Abstract
Background
Glioma is a main cause of brain-cancer relevant death. The present paper designed to reveal the possible role of LSINCT5 in human glioma GL15 cells.
Methods
LSINCT5 and miR-451 expression in glioma tissues was examined using qRT-PCR. The impacts of LSINCT5, miR-451 and Rac1 in GL15 cells were checked by carrying out CCK-8 assay, transwell assay, and flow cytometric analysis. Further, the target gene of LSINCT5 and miR-451 was explored. Accumulation of PI3K/AKT, Wnt/β-catenin and NF-κB pathway proteins was examined using Western blot.
Results
LSINCT5 was highly expressed while miR-451 low expressed in glioma tissues when compared to normal controls. Down-regulating LSINCT5 effectively declined GL15 cells viability, migration and invasion, but accelerated apoptosis. Nonetheless, the above-mentioned effects of LSINCT5 down-regulation were weakened when miR-451 was silenced. Rac1 was a target of miR-451. The tumour-suppressive effects of miR-451 on GL15 cells were weakened when Rac1 was overexpressed. Further, LSINCT5-miR-451-Rac1 axis could impact the activation of PI3K/AKT, Wnt/β-catenin and NF-κB pathways.
Conclusion
Down-regulation of LSINCT5 represses glioma cells growth and metastasis in vitro likely through targeting miR-451 and thereby inhibiting Rac1-regulated PI3K/AKT, Wnt/β-catenin and NF-κB pathways.
Introduction
Glioma, the prevalent and aggressive brain tumour, is a major reason for brain-cancer relevant death [Citation1]. Although a noticeable advancement has been acquired in the management of glioma during the last several decades, the long-term survival of glioma patients remains unsatisfactory, only with an average survival time of 14 months post-diagnosis [Citation2,Citation3]. Knowing more about the mechanisms responsible for glioma growth and metastasis are still required.
Long noncoding RNAs (lncRNAs) are a newly discovered sort of noncoding RNA transcripts with 200 nucleotides to 100 kilobases. LncRNAs are one of the most recent interests of scientists to study underlying pathophysiology of human cancers [Citation4]. Numerous studies provided a correlation between lncRNAs expression and the pathogenesis of cancers [Citation5,Citation6]. Long stress-induced non-coding transcript 5 (LSINCT5), a member of stress-induced lncRNAs family with 12 members (LSINCT1-12), seems to play a role in cancer development [Citation7,Citation8]. LSINCT5 was observed to play significant role in cancer progression and metastasis, and also participate in regulating cellular processes like cell proliferation and apoptosis [Citation8–10]. It was reported that patients with colorectal or gastric cancer who possess higher levels of LSINCT5 behave poor survival rates [Citation8]. In vitro data illustrated that LSINCT5 silence effectively repressed gastrointestinal cancer cells proliferation [Citation8]. Likewise, ectopic expression of LSINCT5 enhanced breast and ovarian cancer cells proliferation [Citation9]. These studies suggested LSINCT5 as a crucial lncRNA implicated in the onset and development of human cancers.
Recently, scientists hypothesize that lncRNAs function to tumour cells by working as molecular sponges for microRNAs (miRNAs), which further quarantining the targeted mRNAs from degradation by miRNAs [Citation11]. These lncRNAs are termed “competing endogenous RNAs (ceRNAs)”, which compose a considering fraction of gene regulation. The ceRNAs hypothesis protestes the impression that an RNA must be translated into a protein to exert its function [Citation12]. miRNAs are essential regulators in several biological procedures like cell differentiation, proliferation, invasion and apoptosis [Citation13,Citation14]. Recently, the aberrant expression of a number of miRNAs in malignant glioma has been confirmed, such as miR-21, miR-451, miR-181d, miR-23b, miR-10b, miR126, and so forth [Citation15–17]. Among which, miR-451 is a frequently investigated miRNA in glioma. According to several research groups, miR-451 expression is repressed in glioma tissues and cells [Citation18,Citation19]. Besides, miR-451 acted as a tentative switch in mediating glioma cells growth and migration [Citation20–22]. Thus, targeting miR-451 has been regarded as a potential adjuvant therapy for glioma besides chemotherapy [Citation23]. In this study, whether miR-451 is related to LSINCT5 in glioma cells is studied.
Herein, the specific role of LSINCT5 in human glioma GL15 cells was explored. Interactions among LSINCT5, miR-451 and Rac1 (a pleiotropic regulator of many cellular processes) and their respective regulatory roles were emphatically investigated. In this study, LSINCT5 may represent a target for gene therapy in glioma.
Methods
Cell culture
GL15 cells (ATCC; Manassas, VA) were cultivated in DMEM (Gibco, Carlsbad, CA) complementing with 10% fetal bovine serum (FBS; Gibco). Cells were cultured at 37 °C in a moistened incubator with 5% CO2.
Cell transfection
shRNAs specifically targeting LSINCT5 (sh-LSINCT5) and Rac1 (sh-Rac1), as well as their scrambled respective control (sh-NC), were provided by GenePharma (Shanghai, China). Full-length Rac1 were intercalated into pEX-2 plasmids (GenePharma) for constructing a Rac-1 expression vector (pEX-Rac1). miR-451 mimic, inhibitor and the respective controls (mimic and inhibitor NC) were from Invitrogen (Carlsbad, CA). Transfections were conducted for 48 h by using Lipofectamine 3000 (Invitrogen).
Clinical specimens
Glioma tissues were collected from 56 glioma patients who went through tumour resection in Jining No.1 People’s Hospital. None of these individuals received any treatment pre-resection. Normal brain tissues from 16 patients with cerebral trauma or epilepsy surgery were used as control. Written informed consent was signed before the usage of clinical specimens and this work was authorized by the ethics committee of Jining No.1 People’s Hospital. The tissues were immediately washed twice by cooling PBS and stored at −80 °C before usage.
qRT-PCR
Cellular and tissue RNAs were extricated by TRIzol (Invitrogen). LSINCT5 expression was detected using One Step SYBR® PrimeScript®PLUS RT-RNA PCR Kit (TaKaRa, Dalian, China). GAPDH served as endogenous control for LSINCT5 expression. For the relative quantification of miR-451 expression, Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and Taqman Universal Master Mix II (Applied Biosystems) were utilized for synthesizing cDNA and conducting PCR. U6 served as endogenous control. For Rac1 detection, RT-PCR was carried using the RNA PCR Kit (AMV) Ver.3.0 (TaKaRa) and GAPDH served as an endogenous control. Relative gene expression was figured out by 2−ΔΔCt method.
Cell viability
The transfected cells in 96-well plate with 5000 cells/well were added with 20 μL CCK-8 solution (Dojindo Molecular Technologies, Gaithersburg, MD). Then, the plates were incubated at 37 °C for 1 h and the optical density (OD) was examined at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA).
Cell apoptosis
The transfected cells were gathered into a centrifuge tube and then stained with PI/FITC-Annexin V (Beijing Biosea Biotechnology, Beijing, China) for 1 h at room temperature away from light. Cell apoptosis detection was carried out using a flow cytometry (Beckman Coulter, Fullerton, CA).
Transwell assay
Cell migration was examined using the transwell system chambers 8 μM pore. Cell invasion ability was examined using the Chamber matrigel invasion 24-well kit (BD Biosciences, San Jose, CA). Briefly, 5 × 104 cells were suspended in serum-free DMEM and placed into the upper side while complete medium was placed to the lower side. 24 h later, the cells in lower chambers were stained with 0.1 mg/mL crystal violet.
Dual-luciferase reporter assay
The luciferase assay was carried out using the Dual-luciferase reporter assay system (Promega, Madison, WI). LSINCT5 sequence containing the forecasted miR-451 binding site was inserted into a pmirGlO Vector (Promega) to constitute LSINCT5-wild-type (LSINCT5-wt) reporter plasmid. The primary sequences of LSINCT5 used in these processes were listed as follows. LSINCT5 forward: 5′-CTCGAGTGTAGTCCCAGCTACTCAG’, containing Xho I restriction site; reverse: 5′-GACGCGTTGGAGACCATCCTGGCTAACA-3′, containing MIu I restriction site. The sequence of presumed binding site was reconstituted as indicated and was referred as LSINCT5-mutated-type (LSINCT5-mt). Likewise, Rac1-wild-type (Rac1-wt) and Rac1-mutated-type (Rac1-mt) were formed. The primary sequences of Rac1 were as follows: Rac1 forward: 5′-GGGTACCTCTTGAGCCGTCCTGCA-3′, containing Kpn I restriction site; reverse: 5′-GGAATTCTCGCTTGCACTTGTAGCGA-3′, containing EchoR I restriction site. The vectors and miR-451 mimics were transfected into cells using Lipofectamine 2000 (Invitrogen). 48 h later, relative luciferase activity was examined.
Western blot
Cell proteins were lysed using RIPA lysis buffer (Thermo Scientific, MA). The primary antibodies against Rac1 (ab97732), Wnt3a (ab28472), Wnt5a (ab72583), β-catenin (ab6302), p-p65 (ab86299), p65 (ab16502), IκBα (ab32518), p-IκBα (ab32518), PI3K (ab86714), p-PI3K (ab182651), AKT (ab18785), p-AKT (ab38449) and GAPDH (ab9485, Abcam, Cambridge, MA) were used for protein immunoblotting. Signals were visualized using ECL Substrates (Pierce, Rockford, IL).
Statistics
The data were expressed as the mean ± SD. Statistical analysis was carried out in Graphpad 6.0 statistical software (GraphPad Software Inc, San Diego, CA). p Values were worked out using ANOVA or independent-Samples T-test. p Values less than .05 were considered statistically significant.
Results
LSINCT5 knockdown repressed cell viability, migration, and invasion but accelerated apoptosis of GL15 cells
First, LSINCT5 expression in glioma tissues was examined using qRT-PCR assay. As seen in , LSINCT5 expression in 56 glioma tissues was dramatically higher than that in 16 normal controls (p < .001). Next, we explored the growth and metastasis of LSINCT5 in glioma GL15 cells. To this end, shRNA specific for LSINCT5 was utilized to repress LSINCT5 expression. qRT-PCR data displayed that sh-LSINCT5 knocked down LSINCT5 expression in GL15 cells as relative to shNC group. Lower expression of LSINCT5 was made by sh-LSINCT5 #1 (p < .01) rather than sh-LSINCT5 #2 (p < .05, ). Therefore, sh-LSINCT5 #1 was selected for use in subsequent assays. Further investigation illustrated that LSINCT5 knockdown reduced GL15 cells viability (p < .05, ). In addition, LSINCT5 knockdown dramatically promoted the apoptosis of GL15 cells (p < .001, ).
Figure 1. Knockdown of LSINCT5 repressed the growth and metastasis of GL15 cells. (A) LSINCT5 expression in 56 glioma tissues and 16 normal controls was detected using qRT-PCR. (B) LSINCT5 expression was analyzed using qRT-PCR after transfection with sh-LSINCT5 #1 and #2. After transfection, (C) cell viability was examined using CCK-8 assay, (D,E) apoptosis was examined using Annexin V-FITC/PI double staining method, and (F) cell migration and invasion were examined using transwell assay. (G) Relative migration and (H) invasion based on the results obtained from transwell assay. *p < .05, **p < .01, ***p < .001.
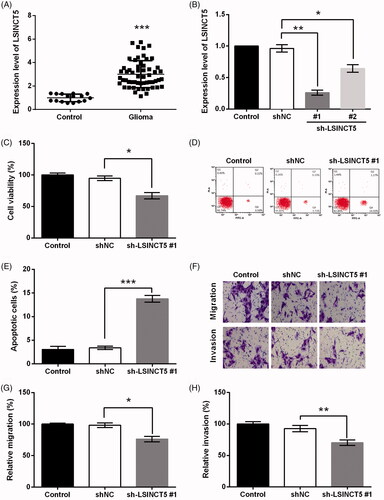
To further explore the impact of LSINCT5 knockdown on GL15 cells metastasis, transwell assay was carried out. The migrating and invasive capacities of GL15 cells transfected with sh-LSINCT5 were significantly decreased as relative to shNC group (p < .05 and p < .01, ).
LSINCT5 acted as a molecular sponge for miR-451
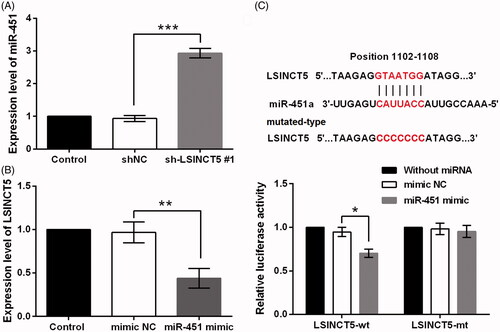
LncRNAs have been found to function as ceRNAs to specific binding with miRNAs [Citation2]. Since miR-451 has been frequently descripted as a tumour suppressive miRNA in glioma [Citation20–22]; the present work attempted to reveal whether LSINCT5 functioned to GL15 cells via regulating miR-451. qRT-PCR data displayed that LSINCT5 knockdown facilitated miR-451 expression in GL15 cells (p < .001, ). Also, LSINCT5 expression was declined in cells transfected with miR-145 mimic (p < .01, ). We suspected that miR-451 might have relevant binding sites in LSINCT5. Luciferase reporter assay result illustrated that miR-451 overexpression declined luciferase activity of the pMIR luciferase reporter containing LSINCT5-wt (p < .05). But, none such significant results were found in LSINCT5-mt group ().
Figure 2. LSINCT5 acted as a molecular sponge for miR-451. (A) miR-451 expression was examined using qRT-PCR after GL15 cells transfected with sh-LSINCT5 #1 or shNC. (B) LSINCT5 expression was examined using qRT-PCR after GL15 cells transfected with miR-451 mimic or mimic NC. (C) The luciferase activity in GL15 cells co-transfected with mIR-451 mimic and luciferase reporters containing LSINCT5-wt or LSINCT5-mt vector. The results were obtained by dual-luciferase reporter assay. *p < .05, ***p < .001.
LSINCT5 knockdown suppressed GL15 cells growth and metastasis via regulating miR-451
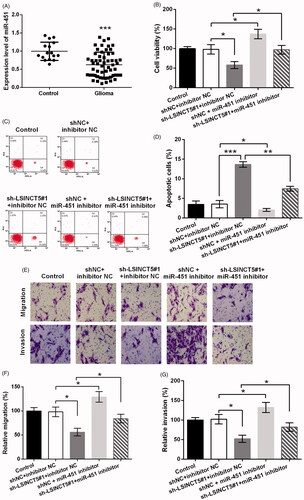
Next, the role of miR-451 in glioma was studied. As seen in , miR-451 expression was remarkably low in glioma tissues as relative to normal controls (p < .001). Then, sh-LSINCT5 and miR-451 inhibitor were co-transfected into GL15 cells, to investigate if miR-451 was implicated in the tumour-suppressive effects of LSINCT5 knockdown. As seen in , miR-451 silence obviously enhanced cell viability even if LSINCT5 was suppressed in cells (p < .05). In addition, miR-451 silence repressed cell apoptosis, as the apoptosis rate was largely decreased (p < .01, ). Furthermore, miR-451 inhibition abolished the suppressive effects of LSINCT5 knockdown on migration and invasion (both p < .05, ).
Figure 3. LSINCT5 suppressed GL15 cells via regulation of miR-451. (A) miR-451 expression in 56 glioma tissues and 16 normal controls was detected using qRT-PCR. (B) The cell viability, (C,D) cell apoptosis, (E) cell migration and invasion were determined when GL15 cells were transfected with sh-LSINCT5, miR-451 inhibitor, or their negative controls (shNC and inhibitor NC). (F) Relative migration and (G) invasion based on the results obtained from transwell assay. *p < .05, **p < .01, ***p < .001.
Rac1 was the target of miR-451
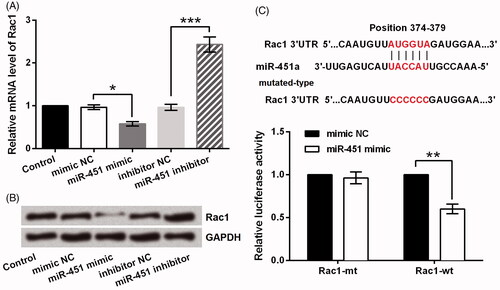
Rac1 is a main member of GTPase family that is regarded as a key regulator in glioma cells growth and metastasis [Citation24,Citation25]. Interestingly, a previous study demonstrated the role of miR-451 in switching between proliferation and migration in glioma cells partially via mediating Rac1 [Citation19]. Thus, this study further verified whether miR-451 functioned to GL15 cells via Rac1. We found that the mRNA level of Rac1 was repressed after miR-451 was overexpressed in GL15 cells (p < .05) while was dramatically increased when miR-451 was silenced (p < .001, ). The protein level of Rac1 was determined and similar results were displayed in . Next, the dual-luciferase reporter assay result illustrated that co-transfection of miR-451 mimic and Rac1-wt strongly declined the luciferase activity (p < .01), whereas miR-451 mimic plus Rac1-mt did not change it significantly ().
Figure 4. Rac1 was a direct target of miR-451. (A) The mRNA and (B) protein levels of Rac1 after transfection with miR-451 mimic, miR-451 inhibitor or the negative controls (mimic NC and inhibitor NC). (C) Luciferase activity in GL15 glioma cells co-transfected with miR-451 mimic and luciferase reporters containing Rac1-wt or Rac1-mt transcript. *p < .05, **p < .01, ***p < .001.
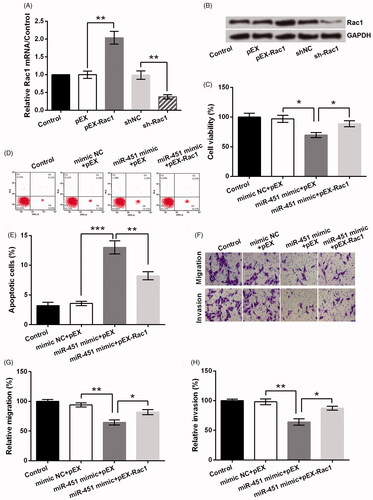
Overexpression of miR-451 suppressed GL15 cells growth and metastasis via regulating Rac1
To confirm the effects of Rac1 on GL15 cells, Rac1 expression was altered by transfection with pEX-Rac1 or sh-Rac1. displayed that the mRNA level of Rac1 was obviously increased by pEX-Rac1 and repressed by sh-Rac1 (both p < .01). Same trends were observed in Rac1 protein expression (). Next, data exhibited miR-451 overexpression significantly inhibited cell viability (p < .05, ), migration and invasion (both p < .01, ), nevertheless largely accelerated apoptosis (p < .001, ), which sufficiently revealed its anti-glioma function. However, co-transfection of miR-451 mimic and pEX-Rac1 inhibited the tumour-suppressive effect of miR-451 on GL15 cells.
Figure 5. miR-451 suppressed GL15 cells via negative regulation of Rac1. (A) The mRNA and (B) protein levels of Rac1 after transfection with pEX-Rac1 and sh-Rac1. (C) The cell viability, (D,E) cell apoptosis, (F) cell migration and invasion were measured after GL15 cells were transfected with miR-451 mimic, pEX-Rac1, or their negative controls (mimic NC and pEX). (G) Relative migration and (H) invasion based on the results obtained from transwell assay. *p < .05, **p < .01, ***p < .001.
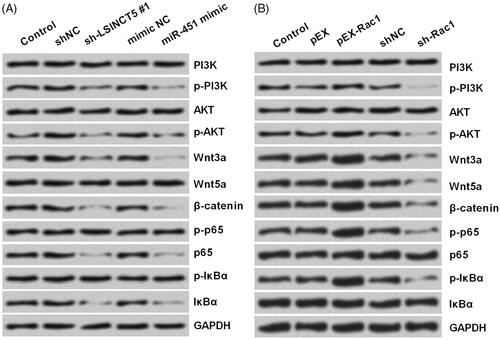
LSINCT5-miR-451-Rac1 axis affected PI3K/AKT, Wnt/β-catenin and NF-κB pathways
Next, we explored the specific function of Rac1 in GL15 cells. As PI3K/AKT, Wnt/β-catenin and NF-κB pathways are the significant signalling pathways involved in glioma [Citation26–28], we examined whether LSINCT5-miR-451-Rac1 axis could regulate the activation of these three pathways. Western blot was utilized to see the protein accumulation of several well-known proteins of these pathways. As seen in , LSINCT5 silence, miR-451 overexpression, as well as Rac1 silence, was capable of reducing the phosphorylation of PI3K, AKT, p65 and IκBα, and repressing the expression of Wnt3a, Wnt5a and β-catenin. However, Rac1 overexpression led to a contrary impact towards these protein accumulations.
Figure 6. Activation of PI3K/AKT, Wnt/β-catenin and NF-κB pathways were affected by LSINCT5-miR-451-Rac1 axis. (A,B) After transfection with sh-LSINCT5 #1, miR-451 mimic, pEX-Rac1, sh-Rac1 or their respective controls, the protein accumulation of main components of PI3K/AKT, Wnt/β-catenin and NF-κB pathways were analyzed using Western blot.
Discussion
LncRNAs are recently-discovered RNA transcripts that are abnormally expressed in various kinds of tumours [Citation5,Citation6]. Numerous researches evidenced that lncRNAs can be used as biomarkers of cancers [Citation4]. LSINCT5 is a stress-regulated lncRNA involved in response to oxidative stress and implicated in cell proliferation and cancer development [Citation29,Citation30]. Here, we designed to see the role of LSINCT5 in glioma cells.
To determine the role of LSINCT5 in GL15 cells, the effect of LSINCT5 knockdown on cell growth and metastasis were firstly analyzed. It was found that LSINCT5 knockdown suppressed cell viability, migration and invasion, and accelerated apoptosis. These data evidenced LSINCT5 as an oncogenic gene in glioma and thereby down-regulating its expression would suppress the development of glioma. LSINCT5 can be dramatically up-regulated in breast and ovarian cancers, and knocking down its expression in cancer-derived cells led to a suppression in cellular proliferation [Citation9]. Likewise, increased LSINCT5 expression was found in gastrointestinal cancer tissues, which was related with the progression of gastric cancer, and knockdown of LSINCT5 significantly inhibited cell metastasis [Citation8]. The findings were in line with our present report, as LSINCT5 expression was also high expressed in glioma tissues. Besides, LSINCT5 exerted as an oncogene and played the tumour-suppressive effect in glioma cells when down-regulating its expression.
In recent years, a novel regulatory mechanism has been widely reported that many lncRNAs can function as ceRNAs to sequester miRNAs, thereby regulating the expression of miRNA targets [Citation11]. In this study, if LSINCT5 can serve as a ceRNA in human glioma was further explored. According to our results, LSINCT5 might regulate miR-451 through directly binding effects, and inhibition of miR-451 abolished the anti-glioma effect of LSINCT5 silence. Therefore LSINCT5 silence might exert the anti-glioma effect by up-regulating miR-451.
The functions of miR-451 in cancers were widely reported. Increasing the level of miR-451 repressed breast cancer cell proliferation and colony formation but promoted cell apoptosis via repressing HER2, EGFR and MAPK signalling [Citation31]. Besides, miR-451 overexpression inhibited liver cancer cells proliferation through inhibiting IKK-β [Citation32]. As for glioma, miR-451 could repress glioma by targeting CAB39 and inhibiting PI3K/AKT pathway [Citation15]. The tumour-suppressive effect of miR-451 was observed, but its specific function in GL15 cells should be further studied.
Rac1, a member of the Rho GTPase family, appears to be deregulated in both expression and activity in diverse tumour cells [Citation33]. Some studies reported that Rac1 was up-regulated in glioma and down-regulating Rac1 could greatly inhibit glioma cell growth [Citation34,Citation35]. In this study, Rac1 was the target of miR-451 which exhibited anticancer effect through negatively regulating Rac1. Thus, Rac1 might be involved in the actions of LSINCT5 and miR-451 in glioma cells. It was reported that Rac1 functioned as a critical mediator of tumour angiogenesis and metastasis through mediating several signal pathways [Citation36]. The PI3K/AKT, Wnt/β-catenin and NF-κB activities regulated by Rac1 were related to many cancer types [Citation37–40]. Since these three signallings were closely related to glioma development [Citation26–28], the effect of LSINCT5-miR-451-Rac1 axis on these pathways in GL15 cells was explored. We found that the pathways were inhibited by LSINCT5 silence, miR-451 overexpression and Rac1 silence. Thereby we speculated that LSINCT5-miR-451-Rac1 axis impacted glioma cells growth and metastasis possibly by indirect mediating PI3K/AKT, Wnt/β-catenin and NF-κB pathways.
In summary, this study illustrated that LSINCT5 knockdown inhibited glioma cells growth and metastasis by up-regulating miR-451. miR-451 overexpression exerted its anti-glioma effects by negative regulating Rac1. LSINCT5 may represent a target for gene therapy in human glioma.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Li Y, Ma X, Wang Y, et al. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed Pharmacother. 2017;93:435–443.
- Ma CC, Xiong Z, Zhu GN, et al. Long non-coding RNA ATB promotes glioma malignancy by negatively regulating miR-200a. J Exp Clin Cancer Res CR. 2016;35:90.
- Chistiakov DA, Chekhonin VP. Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumor Biol. 2014;35:8425–8438.
- Bolha L, Ravnik-Glavac M. Long noncoding RNAs as biomarkers in cancer. Dis Markers. 2017;2017:7243968.
- Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407.
- Perez DS, Hoage TR, Pritchett JR, et al. Long, abundantly expressed non-coding transcripts are altered in cancer. Hum Mol Genet. 2008;17:642–655.
- Smith DI, Jessica SM. Abstract A1: long stress-induced non-coding transcripts (LSINCTs) and cancer. Cancer Res. 2012;72:A1–A1.
- Xu MD, Qi P, Weng WW, et al. Long non-coding RNA LSINCT5 predicts negative prognosis and exhibits oncogenic activity in gastric cancer. Medicine. 2014;93:e303.
- Silva JM, Boczek NJ, Berres MW, et al. LSINCT5 is over expressed in breast and ovarian cancer and affects cellular proliferation. RNA Biol. 2011;8:496–505.
- Zhang X, Sha M, Yao Y, et al. Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1beta signaling pathway. Mol Med Rep. 2015;12:6761–6767.
- Yu F, Lu Z, Cai J, et al. MALAT1 functions as a competing endogenous RNA to mediate Rac1 expression by sequestering miR-101b in liver fibrosis. Cell Cycle (Georgetown, Tex). 2015;14:3885–3896.
- Rubio-Somoza I, Weigel D, Franco-Zorilla JM, et al. ceRNAs: miRNA target mimic mimics. Cell. 2011;147:1431–1432.
- Zhang Y, Dutta A, Abounader R. The role of microRNAs in glioma initiation and progression. Front Biosci. 2012;17:700–712.
- Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159.
- Tian Y, Nan Y, Han LEI, et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol. 2012;40:1105–1112.
- Li R, Li X, Ning S, et al. Identification of a core miRNA-pathway regulatory network in glioma by therapeutically targeting miR-181d, miR-21, miR-23b, β-catenin, CBP, and STAT3. PloS One. 2014;9:e101903.
- Godlewski J, Nowicki MO, Bronisz A, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620–632.
- Nan Y, Guo H, Guo L, et al. MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1alpha/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clini Dev. 2018;29:156–166.
- Zhao K, Wang L, Li T, et al. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol. 2017;50:1989–1999.
- Godlewski J, Bronisz A, Nowicki MO, et al. microRNA-451: a conditional switch controlling glioma cell proliferation and migration. Cell Cycle (Georgetown, Tex). 2010;9:2742–2748.
- Nan Y, Han L, Zhang A, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21.
- Kim Y, Roh S, Lawler S, et al. miR451 and AMPK mutual antagonism in glioma cell migration and proliferation: a mathematical model. PLoS One. 2011;6:e28293.
- Alural B, Ayyildiz ZO, Tufekci KU, et al. Erythropoietin promotes glioblastoma via miR-451 suppression. Vitam Horm. 2017;105:249–271.
- Nakada M, Drake KL, Nakada S, et al. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–8500.
- Cardama GA, Gonzalez N, Ciarlantini M, et al. Proapoptotic and antiinvasive activity of Rac1 small molecule inhibitors on malignant glioma cells. Onco Targets Ther. 2014;7:2021–2033.
- Jiang L, Lin C, Song L, et al. MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-κB/IκBα negative feedback loop. J Clin Invest. 2012;122:33–47.
- Gong A, Huang S. FoxM1 and Wntβ-catenin signaling in glioma stem cells. Cancer Res. 2012;72:5658–5662.
- Li X, Wu C, Chen N, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7:33440–33450.
- Nazari M, Nasiri M, Ghaderi A. Evaluation of long stress-induced non-coding transcripts 5 polymorphism in Iranian patients with bladder cancer. Res Mol Med (Rmm). 2016;4:17–21.
- Dong L, Qi P, Xu M-D, et al. Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015;137:1128–1135.
- Bergamaschi A, Katzenellenbogen BS. Tamoxifen downregulation of miR-451 increases 14-3-3[zeta] and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47.
- Li HP, Zeng XC, Zhang B, et al. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-beta. Carcinogenesis. 2013;34:2443–2451.
- Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019.
- Senger DL, Tudan C, Guiot MC, et al. Suppression of Rac activity induces apoptosis of human glioma cells but not normal human astrocytes. Cancer Res. 2002;62:2131.
- Man J, Shoemake J, Zhou W, et al. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014;9:1812–1826.
- Bid HK, Roberts RD, Manchanda PK, et al. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther. 2013;12:1925–1934.
- Myant Kevin B, Cammareri P, McGhee Ewan J, et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773.
- De P, Carlson JH, Jepperson T, et al. RAC1 GTP-ase signals Wnt-beta-catenin pathway mediated integrin-directed metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget. 2017;8:3072–3103.
- Gastonguay A, Berg T, Hauser AD, et al. The role of Rac1 in the regulation of NF-kB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther. 2012;13:647–656.
- Qu H, Sun H, Wang X. Neogenin-1 promotes cell proliferation, motility, and adhesion by up-regulation of zinc finger E-box binding homeobox 1 via activating the Rac1/PI3K/AKT pathway in gastric cancer cells. Cell Physiol Biochem. 2018;48:1457–1467.
