?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Elicited artificial in vitro cultures are gaining more interest due to their uniform biosynthesis of industrially valuable secondary metabolites. In this study, a unique methodology was applied, in which different ratios of gold (Au) and silver (Ag) nanoparticles (NPs) were supplemented to submerge cultures to investigate sustainable production of biomass and antioxidant secondary metabolites. Cell suspension cultures were exposed to Ag and AuNPs alone or different ratios of AgAuNPs (1:2; 1:3; 2:1; 3:1) in combination with NAA. The combination of AgAuNPs (3:1) with NAA enhanced fresh (9.25 g/100 ml) and dry biomass (0.64 g/100 ml) of suspended cells than control (6.67; 0.233 g/100 ml). AuNPs with NAA-augmented media enhanced biomass accumulation in lag, log and stationary phases in a period of 49 days. Furthermore, AgAu (3:1) and AgAuNPs (2:1; 1:2) with NAA enhanced protein contents, peroxidase and superoxide dismutase enzymes. However, maximum phenolics (TPC; 10.61 mg/g-DW) and flavonoids (7.62 mg/g-DW) were observed in cell cultures exposed to a combination of AgAuNPs (1:3) and NAA than control (6.27, 5.49 mg/g-DW). The combination of AgAuNPs (2:1) with NAA enhanced antioxidant activity (87.85%) in cell cultures. This study will help in illuminating the impact of NPs on cultures development and production of natural antioxidants.
Introduction
Nanotechnology is a multipurpose field that has got ever escalating applications in nearly every field of science. It is advancing rapidly and expected to turn into a trillion-dollar industry by 2018 with employment of 2 million, exceeding the industrial revolution impact [Citation1]. This industry is posing significant impacts on the environment, economy and society worldwide. In turn, it is producing both encouraging and discouraging responses from governments, researchers and social media [Citation2]. Due to their distinctive characteristics, this field is advancing its applications in the diverse fields of biology. Conversely, the use of nanoparticles is novel and requires elaborative research in the area including tissue culture and medicinal plant biotechnology. Currently, the majority of studies regarding plant growth, seed germination and physiological responses are concerned about nanoparticles toxicity [Citation1,Citation3]. It has been reported that seed germination is accelerated in Glycine max by the applications of TiO2 and SiO2 and they also enhanced activities like antioxidation and nitrate reductase [Citation4]. Similarly, Sharma et al. [Citation5] reported that antioxidative enzymes and growth of the seedlings is improved by AgNPs. Contrarily, seed germination and root development are inhibited by ZnONPs in various plants [Citation1]. Furthermore, in Brassica oleracea, synthesis of chlorophyll, metabolism and dry biomass are enhanced by the application of TiO2NPs [Citation6]. However, the phenomenon of plant growth and development by these nanoparticles is still poor and needs further research.
Prunella vulgaris (self heal/all heal) is thermophilic and hygrophilous edible herb (family; Lamiaceae), that has been utilized in North America, China and Europe as a potent herbal medicine [Citation7]. Recent studies emphasized on the potentials of P. vulgaris as promising anti-HIV and against herpes viruses [Citation7]. However, in Unani, Korean and Chinese traditional medicine, it has been widely used against cold and sore throat, headache, oedema goiter and nephritis [Citation8–10]. Due to the occurrence of polyphenols in this plant species, it also exhibits properties including, antitumor, antiviral and anti-inflammatory [Citation11,Citation12]. Plant-based phenolic and flavonoids, and their various activities are associated with antioxidant potential [Citation13]. During extreme conditions, like light fluctuation, osmotic stress and in defense mechanism against injury, the plant antioxidant system plays a protective role against these conditions. DPPH-based antioxidant activity or others have a direct or linear relationship with species resistance toward the stressors [Citation14–16].
During unusual conditions, reactive oxygen species (ROS) interact with biomolecules and therefore hinder the process of growth and differentiation as well as effect secondary metabolism [Citation17]. To neutralize the adverse effects of these stressful ROS, plant cells utilize endogenous enzymes such as POD and SOD, or provoke non-enzymatic system to release polyphenolics [Citation18]. Endogenous enzymes are of great importance in plant development, morphogenesis, catabolism of auxins and differentiation [Citation19]. Likewise, the non-enzymatic machinery helps to quench the toxic free radicals, motivate the immune machinery, control apoptosis, activate gene expression, stimulate the biosynthesis of enzymes and cooperate with cell-cycle arrest [Citation20]. Generally, in plants, the production of bioactive compounds results as a comeback to the biotic and abiotic stresses [Citation21]. Therefore, the exposure of plant cell culture to biotic and abiotic elicitors stimulates the cultured cells to synthesize metabolites in higher quantities [Citation22–25].
Therefore, the major aim of this study was to explore the possible effects of Ag and Au nanoparticles, as metallic elicitors, in combination with NAA on the development of cell suspension culture in P. vulgaris. Moreover, the impact of these nanoparticles on the biosynthesis of the content of total protein (CTP) and polyphenolics was also investigated. Also, enzymatic potentials (SOD and POD) were conducted along with the determination of possible DPPH-based antioxidant activities.
Methods
Establishment of cell suspension from calli cultures
In order to develop calli cultures, leaf explants were exploited from in vitro grown plantlets of P. vulgaris. Here, 0.5–1 cm young leaf pieces were carefully harvested and placed on the Murashige and Skoog [Citation26] media. To obtain maximum callogenesis, the media was augmented with 2.0 mg l−1 naphthalene acetic acid (NAA). Sucrose (30 g l−1) as limiting substrate and good carbon source was added to the cultures media, followed by the addition of gelling agent (8 g l−1 agar; Oxoid, England). The fluctuated pH of the culture media was altered to 5.6–5.8 through the addition of weak acid or base (pH meter, Eutech Instruments, Singapore). The sealed jars containing media and plant growth regulators (PGRs) were autoclaved for 25 min at 121 °C (Systec, Germany). The inoculated cultures were placed in growth room having an optimum temperature (25 °C) and 40–50 µmol m−2 s−1 light intensity for better response. Finally, the 16/8 h photoperiod was applied in growth room to get maximum calli cultures.
To established suspension culture, 30-days old calli was shifted to MS basal media augmented with 2.0 mg l−1 NAA in Erlenmeyer flask (500 ml). The cultured flask was placed on an orbital shaker (120 rpm; Gallenkamp, England) at 25 °C for producing stock cell suspension culture. This culture was filtered after 14-days and transferred to 250 ml flasks for subsequent experiments. Synthesized NPs were procured from the research project of Rahman et al. [Citation27] Accurately, 30 µg l−1 of each NPs along with NAA (2.0 mg l−1) were added to MS media. MS basal media augmented with AuNPs with NAA (T1), AgNPs with NAA (T2), AgAu (1:2) with NAA (T3), AgAu (1:3) with NAA (T4), AgAu (2:1) with NAA (T5), AgAu (3:1) with NAA (T6) and NAA alone (T7) were placed on orbital shaker for the establishment of cell suspension culture ().
Table 1. Application of various treatments of metallic nanoparticles alone or different ratios of silver and gold nanoparticles in combination with naphthalene acetic acid [AuNPs + NAA (T1); AgNPs + NAA (T2); AgAuNPs 1:2 + NAA (T3); AgAuNPs 1:3 + NAA (T4); AgAuNPs 2:1 + NAA (T5); AgAuNPs 3:1 + NAA (T6) and control) for accumulation of biomass and biosynthesis of desirable secondary metabolites in cell cultures of P. vulgaris.
Growth kinetics and biomass determination
The data for suspended cells growth kinetics were obtained with 07 days interval for 07 weeks period. From these data, the suspended cells growth curve was developed upon exposure to each ratio of metallic nanoparticles (1:2; 1:3; 2:1; 3:1) in combination with 2.0 mg l−1 NAA. In order to investigate fresh weight, the suspended cells were slowly washed with sterile distilled water and slightly dried with filter paper and then weighed. The harvested cells were then weighed (Sortorious; Germany) for the estimation of FB. The suspended cells were placed in an oven (55 °C; Thermo Scientific; Germany) to investigate the dried biomass. Herein, the FW and DW of suspended cells are represented in gram/100 ml.
Determination of antioxidative enzymes
Antioxidative enzymes or stress enzymes in suspended cells were determined according to the established protocol of Nayyar and Gupta [Citation28]. One gram fresh calli cultures in the presence of 1% PVPP (pH 7) was carefully blended with 10 ml of extraction buffer (50 mM) prepared from KH2PO4 in autoclaved tubes. The solution was centrifuged at 14,000 rpm under chilling temperature (4 °C) for approximately 20–30 min. After centrifugation, the upper portion of the mixture was used for the investigation of antioxidative enzymes. The protocol of Lagrimini [Citation29] was followed for the evaluation of peroxidase (POD) activity with some modifications. Furthermore, for investigating superoxide dismutase (SOD), the recent method of Ahmad et al. [Citation14] was used.
Determination of polyphenolics content
To prepare extract for polyphenolics content, the dried suspended cells were powdered and mixed with ethanol solvent. To obtain maximum extraction, the powdered solution was placed in a dark room for seven days period. The supernatant was collected in a separate container after centrifugation at 14,000 rpm for 10 min and the cells debris were discarded. The collected supernatant was exploited for polyphenolics determination. The polyphenolics content were investigated in dried extracts according to the method of Ahmad et al. [Citation14]. Accurately, the supernatant (0.03 ml) from each treatment was combined with 0.03 ml of FCR (2 normal Folin–Ciocalteus reagent) for in vitro activity along with 2.55 ml sterile distilled water. Before filteration (through 45 µm membrane) the solution was centrifuged (10,000 rpm) for 10 min. A wavelength of 760 nm was applied for phenolics estimation through double-beam spectrophotometer (UV visible; Shimadzu, Japan). A known concentration (1 to 10 mg/ml) ofGallic acid as standard for phenolics was exploited for curve and regression (R2 = 0.9878). The TPC mean values were indicated as Gallic acid equivalent (GAE) milligram/gram of dry weight (DW) using the following formulae.
Here, AbSC represents suspended cells absorbance; AbC represents control absorbance, while the CnF and DnF represent the conversion and dilution factors from the calibration curve.
The Ahmad et al. [Citation14] protocol was used for flavonoid determination. In this activity, 0.25 ml of cells solution and 1.25 –ml of sterile distilled water was carefully combined with 0.075 –ml of aluminum chloride (5%). The mixture was then combined with 0.5 ml of 1 M NaOH and placed in a dark room to minimize the oxidation reaction. The mixture was centrifuged at 10,000 revolutions per min and the supernatant absorbance was checked at 510 nm for the investigation of flavonoids content. The different known concentrations (1–10 mg/ml) of Rutin as standard for flavonoids were exploited for obtaining the calibration curve. The regression obtained from the calibration curve was R2 = 0.9866. Finally, the flavonoid results were represented as Rutin equivalent mg/g-DW (milligram per gram of dried extract).
Estimation of content of total protein
Prunella cell cultures were extracted through the methods of Giri et al. [Citation30]. Fresh and viable suspended cells from each treatment (4 g) were grinded with liquid nitrogen. Accurately, 200 mg of each crushed sample was mixed with 1 ml of methanol and then incubated for 5 min in a cold room. For the removal of intracellular protein, the samples were exposed to Sonicator (Toshiba, Japan) for 5 min. The solution was then vortexed for approximately 5 min to get homogenous metabolites. Centrifugation for 20 min at 13,000 rpm for the removal of cell debris and the supernatant was used for the determination of CTP. The method of Lowry et al. [Citation31] was used for calculating total protein (CTP), following which the supernatant was exposed to 650 nm wavelength in Shimadzu UV-spectrophotometer. BSA-standard curve was established from protein absorbance data.
Determination antioxidant activity in cell cultures
DPPH-radicals scavenging activity in suspended cells were investigated according to the method of Ahmad et al. [Citation32]. Herein, the pure extract from each treatment (2.0 ml) was slowly combined with DPPH solution in a test tube. The test tube containing mixture was placed in a dark condition to minimize oxidation reactions. The DPPH solution was obtained by the addition of 0.25 mg of powdered DPPH in 20–80 ml of HPLC grade ethanol. After incubation in dark, the solution was applied for antioxidant activity. The readings of the samples were taken at 517 nm using double-beam UV-visible system (Shimadzu, Japan). The DPPH-based antioxidant activity was investigated using the given formul:
Here, ASC indicates suspended cells absorbance and ADRS represents DPPH radicals solution absorbance
Analysis of data
The data for each treatment was obtained from twice-revised independent experiments. Here, one-way analysis of variance (ANOVA) was used for obtaining the mean values from each treatment. To obtain least significant difference and SE (±), Statistix 8.1 (USA) was applied. Further, Origin Lab (8.5; USA) software was applied for graphical presentation of the mean data.
Results
Production of biomass during growth kinetics in cell suspension cultures
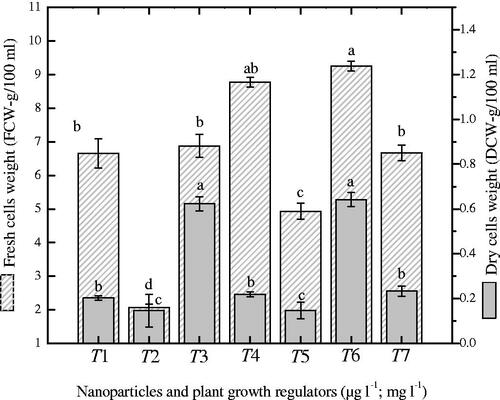
In the current study, combination of singlet NP (Ag or Au) with NAA or a synergistic combination of NPs (AgAu) and NAA were investigated for biomass accumulation. During cell suspension culture development, maximum accumulation of fresh biomass (9.25 g) was observed when liquid media were augmented with a combination of AgAuNPs (3:1) and NAA after 30-days of growth kinetics (. The addition of AgAuNPs (1:3) with NAA produced 8.77 g fresh and 0.64 g/100 ml dry biomass as compared to control (6.67; 0.233 g). However, the combination of AgAuNPs (3:1) and NAA did not show a positive correlation with dry biomass accumulation.
Figure 1. Investigation of fresh and dry biomass (gram/100 ml) in suspended cells of Prunella vulgaris exposed to different ratios of nanoparticles and NAA. The data of mean values and SE were collected from revised independent experiments for each treatment. Mean data with common alphabets are significantly different at p < .05.
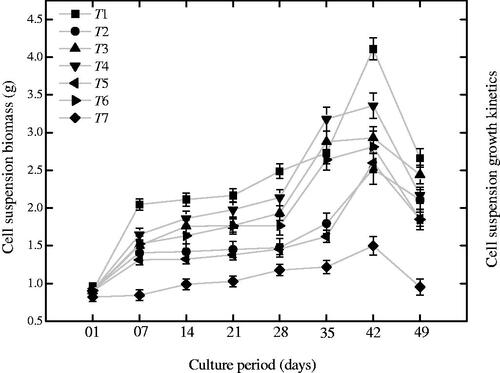
The growth kinetics of cell suspension culture was investigated for 49 days period with 7 days interval ( and ). The lag phases took maximum days (1–28) and the growth was found relatively slower. The log phases take 14 days, while the decline phases were found shorter (7 days). The color of cell culture showed variation at each growth phase and become dark brown at the closing stages (. During growth kinetics, higher fresh biomass (20.14 g) was observed at 42nd day on MS medium incorporated with AuNPs in combination with NAA as compared to control (8.42 g). The application of different ratios of AgAuNPs (1:2; 1:3) with NAA also improved fresh biomass accumulation (16.5 and 18 g). Moreover, other treatments also accumulated optimum quantities of biomass as compared to control (.
Figure 2. Establishment of growth curve (49 days period) for suspended cells for exposed to different ratios of metallic nanoparticles along with NAA in cell cultures of P. vulgaris. Mean data was taken on weekly basis. The data of mean values and SE were collected from revised independent experiments for each treatment. Mean data with common alphabets are significantly different at p < .05.
Effect of NPs on total protein content and stress enzymes in suspended cells
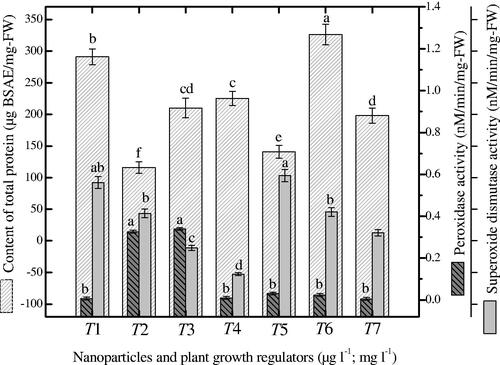
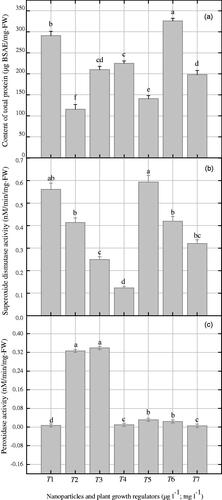
Herewith, peroxidase enzymes, superoxide dismutase enzymes and content of total protein were determined in cell suspension cultures of Prunella exposed to combinations of NAA and NPs (. In cell suspension cultures, the combination of AgAuNPs (3:1) with NAA (2.0 mgl−1) induced maximum CTP (326 µg BSAE/mg-FW) as compared to control (). Higher production of SOD enzyme (0.59 nM/min/mg-FW) was observed when AgAuNPs (2:1) with 2.0 mg l−1 NAA was applied (). Significantly similar enzyme production (0.56 nM/min/mg-FW) was observed when AuNPs (30 µg l−1) with NAA (2.0 mg l−1) was applied as compared to control (0.32 nM/min/mg-FW) and other treatments. An increment in POD enzyme production (0.33 and 0.334 nM/min/mg-FW) was observed on medium containing combinations of AgNPs and AgAuNPs (1:2) along with NAA, which is comparatively higher than control (0.005 nM/min/mg-FW) (). Herewith, the stress enzymes showed autonomous behavior and hence suggest that they are not restricted to the application of single type of NP and plant growth regulators, but varied upon each exposure. In contrast, SOD enzyme did not showed linear correlation with POD production. Similarly, the CTP did not show positive correlation with stress enzymes production (.
Figure 4. Estimation of stress enzymes (superoxide dismutase and peroxidases) and protein content in suspended cells of P. vulgaris exposed to metallic nanoparticles along with naphthalene acetic acid. Mean values were collected from three replicates and also containing ± standard error. Bars with common alphabets are significantly different at p < .05.
Effect of NPs on polyphenolics content and antioxidant activity in suspended cells
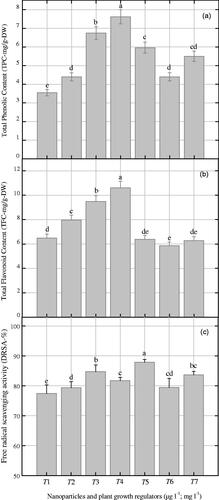
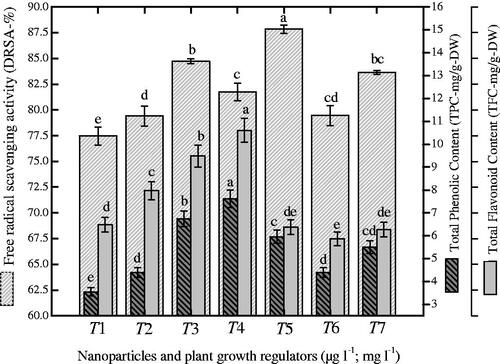
Herewith, TPC, TFC and DPPH-radical scavenging activity (DRSA) were investigated in cell suspension cultures in response to various combinations of NPs + NAA (. Maximum accumulation of TPC (7.62 GAE-mg/g-DW) was observed with the combination of AgAuNPs (1:3; 30-µg l − 1) and 2.0 mg l−1 NAA supplemented to MS medium (). The same combination of NPs and NAA enhanced TFC production (0.61 RE-mg/g-DW) in cell suspension culture of P. vulgaris (). In contrast, maximum DRSA (87.8%) was observed on medium containing AgAuNPs (2:1) as compared to control (83.64%), as shown in . Here, we observed a clear correlation between TPC and TFC production when exposed to the same NPs combination as shown in . In contrast, we did not observe correlation between TPC and free radicals scavenging activity ().
Discussion
Application of nanoparticles significantly influenced the establishment of cell cultures in P. vulgaris. The literature is still limited that know the effect of nanoparticles on the progression and development of different plant tissues and cells. The effect of nanoparticles not only restricted to growth regulation but also prominently affected the pathways of secondary metabolism. Here, the combination of nanotechnology and plant tissue culture produce promising results in suspended cells of P. vulgaris that further need insight view of molecular mechanism. In some plant species, the application of nanoparticles produced positive results but their accumulation in plant tissues and its subsequent release to the environment are still contradictory [Citation2]. The effect of nanoparticles varies with plant species, with age and type of tissues selected for applications [Citation33]. Previously, in plant species like Hordeum vulgare and Linum usitatissimum, AgNPs negatively affected the process of seed germination [Citation5,Citation34]. Contrarily, the growth of Zea mays and Phaseolus vulgaris were enhanced using the same NPs [Citation35,Citation36]. Herewith, the synergistic application of Ag and Au NPs along with NAA showed positive effects on cell culture development. Moreover, the same combinations during phases of growth kinetics (lag, log, stationary and decline) enhanced fresh and dry biomass biosynthesis. On 30–42 days of cell cultures, biomass accumulation as compared to control was found maximum on media with variable ratios of nanoparticles and NAA.
Inside plant morphogenesis, the unconditional release of reactive oxygen species (ROS) reduced cell dedifferentiation and redifferentiation, which directly or indirectly produced toxic metabolites [Citation36]. Plants produce tissue-specific stress enzymes to avoid such oxidative stress conditions [Citation37]. These enzymes form an intervertebral protection system, which protect plant tissues from mono-oxygen related species [Citation36]. In the current experiment, the exposure of suspended cells to different metallic NPs improved the production of these stress enzymes in comparison to control, but here we did not observe linear correlation between SOD and POD enzymes. Similar reports regarding the stress enzymes (SOD and POD) are widely available in various species including Prunus and Solanum [Citation38,Citation39]. In Brassica juncea the application of silver NPs enhanced the synthesis of stress enzymes [Citation5]. Furthermore, the exposure of Glycine max to different NPs improved germination rate and stimulate the production of stress enzymes [Citation4]. It is also reported that Acanthophyllum sordidum callus cultures produce small amount of stress enzyme (SOD) during dedifferentiation but gradually increases in redifferentiation [Citation40]. Moreover, the role of stress enzymes (SOD and POD) and their production are widely reported in many elite plant species [Citation41,Citation42]. During the stress situation, singlet free radicals are released in plant cells, which have the ability to react with macromolecules and finally reduce the developmental processes of various plant tissues [Citation43]. In response to highly singlet reactive radicals, selected plant tissues stimulate the production of polyphenolics, which scavenge free radicals for further reaction [Citation44].
In this study, the addition of nanoparticles to broth cultures induced the accumulation of biomass as well as the biosynthesis of secondary metabolites in suspended cells of P. vulgaris. This study is not restricted to Prunella but helpful for the synthesis of novel or other important secondary cell products in high-valued medicinal species. Information of nanoparticle-induced biosynthesis of secondary metabolites in liquid cultures of medicinal plants are limited in the already published work; however, the biosynthesis of secondary metabolites in various elite medicinal species is widely reported. In this context, the addition of exogenous biotic or abiotic elicitors and growth regulators not only effect the architecture development of plant but also regulate the biosynthesis and release of high valued secondary cell products [Citation45]. In continuation, the calli cultures of Cicer arietinum accumulate higher quantities of secondary cell products than other plant in vitro tissues [Citation46]. A similar variation in the synthesis and release of secondary cell products were also documented in two species of Zingiber officinale [Citation13]. Therefore, the current research showed a clear picture of nanobiotechnology that enhanced the biosynthesis secondary cell products by regulating the growth behavior of various tissues. Different ratios of NPs and NAA significantly alter the DRSA in cell suspension culture. Various biotechnological techniques have been used for the biosynthesis of valuable bioactive compounds [Citation47]. Here, the applications of NPs along with NAA have displayed maximum DRSA in suspended cells than control. Previous literature also proved that NPs-induced antioxidant system enhanced malon-di-aldehyde content in medicinal plants [Citation48].
It is believed that the movement of nanoparticles in solid medium is comparatively slower to interact with the cells to enhance the secondary metabolism. However, the previous study of Riaz et al. [Citation49] confirmed that the addition of melatonin to the solid culture media modulate the synthesis of ZnONPs in callus cultures of Catharanthus roseus. In this study, we directly exposed the suspended cells of P. vulgaris to the silver and gold nanoparticle to investigate its possible mechanism on secondary metabolites biosynthesis in liquid medium rather than solid one. At this stage, it is very difficult to understand the overall mechanism that how nanoparticle modulates the secondary metabolism at the molecular level. However, some previous studies endorse that nanoparticles interact with plant cell and provoke the defense system of plant by releasing higher quantities of secondary metabolites [Citation50]. In the direct interaction of nanoparticles with suspended cells in liquid media, it raised the level of cytoplasmic calcium, ROS and the expression of mitogen-activated protein kinas cascades (). According to Sosan et al. [Citation51], the nanoparticles bind with the cell membrane of Arabidopsis thaliana and triggered ROS activation and calcium burst. Mirzajani et al. [Citation52] proved the expression of calcium and other factors associated with biosynthetic pathways of secondary metabolism in response to nanoparticles in O. sativa roots. Therefore, these calcium spike, elevated ROS and MAPK are involve in the reprogramming of secondary metabolism that activate the defense system of plant cell to release higher amount of secondary metabolites to cope with the oxidative stress [Citation50,Citation53–55].
Figure 8. The effect of nanoparticles on the synthesis of secondary metabolites in cell cultures (Adopted from Marslin et al. [50]). After the supplementation of silver and gold nanoparticles to the culture media, the NPs directly interact with the suspended cells producing pores in the cell membrane and provoke the antioxidant system by releasing reactive oxygen species. The NPs induced ROS mediate mitogen-activated protein kinase cascade and calcium spikes, which possibly modulate the transcription of secondary metabolism. In response to ROS, the plant cell activates the defensive machinery to prevent ROS damage. In this way, the quantity of secondary metabolites increases as compared control cultures having no nanoparticles. The nanoparticle-secondary metabolites complexes exit the plant cell or sometimes persist inside the cell in the form of intracellular metabolites. However, the effect of nanoparticles on alteration of metabolites, plant growth and yield and its interaction with environment is still unknown.
![Figure 8. The effect of nanoparticles on the synthesis of secondary metabolites in cell cultures (Adopted from Marslin et al. [50]). After the supplementation of silver and gold nanoparticles to the culture media, the NPs directly interact with the suspended cells producing pores in the cell membrane and provoke the antioxidant system by releasing reactive oxygen species. The NPs induced ROS mediate mitogen-activated protein kinase cascade and calcium spikes, which possibly modulate the transcription of secondary metabolism. In response to ROS, the plant cell activates the defensive machinery to prevent ROS damage. In this way, the quantity of secondary metabolites increases as compared control cultures having no nanoparticles. The nanoparticle-secondary metabolites complexes exit the plant cell or sometimes persist inside the cell in the form of intracellular metabolites. However, the effect of nanoparticles on alteration of metabolites, plant growth and yield and its interaction with environment is still unknown.](/cms/asset/e7420e87-5b93-4186-bb77-fde41ed4a6fc/ianb_a_1625913_f0008_c.jpg)
Conclusion
In conclusion, NPs are not only modulating biomass accumulation but also enhance the production of polyphenolics content in suspended cells of P. vulgaris. This is one of the best applications of Nanobiotechnology for the synthesis of high-valued secondary metabolites in liquid cultures and can be easily scaled up to bioreactor for enhanced productivity for pharmaceutical and nutraceuticals industries. However, the molecular mechanism of nanoparticles in various plants tissues, their translocation to other parts and its effect on modulating the chemistry of other secondary metabolites are yet to be explore and need further research.
Acknowledgements
We acknowledge the support of Latif-ur-Rahman team for providing different nanoparticles for the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;50:243–250.
- Ma X, Geisler-Lee J, Geiser-Lee J. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408:3053–3061.
- Larue C, Laurette J, Herlin-Boime N. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ. 2012;431:197–208.
- Lu CM, Zhang CY, Wen JQ, et al. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002;21:168–172.
- Sharma P, Bhatt D, Zaidi MG, et al. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol. 2012;167:2225–2233.
- Singh D, Kumar S, Singh SC, et al. Applications of liquid assisted pulsed laser ablation synthesized TiO2 nanoparticles on germination, growth and biochemical parameters of Brassica oleracea var Capitata. Sci Adv Mater. 2012;4:522–531.
- Fazal H, Abbasi BH, Ahmad N, et al. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl Biochem Biotechnol. 2016;180:1076–1092.
- Fazal H, Shinwari ZK, Ahmad N, et al. Factors influencing in vitro seed germination, morphogenetic potential and correlation of secondary metabolism with tissue development in Prunella vulgaris L. Pak J Bot. 2016;48:193–200.
- Fazal H, Abbasi BH, Ahmad N, et al. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J Photochem Photobiol B. 2016;159:1–7.
- Fazal H, Abbasi BH, Ahmad N, et al. Exogenous melatonin trigger biomass accumulation and production of stress enzymes during callogenesis in medicinally important Prunella vulgaris L. (Selfheal). Physiol Molecular Biol Plant 2018;24:1307–1315.
- Liu GM, Jia XB, Wang HB, et al. Review about current status of cancer prevention for the chemical composition or composition and function mechanism of Prunella vulgaris. J Chinese Med Mater. 2009;3:1920–1926.
- Chen CY, Wu G, Zhang MZ. The effects and mechanism of action of Prunella vulgaris L., extract on Jurkat human T lymphoma cell proliferation. Chin Ger J Clin Oncol. 2009;8:426–429.
- Ghasemzadeh A, Jaafar HZE, Rahmat A, et al. Effect of different light intensities on total phenolics and flavonoids synthesis and antioxidant activities in young ginger varieties (Zingiber officinale Roscoe). IJMS. 2010;11:3885–3897.
- Ahmad N, Abbasi BH, Fazal H, et al. Effects of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum. CR Biol. 2014;337:19–28.
- Ali M, Abbasi BH, Haq IU. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind Crop Prod. 2013;49:400–406.
- Morkunas I, Marczak L, Stachowiak J, et al. Sucrose-induced lupine defense against Fusarium oxysporum sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem. 2005;43:363–373.
- Shoji H, Yamashiro Y, Koletzko B. Oxidative stress and antioxidants in the perinatal period. In: Sies H, editor. Oxidative stress and inflammatory mechanisms in obesity, diabetes, and the metabolic syndrome. Boca Raton, FL: Taylor & Francis Group; 2008. p. 72.
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2006;7:45–78.
- Manohari J, Indra M, Muthuchelian K. Enzymatic activities of mother plant and tissue cultured plants of Cassia siamea (Fabaceae). J Biosci Res. 2011;2:183–188.
- Joo SS, Kim Y, Lee DI. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant Pathol J. 2010;26:57–62.
- Sivanandhan G, Arun M, Mayavan S, et al. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crop Prod. 2012;37:124–129.
- Eilert U. Elicitation: methodology and aspects of application. In: Constabel F, Vasil I, editors. Cell culture and somatic cell genetics of plants. San Diego: Academic Press. 1987. Vol. 4, p.153–196.
- Barz W, Daniel S, Hinderer W, et al. Elicitation and metabolism of phytoalexins in plant cell cultures. In: Pais M, Mavituna F, Novais J, editors. Plant cell biotechnology. NATO ASI Series. Berlin: Springer-Verlag, 1988, p. 211–30.
- Fazal H, Abbasi BH, Ahmad N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl Biochem Biotechnol. 2014;174:2086–2095.
- Fazal H, Abbasi BH, Ahmad N, et al. Sucrose induced osmotic stress and photoperiod regimes enhanced the biomass and production of antioxidant secondary metabolites in shake-flask suspension cultures of Prunella vulgaris L. Plant Cell, Tissue Organ Cult. 2016;124:573–581.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497.
- Rahman LU, Shah A, Khan SB, et al. Synthesis, characterization, and application of Au–Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene. J Appl Electrochem. 2015;45:463–472.
- Nayyar H, Gupta D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ Exp Bot. 2006;58:106–113.
- Lagrimini LM. Wound-induced deposition of polyphenols in transgenic plants over expressing peroxidase. Plant Physiol. 1991;96:577–583.
- Giri L, Dhyani P, Rawat S, et al. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii, a rare Himalayan medicinal orchid. Ind Crop Prod. 2012;39:1–6.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein Measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Ahmad N, Fazal H, Abbasi BH, et al. Efficient regeneration and antioxidant potential in regenerated-tissues of Piper nigrum L. Plant Cell Tiss Organ Cult. 2010;102:129–134.
- Vannini C, Domingo G, Onelli E, et al. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PlosOne 2013;8:e68752.
- El-Temsah YS, Joner EJ. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 2012;27:42–49.
- Abbasi BH, Khan M, Guo B, et al. Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tiss Organ Cult. 2011;105:337–344.
- Abbasi BH, Saxena PK, Murch SJ, et al. Echinacea biotechnology: challenges and opportunities. In Vitro Cell Dev Biol Plant. 2007;43:481–492.
- Kumar GNM, Knowles NR. Changes in lipid peroxidation and lipolytic and free radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol 1993;102:105–124.
- Franck T, Kevers C, Gaspar T. Protective enzymatic systems against activated oxygen species compared in normal and hyperhydric shoots of Prunus avium L. raised in vitro. Plant Growth Regul. 1995;16:253–256.
- Meratan AA, Ghaffari SM, Niknam V. In vitro organogenesis and antioxidant enzymes activity in Acanthophyllum sordidum. Biol Plant. 2009;53:5–10.
- Thakar J, Bhargava S. Seasonal variation in antioxidant enzymes and the sprouting response of Gmelia arborea nodal sectors cultured in vitro. Plant Cell Tissue Org Cult. 1999;59:81–187.
- Zavaleta-Mancera HA, López-Delgado H, Loza-Tavera H, et al. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J Plant Physiol. 2007;164:1572–1582.
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 2009;64:303–311.
- Hong Y, Lin S, Jiang Y, et al. Variation in contents of total phenolics and flavonoids and antioxidant activities in the leaves of 11 Eriobotrya Species. Plant Foods Hum Nutr. 2008;63:200–204.
- Kosar M, Goger F, Baser KHC. In vitro antioxidant properties and phenolic composition of Salvia halophila Hedge from Turkey. Food Chem. 2011;129:374–379.
- Liu CZ, Guo C, Wang YC, et al. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua. Process Biochem. 2002;38:581–585.
- Naz S, Ali A, Iqbal J. Phenolic content in vitro cultures of chick pea (Cicer arietinum L. ) during callogenesis. Pak J Bot. 2008;40:2525–2539.
- Abouzid SF, El-Bassuon AA, Nasib A, et al. Withaferin, a production by root cultures of Withania coagulans. Int J Appl Res Nat Prod. 2008;3:23–27.
- Lei Z, Mingyu S, Xiao W, et al. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res. 2008;121:69–79.
- Riaz HR, Hashmi SS, Khan T, et al. Melatonin-stimulated biosynthesis of anti-microbial ZnONPs by enhancing bio-reductive prospective in callus cultures of Catharanthus roseus var. Alba. Artificial Cells Nanomed Biotechnol. 2018; 46:936–950.
- Marslin G, Sheeba CJ, Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front Plant Sci. 2017; 8:832.
- Sosan A, Svistunenko D, Straltsova D, et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 2016;85:245–257.
- Mirzajani F, Askari H, Hamzelou S, Schober Y, Rompp A, Ghassempour A, Spengler B. Proteomics styudy of silver nano-particles toxicity on Oryza sativa L. Ecotoxicol Environ Saf. 2014;108:335–339.
- Phukan UJ, Jeena GS, Shukla RK. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 2016;7:760.
- Schluttenhofer C, Yuan L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015;167:295–306.
- Vasconsuelo A, Boland R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007;172:861–875.

![Figure 3. Different phases of growth kinetics during cell suspension culture in P. vulgaris[Mismatch].](/cms/asset/d2e67970-95c0-4a6b-82ed-98adec71d099/ianb_a_1625913_f0003_c.jpg)