DMBA-scramble, *p < .05, **p < .01).
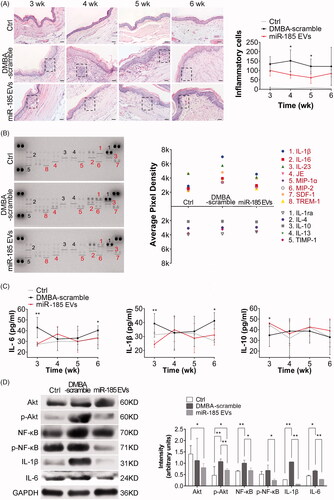
Our previous studies had shown that Akt transcriptional activity is blocked by the direct targeting of miR-185 to its binding site in the Akt 3′-UTR (unpublished data). In DMBA induced OPMD model, we found that DMBA stimulated Akt activation was represented by increased levels of phosphorylated Akt and its correlated phosphorylated NF-κB, a key downstream gene in the Akt signalling pathway. However, miR-185-EV treatment significantly suppressed the levels of phosphorylated Akt and phosphorylated NF-κB as assessed by Western blotting (), confirming that miR-185-EVs inhibited inflammation via targeting of the Akt signalling pathway.
MiR-185-EVs inhibit proliferation and angiogenesis
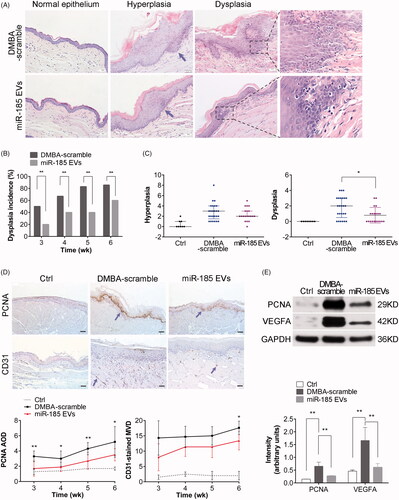
Emerging evidence suggests that inflammation and cancer share intrinsic and extrinsic pathways. We have demonstrated here that OPMD progression in hamsters is accompanied by alteration in gene expression, which subsequently affected proliferation, angiogenesis and apoptosis. Due to a persistently elevated inflammatory response caused by DMBA, the buccal tissue epithelium changed from hyperplasia to mild dysplasia within 2 weeks and displayed a loss of polarity of basal cells and irregular epithelial stratification. The degree of dysplasia was further aggravated during weeks 4–5 as shown by drop-shaped rete ridges, increased nuclear to cytoplasmic ratio, and atypical mitotic figures (MFs). By week 6, severe dysplasia was present (). In contrast, miR-185-EV treatment led to marked alleviation of hyperplasia and dysplasia based on the diagnosis criteria [Citation19] (). Cytological and histological analysis showed that even in the presence of constant DMBA exposure, the mucosa lesion treated with miR-185-EVs had significantly decreased the incidence and the number of dysplasia (). Furthermore, immunohistochemistry and Western blotting showed that administration of miR-185-EVs resulted in a significant decreased expression of the proliferative marker PCNA and the decreased expression of microvascular marker VEGFA in the buccal tissue (), suggesting that miR-185-EVs suppressed cell proliferation and angiogenesis in the DMBA-induced OPMD model.
Figure 5. MiR-185 MSC-EVs alleviate pathological features and inhibit proliferation and angiogenesis. (A) Representative images of histopathological grading in buccal sections by H&E staining (arrows indicate the location of hyperplasia, dotted squares indicate the location of typical dysplasia with partially magnified images (right), 10 sections per animal, n = 5 in each group, scale bar = 50 μm (100 μm for Ctrl)). (B) Incidence of dysplasia on weeks 3, 4, 5 and 6 of DMBA exposure (n = 5 in each group, **p < .01). (C) Hyperplasia and dysplasia were quantified and visualized by scatter-plot diagram (n = 5 in each group, *p < .05). (D) IHC analysis of PCNA and CD31 protein expression levels in buccal sections. Homogenous brown nuclear and vascular endothelial staining indicate respectively the positive expression for PCNA and CD31 (upper, arrows indicate positive expression, scale bar = 50 μm). Staining was then quantified (lower, MOD: mean optical density, MVD: microvessel density determined by CD31 staining, n = 5 in each group, miR-185 EVs versus DMBA-scramble, *p < .05, **p < .01). (E) The protein levels of PCNA and VEGFA in buccal tissue were determined by Western blotting (upper) and then quantified (lower, n = 5 in each group, **p < .01).
MiR-185-EVs induce apoptosis
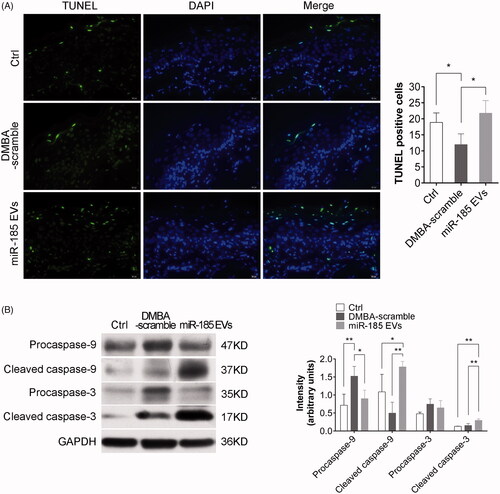
We next investigated the effect of miR-185-EVs on cell apoptosis and found that the hamsters receiving miR-185-EVs treatment had substantially increased TUNEL stain-positive cells in the mucosa lesions as compared to the DMBA-scramble group (), indicating that miR-185 induced cell apoptosis. Considering the epiregulatory effect of miR-185 on Akt pathway activation, we next investigated the effects of miR-185 on the levels of cleaved caspase-9, and caspase-3, the downstream genes of Akt. We found that miR-185-EVs led to significantly increased expression of cleaved caspase-9 and cleaved caspase-3 ().
Figure 6. MiR-185 MSC-EVs induce apoptosis. (A) Apoptotic cells in buccal sections were determined by TUNEL assay (10 sections per animal, green indicates apoptotic bodies, blue indicates DAPI-stained nuclei, scale bar = 20 μm). Positive (apoptotic) cells were quantified (right, n = 5 in each group, *p < .05). (B) Pro-caspase-9, cleaved caspase-9, pro-caspase-3 and cleaved caspase-3 protein levels in buccal tissue were assessed by Western blotting (left) and were quantified (right, n = 5 in each group, *p < .05, **p < .01).
Discussion
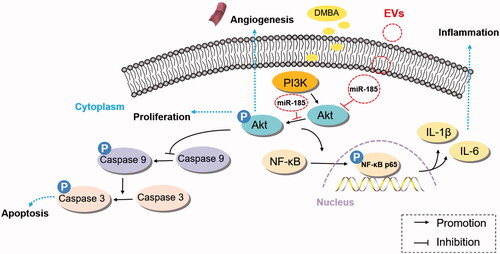
The main finding of the present study is that engineered EVs with a high copy of miR-185 effectively suppressed the occurrence of inflammation and delayed the pathological development of OPMD. We have shown for the first time the positive abilities of miR-185-EVs to inhibit inflammation, angiogenesis and proliferation, and to promote apoptosis in vivo. After continuous exposure to the carcinogen DMBA, animal buccal mucosa first developed into inflammatory lesions, and later incurred white patches, which has been characterized as typical OPMD lesions. We monitored the entire histological progress of DMBA-induced OPMD, from the formation of the initial inflammatory lesion to the occurrence of hyperplasia, followed by progression to dysplasia. This typical OPMD is eventually developed into OSCC (unpublished data). We report in this study that MSC-derived EVs carrying high copies of miR-185 exerted therapeutic effects on DMBA induced OPMD. Treatment with miR-185-EVs alleviated inflammation between weeks 3 and 6, decreased the number of dysplasia and attenuated dysplasia grade. miR-185-EV treatment led to the activation of the apoptotic pathway via direct targeting on Akt, which is an upstream regulator of caspase-9. Overall, our results show for the first time that enriched miR-185 carried in engineeringly modified MSC-derived EVs have therapeutic potential to modulate inflammation and delay malignant transformation ().
Figure 7. Schematic representation of the mechanism by which miR-185 encapsulated by MSC-EVs ameliorates OPMD by inhibition of inflammation and induction of apoptosis via Akt/NF-κB and Akt/caspase-9 related pathways.
Currently, EVs are emerging as a promising therapeutic tool for various diseases, especially cancer. This is mainly due to the fact that these biological vesicles have low immunogenicity and toxicity, and have a high therapeutic efficacy [Citation20]. Engineered EVs or EVs from healthy subjects can transport biologically active anti-cancer molecules, that in turn regulate gene expression and cellular function in target cells [Citation21]. In particular, the administration of MSC-derived EVs exerts various effects on the development and progression of different type of cancers [Citation22,Citation23]. The safety and feasibility observed in early clinical trials using MSCs has resulted in an increased interest in the translation of the use of these cells to the clinic [Citation24]. Likewise, increasing evidences suggest that the MSC-derived EVs therapeutic effects are mainly mediated in a paracrine manner by EVs [Citation25,Citation26]. In this study, we designed a therapeutic system that is capable of delivering high copies of miR-185 into MSC derived EVs by painting the “drug” directedly to the surface of the diseased lesion in our OPMD model. We found that these genetically modified MSC-derived EVs have strong binding affinities to oral epithelial cells as they majorly targeted the epithelial cells after application and significantly attenuate proinflammatory cytokine release. This delivery method is noninvasive, easy operable and effective. In addition, the topical application of engineered EVs on oral mucosal lesions has no observable side effects on liver or kidney function even after 6 weeks pasting. Therefore, the topical application of engineered EV is an ideal delivery method for OPMD treatment.
Located on the 22q11.2 gene locus, miR-185 is reported as a regulator involved in the biological processes of carcinoma. The targets of miR-185 include Akt, Wnt1/β-catenin, WNT2B, TGF-β1 and STIM1 [Citation27–30] which are associated with cancer-related signalling pathways. Our previous in vitro study demonstrated that miR-185 directly targets the 3′-UTR of Akt mRNA, resulting in mRNA degradation and subsequent inhibition of expression of Akt target genes. In this study, we showed the evidence that delivery of high copies of miR-185 to the injured recipient cells significantly decreased the levels of Akt and effectively inhibited phosphorylation of Akt. Since NF-κB is a downstream target of Akt and a critical regulator of the release of proinflammatory cytokines [Citation31], we additionally found that delivery of MSC-EV-miR-185 inhibited the phosphorylation of NF-κB, followed by the attenuation of carcinogen-induced local inflammatory response. This regulatory mechanism was evidenced together with other reports in neuro-inflammation, osteoarthritis and placental inflammation [Citation32–34].
As a tumour suppressor miRNA, miR-185 inhibits carcinoma growth by targeting the Akt pathway [Citation35]. The PI3K/Akt signalling pathway also regulates a variety of cellular activities, including proliferation, differentiation, metabolism and apoptosis [Citation36,Citation37]. Abnormal regulation of these cellular activities can mediate neoplastic transformation, including oral carcinoma [Citation38]. Previous report showed that lncRNA PDIA3P interacts with miR-185-5p to modulate OSCC progression by targeting Cyclin D2 [Citation14]. In our study, we demonstrated clearly that miR-185-EVs administration resulted in a marked decrease of dysplasia incidence and a significant increase in apoptosis. As one of the substrates of Akt, procaspase-9 is cleaved into active fragments when the apoptosis pathway is activated, leading to an increase in the level of cleaved caspase-9 and its downstream target caspase-3. Akt inhibits the processing and activation of procaspase-9 via Akt-mediated phosphorylation of Ser196-procaspase-9 [Citation39]. Previous studies show that radiation enhanced apoptosis can be achieved by the depression of Akt-related signalling in human oral cancer cells [Citation40], and the resveratrol triggered apoptosis in OSCC is via Akt/mTOR signalling [Citation41]. Thus, the inhibition of Akt phosphorylation by miR-185 may lead to cascading activation of caspase-9, which is essential for the induction of the apoptosis in the treatment of OPMD. Replenished delivery of MSC-EVs-miR-185 is a promising therapeutic strategy to reduce malignant transformation of OPMD by inhibiting proliferation and inducing apoptosis via the Akt pathway.
In conclusion, this study tested the feasibility of local delivery of EV carried miR-185 to treat OPMD in hamster model as a potential therapeutic strategy. Genetically modified EVs from MSCs modulate inflammation, inhibit cell proliferation and promote apoptosis. Future studies on the use of other therapeutic molecules in the MSC-EV payload and EV homing will help to understand the mechanisms of precancerous lesion development and the utility of engineered MSC-derived EVs as a comprehensive anti-OPMD strategy.
Disclosure statement
Dr. Yu Zhou is the CEO and shareholder of Genexosome Technologies Inc. The other authors declare that they have no conflict of interest.
Additional information
Funding
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548.
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Raposo TP, Beirão BC, Pang LY, et al. Inflammation and cancer: till death tears them apart. Vet J. 2015;205:161–174.
- Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Front Oncol. 2012;2:58.
- Zhao Y, Yao J, Wu XP, et al. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog. 2015;54(Suppl. 1):E81–E93.
- Jung IH, Choi JH, Chung YY, et al. Predominant activation of JAK/STAT3 pathway by interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia. 2015;17:586–597.
- Gasche JA, Hoffmann J, Boland CR, et al. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129:1053–1063.
- Lewis AM, Varghese S, Xu H, et al. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48.
- Lee CH, Chang JS, Syu SH, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230:875–884.
- Josse C, Bours V. MicroRNAs and inflammation in colorectal cancer. Adv Exp Med Biol. 2016;937:53–69.
- Niu J, Shi Y, Tan G, et al. DNA damage induces NF-κB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J Biol Chem. 2012;287:21783–21795.
- Jin K, Li T, Sánchez-Duffhues G, et al. Involvement of inflammation and its related microRNAs in hepatocellular carcinoma. Oncotarget. 2017;8:22145–22165.
- Sun CC, Zhang L, Li G, et al. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting cyclin D2. Mol Ther Nucleic Acids. 2017;9:100–110.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383.
- Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15.
- Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122.
- Pakravan K, Babashah S, Sadeghizadeh M, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017;40:457–470.
- Müller S. Oral epithelial dysplasia, atypical verrucous lesions and oral potentially malignant disorders: focus on histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:591–602.
- EL Andaloussi S, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357.
- Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78.
- Suchorska WM, Lach MS. The role of exosomes in tumor progression and metastasis. Oncol Rep. 2016;35:1237–1244.
- Zhang X, Tu H, Yang Y, et al. Mesenchymal stem cell-derived extracellular vesicles: roles in tumor growth, progression, and drug resistance. Stem Cells Int. 2017;2017:1758139.
- Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848.
- Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157.
- Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287.
- Li S, Ma Y, Hou X, et al. MiR-185 acts as a tumor suppressor by targeting AKT1 in non-small cell lung cancer cells. Int J Clin Exp Pathol. 2015;8:11854–11862.
- Liu C, Li G, Ren S, et al. miR-185-3p regulates the invasion and metastasis of nasopharyngeal carcinoma by targeting WNT2B in vitro. Oncol Lett. 2017;13:2631–2636.
- Zhao P, Wang S, Zhou Y, et al. MicroRNA-185 regulates spinal cord injuries induced by thoracolumbar spine compression fractures by targeting transforming growth factor-β1. Exp Ther Med. 2017;13:1127–1132.
- Zhang Z, Liu X, Feng B, et al. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34:4808–4820.
- Markopoulos GS, Roupakia E, Tokamani M, et al. Roles of NF-κB signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines. 2018;6:40.
- Himaya SW, Ryu B, Qian ZJ, et al. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicol In Vitro. 2012;26:878–887.
- Wang C, Zeng L, Zhang T, et al. Tenuigenin prevents IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-κB signaling pathway. Inflammation. 2016;39:807–812.
- Zhou J, Miao H, Li X, et al. Curcumin inhibits placental inflammation to ameliorate LPS-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated Akt. Inflamm Res. 2017;66:177–185.
- Qadir XV, Han C, Lu D, et al. miR-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am J Pathol. 2014;184:2355–2364.
- Huang H, Song Y, Wu Y, et al. Erbin loss promotes cancer cell proliferation through feedback activation of Akt-Skp2-p27 signaling. Biochem Biophys Res Commun. 2015;463:370–376.
- Zhang C, Lan T, Hou J, et al. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation of PI3K/Akt signaling. Oncotarget. 2014;5:4392–4405.
- Martins F, de Sousa SC, Dos Santos E, et al. PI3K-AKT-mTOR pathway proteins are differently expressed in oral carcinogenesis. J Oral Pathol Med. 2016;45:746–752.
- Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321.
- Nakagawa Y, Takahashi A, Kajihara A, et al. Depression of p53-independent Akt survival signals in human oral cancer cells bearing mutated p53 gene after exposure to high-LET radiation. Biochem Biophys Res Commun. 2012;423:654–660.
- Chang CH, Lee CY, Lu CC, et al. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol. 2017;50:873–882.
