Abstract
Mortality attributable to atherosclerosis can be reduced significantly with timely diagnosis and treatment. It is meaningful to find a proper way to diagnose and prevent the progression of atherosclerosis. Vascular cell adhesion molecule-1 (VCAM-1) expressed by endothelial cells is a prominent marker of atherosclerotic plaques. There are a number of researches on VCAM-1 based probes for targeted imaging, but rarely on a system with both targeting and drug delivery. Here, we report a novel magnetic mesoporous silicon nanoparticle that is capable of drug delivery and targeting at atherosclerosis plaque. The nanoparticles were constructed using incorporated FITC (fluorescein isothiocyanate) and VHPKQHR peptide into Fe3O4@SiO2 (FITC-VHP-Fe3O4@SiO2). The FITC-VHP-Fe3O4@SiO2 nanoparticles showed great morphological characteristics, superior targeting ability, low toxicity and good biocompatibility in vitro and in vivo. The in vivo experiments showed that FITC-VHP-Fe3O4@SiO2 is a superior contrast agent of magnetic resonance imaging (MRI) for diagnosis of atherosclerosis plaques.
Introduction
Atherosclerosis is one of the most frequently occurred cardiovascular disease with high morbidity for elder people and presently spreads to young people who smoke and have hypertension and high blood lipids and glucose [Citation1,Citation2]. Atherosclerosis does not cause fatal damage to body at early stage but can block blood circulation prevailingly in the vessels of heart and brain at later stage and may thus cause sudden death by destroying transport of oxygen [Citation3]. Thus, timely and precise diagnosis and effective treatment are essential to delay the progression of the disease and to improve the survival rate. In comparison with other diagnostic methods, such as computed tomography (CT), ultrasonic imaging, and positron emission computed tomography (PET), magnetic resonance imaging (MRI) is widely used in clinical for its advantages of no invasion, no radiation and high soft tissue contrast in imaging [Citation4,Citation5]. Although the contrast agents such as iron and gadolinium agent increase the imaging effectiveness [Citation6], MRI is still difficult to distinguish between small abnormal and normal tissues.
In recent years, nanoscale contrast agents for imaging have attracted increasing attention of researchers worldwide for the advantages of biocompatibility, biodegradability, non-toxicity and prolonged circulation, providing a versatile platform for diagnosis and disease treatment [Citation7]. Suitable targeting sites for contrast agents play important roles in diagnostic accuracy and alleviating side effect of disease treatment. Since atherosclerosis is an inflammatory disease, high expression of inflammatory molecules can be used as a characteristic site for targeting. The vascular cell adhesion molecules 1 (VCAM-1, CD106) expressed in endothelial cells are thus great targets to track the development of atherosclerosis [Citation8]. At the early stages of atherosclerosis, injured endothelial cells will increase the expression of VCAM-1 and monocytes will evolve into foam cells after phagocytosis of oxidized low-density lipoprotein (oxLDL) [Citation9]. After apoptosis and necrosis of foam cells, lipid deposited on the arterial wall leading to the formation of atherosclerotic plaque [Citation10]. If the inflammation which involved adhesion molecules including VCAM-1 on endothelial cells persist, atherosclerosis will be advanced to late stage [Citation11]. In general, the high expression of VCAM-1 is closely related to the early and late stages of atherosclerosis. VCAM-1 targeting has previously been showed to enhance the diagnostic ability of MRI, PET and CT in different stages of atherosclerosis [Citation12–14]. While only a few nanoparticles can deliver drugs to the plaque site according to this special target.
Mesoporous silica nanoparticles (MSN) have large specific surface area and pore volume, controllable particle size and good biocompatibility, and have been widely used for delivery of both hydrophilic and hydrophobic drugs such as doxorubicin (DOX) and rapamycin [Citation15]. Zhang and Xu modified MSN with active targeting hyaluronic acid (HA) to deliver DOX to breast cancer site [Citation16]. Wei et al. designed a DOX-carrying MSN with polydopamine and peptide to treat bladder cancer [Citation17]. However, the drug loading MSN has rarely applied to atherosclerosis.
Here, we designed a mesoporous silica nanoparticle that is capable of drug delivery to atherosclerosis plaque. To increase the targeting capability of MSN to atherosclerosis, we modified the peptide VHPKQHR (Val-His-Pro-Lys-Gln-His-Arg), which contains homology to very late antigen-4 (VLA-4), a known ligand of VCAM-1 [Citation18,Citation19]. In addition, iron nanoparticles were added to the mesoporous silicon for magnetic resonance imaging since iron nanoparticles are less toxic and better contrast enhancing agent than gadolinium [Citation20,Citation21]. In this study, we test the nanoparticles of FITC-VHP-Fe3O4@SiO2 to target atherosclerotic plaques in vivo and in vitro. The results indicated that FITC-VHP-Fe3O4@SiO2 can accurately target atherosclerotic plaque site and thus provides a possibility for subsequent drug delivery.
Materials and methods
Ethical approval
All animal procedure was performed in accordance with National Institutes of Health (NIH) Guideline for the Care and Use of Laboratory Animal of South China Normal University, and the experiments were approved by Animal Ethics Committee of South China Normal University.
Synthesis of mesoporous silica nanoparticles
Step 1: synthesis of iron oleate precursors
Four grams FeCl3 was dissolved in 150 ml methanol solution and oleic acid was added. Then, 2.4 g NaOH was dissolved in 200 ml methanol and then added to the FeCl3 solution. The precipitant was obtained by centrifugation, washed with methanol repeatedly and dried in vacuum.
Step 2: Fe3O4 nanoparticles were synthesized by thermal decomposition
The above ferric oleate precipitation was dissolved in 30 g octadecene, heated at 60 °C for 2 h. After adding 2 g oleic acid, the mixture was reacted under nitrogen protection at 300 °C for 30 min. The solutions were cooled down to room temperature, and then 35 ml methanol was added to precipitate them. Finally, the precipitant was washed by the mixture of hexane and acetone (volume ratio, 1:4) three times and dispersed in trichloromethane.
Step 3: synthesis of Fe3O4@SiO2
One millilitre Fe3O4 nanoparticle (34 mg/mL) was poured into 10 ml hexadecyl trimethyl ammonium bromide (CTAB, 0.55 M) aqueous solution and vigorously stirred for 30 min. Trichloromethane was evaporated away under stirring at 60 °C for 30 min. Then, 100 ml of deionized water and 0.62 ml of NaOH solution (2 M) were added. After the mixture was heated to 70 °C, 1 ml of TEOS was added. Two hours later, the mixture was washed by ethanol for three times. The precipitate was obtained by centrifugation and dried in vacuum. The obtained solids reflux in 6 mg/mL ammonium nitrate solution for 12 h and CTAB was removed. Finally, the mixture was washed and dried in vacuum to yield Fe3O4@SiO2.
Step 4: synthesis of FITC-labeled Fe3O4@SiO2
6.6 g EDC (1–(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride), 9.6 mg NHS (N-Hydroxysuccinimide) and 1 mg FITC were dissolved in 8 ml water and added 130 μL 3-aminopropyl)triethoxysilane (APTES). The mixture was stirred at room temperature for 8 h, and then 10 mg of Fe3O4@SiO2 was added and centrifuged 24 h later. The products were collected, washed for three times, and dried in vacuum.
Step 5: synthesis of FITC-VHP-Fe3O4@SiO2
Targeting peptide VHPKQHR(VHP) and unrelated peptides MEHFRWG (Met-Glu-His-Phe-Arg-Trp-Gly) (A synthetic peptide according to the amino acids 4–10 of the human hormone ACTH/Adrenocorticotropic hormone) with a purity >95% were synthesized by Sangon Biotech (Shanghai) Co., Ltd. The procedure was similar to that in step 4. 19.8 g EDC, 28.8 mg NHS and 1 mg FITC and 3 mg VHPKQHR polypeptide were dissolved in 10 ml water followed by adding 130 μL APTES. The mixture was stirred at room temperature for 8 h, and then 10 mg of Fe3O4@SiO2 was added and kept for 24 h before centrifugation. The product was collected, washed for three times, and dried in vacuum. The synthesis of FITC-ACTH-Fe3O4@SiO2 was the same as the above process. All samples were dissolved in stroke-physiological saline solution (SPSS, 0.9% NaCl) before use.
Characterization of nanoparticles
TEM (transmission electron microscopy) was used to observe the shape of Fe3O4@SiO2. Zeta potential was measured by dynamic light scattering. The inclusion of VHPKQHR in Fe3O4@SiO2 nanoparticles was examined by ultraviolet visible spectroscopy (UV-Vis) and Fourier Transform Infrared Spectroscopy (FTIR).
Cell culture and viability assay
Raw264.7 cell line and mouse aortic endothelial cells (MAECs) were obtained from CHI Scientific, Inc. The cells were cultured in DMEM (Gibico) with 10% FBS (Gibico) and 1% Penicillin/streptomycin solution in 5% CO2 atmosphere at 37 °C. CCK-8 was used to assay the viability of MAECs and Raw264.7. MAECs and Raw264.7 were cultured in 96-well plates at 2 × 104 cells per well under various concentrations (0, 10, 25, 50, 100, 200 μg/ml) of FITC-VHP-Fe3O4@SiO2 for 24 h. After that, the absorbance was measured at a wavelength of 450 nm in a microplate reader (INFINITE M200, Tecan, Switzerland).
Western blot
Endothelial cells treated with lipopolysaccharide (LPS) can increase the expression of VCAM-1 [Citation22]. In this study, we used 1 μg/ml LPS to treat MAECs for 24 h. The culture medium was removed and cells were washed with PBS for three times. Then, the cells were quickly separated by cells spatulas and lysed with cell lysate solution for 45 min under ice. The supernatant was collected after centrifugation at 7000 g for 10 min. 10% polyacrylamide gel was used to separate protein.
Confocal microscopy imaging
The adhesion between nanoparticles and endothelial cells was verified by confocal microscopy. 1 × 106 MAECs were cultured in each confocal dish. We incubated cells with 1 μg/ml LPS for 24 h. When the cells density reaches 70–80%, 25 μg/ml FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 were added, respectively. After incubated for 3 h, the cells were washed by PBS for three times. Then, the cells were treated with 4% paraformaldehyde (Biosharp) for 30 min and incubated with 4',6-diamidino-2-phenylindole (DAPI) (Genview) for a nuclear stain for 5 min. Finally, the cells were washed for three times after DAPI solution was removed. The fluorescence intensity of FITC, used to assess the binding of nanoparticles, was observed under Confocal microscopy (Zeiss 880) and analyzed using Image J software.
Flow cytometry analysis
MAECs were placed in 6-well plates. After the cells density reaches 70–80%, the cells were incubated with 25 μg/ml FITC-VHP-Fe3O4@SiO2 in each well for 0, 0.5, 1 and 2 h, respectively. The cells were washed three times with PBS, digested with trypsin, and finally analyzed on FACS of BD-Biosciences.
Hemolytic test
Fresh blood was obtained from 8-week old C57BL/6 mice, added to a tube with heparin sodium. Erythrocyte was harvested by centrifuging at 650 g for 10 min. The erythrocyte was diluted to 0.25% with PBS and incubated with different concentrations of FITC-VHP-Fe3O4@SiO2 (10, 20, 50, 100, 200 μg/ml) for 12 h at 37 °C. Erythrocyte diluted with deionized water was used as negative control, and erythrocyte diluted with PBS was used as positive control. After the treatment, the erythrocyte was centrifuged at 10,000 g for 5 min, and 200 μL supernatant of each sample was placed in a 96-well plate. The absorbance at 542 nm was measured using a microplate reader. The hemolysis rate is obtained by the following formula: Hemolysis rate (%) = (OD valuematerial hemolysis absorbance − OD valuenegative control group absorbance)/(OD valuepositive control group absorbance − OD valuenegative control group absorbance) 100%.
Establishment of atherosclerosis model
8-week-old ApoE−/− mice (Nanjing Biomedical Research Institute, Nanjing, Jiangsu, China) were fed with high-fat diet containing 1% cholesterol, 15% lard, 0.25% sodium cholate, 5% yolk powder, 2.5% sugar and basal diet for 20 weeks. As a control, 8-week-old C57BL/6 mice were fed with basal diet for 20 weeks. Mice were dwelled at 22 ± 2 °C, 55 ± 5% relative humidity, with a light/dark cycle of 12 h.
Magnetic resonance imaging
We compared the retention of nanoparticles (NPs) (FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2) in vivo before and after 24 h injection by magnetic resonance imaging (2.5 mg NPs/kg body weight). The concentration of NPs used for tail vein injection was 500 μg/mL. The average weight of the high-fat fed mice was about 30 g, so the volume of injected nanoparticles was about 150 μL per mouse. T2-weighted imaging was conducted with a pharma Scan70/60 US 7.0 T superconductor experimental system (Bruker Biospin MRI GmbH, Switzerland). The following parameters were used for the T2-weighted imaging: repetition time [TR]/echo time [TE] 2500/30 ms, field of view 2.3 cm × 2.3 cm, matrix 256 × 256, slice thickness 0.8 mm. And, the following parameters were used for T2-mapping imaging: repetition time [TR]/echo time [TE] 2200/9.5 ms, field of view 2.3 cm × 2.3 cm, matrix 256 × 256, slice thickness 0.8 mm.
Ex vivo fluorescence imaging
The targeting capability of FITC-VHP-Fe3O4@SiO2 was also measured by ex vivo fluorescence imaging. We isolated the aortic vessels of the mice after MRI and carefully dissect the fatty tissue around the blood vessels. The fluorescence intensity of blood vessels was measured using a Small Animal Multi-mode Imaging machine (Bruker MI) with 470 nm excitation wavelength and 535 nm emission wavelength.
Histological examination
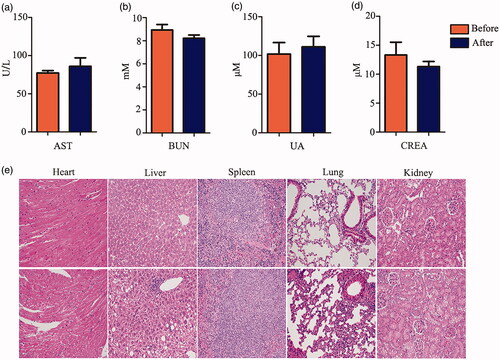
Atherosclerosis plaque was detected using Hematoxylin-eosin staining (HE staining). Blood vessels and other organs (heart, liver, spleen and kidney) of mice were carefully obtained from anesthetized ApoE−/− mice and soaked with formalin for 24 h. The samples were dehydrated with gradient alcohol and embedded in an implanter, cut into sections (4 μm) with a cryogenic thermostat. Slices were successively collected on gelatin-coated glass slides and stained with HE staining and Prussian blues staining.
Blood biochemical analysis
Fresh blood obtained from treated mice was dealt with static settlement at room temperature for 30 min and then centrifuged at 650 g for 10 min at 4 °C. The supernatant was collected and sent to Overseas Chinese hospital (Guangzhou, China) for examination of triglycerides (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol, blood urea nitrogen (BUN), creatinine (CREA), Uric Acid (UA) and Aspartate transaminase (AST).
Data analysis
Each experiment was repeated for three times at least. GraphPad Prism 6.0 software was used for statistical analysis. The data were presented as mean ± standard deviation (SD). p < .05, p < .01 and p < .001 were thought to be statistically significant.
Result
Characterization of Fe3O4@SiO2
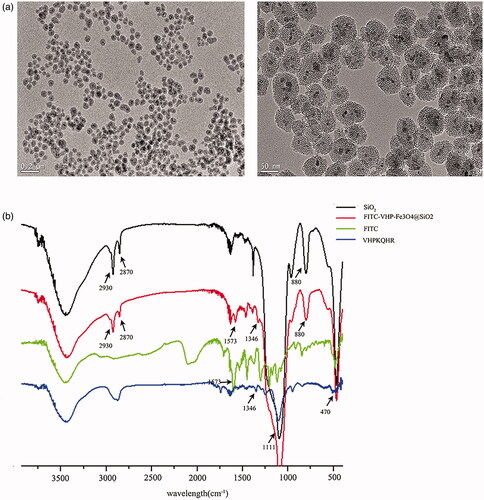
Amino Fe3O4@SiO2 was synthesized and showed a structure by transmission electron microscopy (TEM) with approximately uniform size (). Fe3O4@SiO2's Zeta potential is about +21.6 ± 0.6 mv (Figure S1a), indicating that it has good dispersion and the amino (positive charge) was modified successfully.
Figure 1. Characterization of FITC-VHP-Fe3O4@SiO2. (a) The morphology of Fe3O4@SiO2 with different magnification under TEM (left: the scale bar is 0.2 μm; right: the scale bar is 50 nm). (b) FITR analyse of SiO2 (black line), FITC (green line), VHPKQHR (blue line) and FITC-VHP-Fe3O4@SiO2 (red line).
Characterization of FITC-VHPKQHR-Fe3O4@SiO2
As shown by UV-Vis, VHPKQHR labelled Fe3O4@SiO2 had a characteristic absorption peak at 220 nm, suggesting that peptide VHPKQHR was successfully incorporated with Fe3O4@SiO2 (Figure S1b). FITR analysis results are as follows (). The absorption peaks around 1548 and 1632 cm−1 were caused by N–H bending vibration, indicating that the surface of mesoporous SiO2 was modified by amino. The absorption peaks at 2870 and 2930 cm−1 result from respectively asymmetric and symmetrical − CH2 stretching, which confirmed that the polymerization of TEOS and APTES formed the SiO2 nanostructure. Mesoporous SiO2 nanoparticles showed strong absorption peaks at 470, 800 and 1111 cm−1, which was caused by the asymmetric vibration of Si–O and Si–O–Si bonds in SiO2. The absorption peak at 1573 cm−1 belongs to the stretching vibration of anthracyclcarbonyl group in FITC. The 1346 cm−1 absorption peak was attributed to the α-helix structure of the peptide. All the data suggest successful construction of FITC and VHPKQHR modified Fe3O4@SiO2.
FITC-VHP-Fe3O4@SiO2 was first synthesized and could target atherosclerosis in vitro
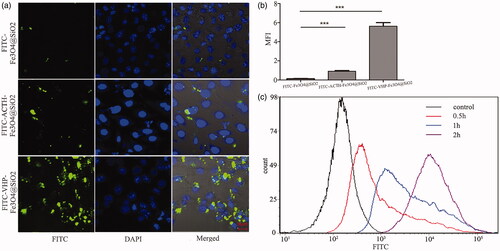
Endothelial cells are characteristic cells at the site of atherosclerosis which express high level of VCAM-1. The binding ability of FITC-VHP-Fe3O4@SiO2 was determined using MAECs. The result indicated that stimulated MAECs with LPS overexpress VCAM-1 (Figure S2a,b). To demonstrate the binding of VCAM-1 targeting nanoparticles, the flow cytometry was used to analyze the FITC fluorescence intensity of stimulated MAECS after incubated with FITC-VHP-Fe3O4@SiO2 for 0, 0.5, 1, 2 h. The results showed that FITC-VHP-Fe3O4@SiO2 strongly bound MAECs (). To test the specificity and high efficiency of VHPKQHR targeting, an unrelated peptide (ACTH) was incorporated into FITC-Fe3O4@SiO2. 25 μg/ml FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 were incubated with stimulated MAECS for 3 h and show increased intensity of FITC in comparison with FITC-Fe3O4@SiO2 and FITC-ATCH-Fe3O4@SiO2 under confocal microscope (). Quantitative analysis using Image J software showed a significant increase of FITC in cells received FITC-VHP-Fe3O4@SiO2 ().
Figure 2. The binding ability of FITC-VHP-Fe3O4@SiO2 to endothelial cells with high expression of VCAM-1. (a) FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 were incubated with stimulated MAECs and observed under confocal microscope. (b) The FITC fluorescence intensity corresponding to confocal microscope was calculated using Image J software. (c) The fluorescence intensity of MAECs after incubation with FITC-VHP-Fe3O4@SiO2 for 0, 0.5, 1, 2 h was analyzed by flow cytometry. ***p < .001.
FITC-VHP-Fe3O4@SiO2 has low toxicity in vitro
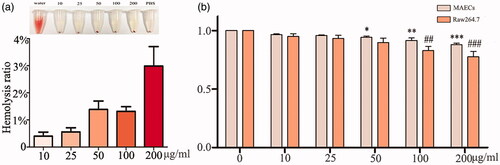
We selected two typical cells of Raw264.7 (immune cell) and MAECs (non-immune cell) to test in vitro nanoparticles toxicity. CCK-8 was used to assess cell viability after incubated with FITC-VHP-Fe3O4@SiO2 (0,10,25,50,100,200 μg/ml) at different concentrations for 24 h. The results demonstrated that the viability of MAECs and Raw264.7 was not affected until the concentration reached 50 μg/ml (p < .05) or 100 μg/ml (p < .001) (), respectively, implying the low toxicity of FITC-VHP-Fe3O4@SiO2. The hemocompatibility of the nanoparticles was tested by hemolysis of the erythrocyte rupture rate at different concentrations of FITC-VHP-Fe3O4@SiO2. Our data showed that the hemolysis rate increased slightly after addition of the nanoparticles. However, FITC-VHP-Fe3O4@SiO2 at all concentrations induced <5% lysis of the erythrocyte, indicating that FITC-VHP-Fe3O4@SiO2 has good blood compatibility (). All the data suggest that FITC-VHP-Fe3O4@SiO2 has low toxicity in vivo and in vitro and has good blood compatibility.
Figure 3. Biosafety of FITC-VHP-Fe3O4@SiO2 in vitro. (a) The hemolysis rate under FITC-VHP-Fe3O4@SiO2 for 10, 25, 50, 100, 200 μg/ml. (b) CCK8 was used to assess the effect of 10, 25, 50, 100, 200 μg/ml FITC-VHP-Fe3O4@SiO2 on viability of MAECs and Raw264.7. *p < .05; **p < .01; ##p < .01; ###p < .001.
FITC-VHP-Fe3O4@SiO2 target at plaque by ex vivo assessment and diagnosed the occurrence of plaques by MRI in vivo
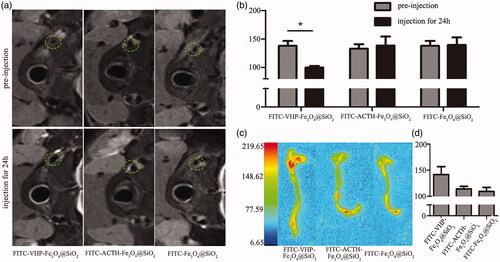
After confirming that the VCAM-1 targeted nanoparticles are biosafe and capable of binding to stimulated MAECs, we conducted in vivo targeting experiments. Atherosclerosis models were established in order to conduct in vivo experiments. Blood biochemical index and thickened vessel wall indicated successful construction of the atherosclerosis model (Figure S3a-b). MRI was taken at 0 and 24 h after nanoparticles administration. It was clearly observed that T2 image signal intensity of plaque regions for the FITC-VHP-Fe3O4@SiO2 group was affected compared to other two groups (). FITC-VHP-Fe3O4@SiO2 can effectively reduce the T2 relaxation time and consequently produce negative enhancement on T2-weighted images (). After imaging with MRI, the mice were euthanized and dissected. Aortic vessels were immediately immersed in formalin. The fluorescence intensity of aortic vessels was measured under Small Animal Multimode Imaging System. The results showed that the FITC-VHP-Fe3O4@SiO2 group has stronger intensity on plaque than others, suggesting accumulation of the VCAM-1 target NPs on atherosclerosis sites.
Figure 4. FITC-VHP-Fe3O4@SiO2 can target atherosclerotic plaques ex vivo and in vivo. T2 signal intensity (a) and T2 value (b) before and after treatment with FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 for 24 h. The fluorescence intensity distribution of blood vessels (c) and Quantitative analysis of fluorescence intensity (d) was measured using a Small Animal Multi-mode Imaging System after injecting with FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 for 24 h. * p < .05
FITC-VHP-Fe3O4@SiO2 has low toxicity in vivo
The above results show that FITC-VHP-Fe3O4@SiO2 can actively target at atherosclerotic plaques as shown by MRI and is not toxic in the in vitro experiment. In order to prove that FITC-VHP-Fe3O4@SiO2 is also non-toxic in vivo, we examined its effect on major organs in vivo. In vivo nanoparticle toxicity was estimated by blood biochemical analysis and morphological analysis after injected with FITC-VHP-Fe3O4@SiO2 for 15 days. Blood biochemical indexes include AST (an indicator of liver function) and BUN, UA, CREA (indicators of kidney function). The results showed that FITC-VHP-Fe3O4@SiO2 had no significant effect on both organs (). Morphological analysis also showed that FITC-VHP-Fe3O4@SiO2 did not significantly impact on heart, liver, spleen, lung and kidney ().
Discussion
Atherosclerosis is mainly caused by long-term intake of high-cholesterol and obesity. Since atherosclerosis at early stage does not cause excessive damage to the body, timely diagnosis is thus important for the prevention of the progress of the disease. In order to improve the accuracy of diagnosis and treatment, molecular targeting has emerged as a novel tool to interact with molecular and cellular components critical for the pathogenesis of atherosclerosis [Citation23]. It is generally believed that vascular endothelial cell injury is the main cause of atherosclerosis [Citation11]. Injured endothelial cells will increase the expression of adhesion molecules such as VCAM-1 which provide targeting for nanoparticles to target at disease site [Citation24]. However, no nanoparticles for both purposes of diagnosis and treatment have been designed. In this study, we have successfully synthesized a VHPKQHR carrier nanoparticles that carry drug vectors with targeted diagnostic function.
The binding between the probe and its target marker is the essence of molecular targeting [Citation25]. Nanoparticles attached with antibodies, peptides or ligands can host particular cell types or structures within an atherosclerotic plaque [Citation24]. Peptides different from antibodies do not destroy specificity or affinity and are small enough to weaken the vascular barrier to the lesion sites conveniently. The combination of peptides and nanoparticles overcomes the delivery barriers of many traditional drugs and provides a powerful strategy for the study of nanomedicines [Citation26]. The previous study had demonstrated that the peptide VHPKQHR has high affinity with injured endothelial cells that are characteristic of atherosclerosis [Citation27]. In order to verify that our synthesis methods do not break the targeting function of VHPKQHR, we evaluated the binding ability of FITC-VHP-Fe3O4@SiO2 to stimulated endothelial cells in vitro. The results met our expectations, demonstrating that our synthesis method does not destroy the binding ability of VHPKQHR. Prolonging circulation time of nanoparticles in vivo is beneficial to the non-specific aggregation and unrelated peptides also seem to have some ability to adhere to the lesion areas [Citation28]. Our results showed that VHPKQHR nanoparticles tightly interact with VCAM-1 in comparison with other non-VCAM-1 targeted peptides (ACTH) nanoparticles and nanoparticles with no peptide. In vitro cell binding experiments using confocal microscopy imaging and flow cytometry had proved that VHPKQHR has high affinity for atherosclerotic plaques.
There are many methods to diagnose vascular stenosis caused by plaque including digital subtraction angiography (DSA), CT, ultrasound, PET and MRI, among which DSA is mostly favoured for detection of arterial stenosis. However, its clinical application is limited because it is an invasive test and unable to estimate the internal condition of the vessel wall and plaque [Citation29,Citation30]. Besides, CT cannot provide the internal information of plaque, unable to evaluate treatment result and prognosis [Citation31]. The accuracy and reproductivity of ultrasonic testing are in question because it depends on operators’ subjective diagnosis. Although PET with a high sensitivity can determine the development of plaques at the molecular level [Citation32], it has radiation and requires high cost. MRI can detect the morphological and functional information of plaques, without radiation and toxicity on kidney [Citation4]; thus, it is the best for the diagnosis of atherosclerosis. However, it is difficult for MRI to detect early plaques due to the limitation of resolution. The combination of targeted probe and contrast agent can significantly increase the resolution of MRI [Citation33]. In our study, T2 weight imaging and T2 mapping were adopted to evaluate the imaging effects of nano-particles of FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 on vascular plaque detection. The experimental results showed that VHP targeted the plaques, weakened the signal and intensity significantly increased the contrast of imaging.
In clinic, the treatment with antiplatelet and lipid-lowering drugs is the most common method for alleviating atherosclerosis [Citation34,Citation35]. However, long-term use of these drugs can cause significant side effects such as damaging liver and kidneys. Side effects can be reduced by precise delivery of drugs especially antiplatelet drugs to the lesions. It is difficult to directly combine probes with drugs but can be solved by loading drugs to a carrier that can be modified with targeting capability [Citation36]. Previous studies have demonstrated that silicon is harmless to human, which has a daily intake and maintain the normal metabolisms [Citation37,Citation38] and MSN is a good drugs carrier [Citation15]. Also, our nanoparticles had low toxicity to endothelial cells and macrophages that are two types of cells at the site of atherosclerosis [Citation11]. In addition, the nanoparticles were tested for blood compatibility. The results showed that FITC-VHP-Fe3O4@SiO2 had no hemolytic effect. By the observation of nanoparticles injection in vivo, we found that long-term intravenous injection did not cause obvious damage to the body. Our results suggest that the FITC-VHP-Fe3O4@SiO2 are indeed non-toxic, biocompatible and suitable for diagnosis.
Conclusion
We constructed mesoporous silicon nanoparticles with VCAM-1 targeting. Our results demonstrate that FITC-VHP-Fe3O4@SiO2 successfully targeted at atherosclerotic plaques by in vitro cell assays, ex vivo fluorescence imaging assays and in vivo magnetic resonance imaging assays. In addition, FITC-VHP-Fe3O4@SiO2 can increase the contrast of magnetic resonance imaging and is a good carrier for future accurate delivery of drugs.
supplement_data.docx
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546.
- Fausto N. Atherosclerosis in young people: the value of the autopsy for studies of the epidemiology and pathobiology of disease. Am J Pathol. 1998;154:1021–1022.
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013.
- Kramer CM, Anderson JD. MRI of atherosclerosis: diagnosis and monitoring therapy. Exp Rev Cardiovasc Ther. 2007;5:69–80.
- Raggi P, Baldassarre D, Day S, et al. Non-invasive imaging of atherosclerosis regression with magnetic resonance to guide drug development. Atherosclerosis. 2016;251:476–482.
- Estelrich J, Sanchez-Martin MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomedicine. 2015;10:1727–1741.
- Jin K, Luo Z, Zhang B, et al. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33.
- Nakashima Y, Raines EW, Plump AS, et al. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851.
- Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85.
- Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50 (Suppl):S382–S387.
- Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16:1978–1990.
- Nahrendorf M, Keliher E, Panizzi P, et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–1222.
- Serres S, Mardiguian S, Campbell SJ, et al. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. FASEB J. 2011;25:4415–4422.
- Zhang X, Liu C, Hu F, et al. PET imaging of VCAM-1 expression and monitoring therapy response in tumor with a (68)Ga-labeled single chain variable fragment. Mol Pharm. 2018;15:609–618.
- Wang Y, Zhao Q, Han N, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11:313–327.
- Zhang Y, Xu J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R Soc Open Sci. 2018;5:1–10.
- Wei Y, Gao L, Wang L, et al. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 2017;24:681–691.
- Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511.
- Kelly KA, Nahrendorf M, Yu AM, et al. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol Imaging Biol. 2006;8:201–207.
- Swaminathan S. Gadolinium toxicity: iron and ferroportin as central targets. Magn Reson Imaging. 2016;34:1373–1376.
- Henderson L, Neumann O, Kaffes C, et al. Routes to potentially safer T1 magnetic resonance imaging contrast in a compact plasmonic nanoparticle with enhanced fluorescence. ACS Nano. 2018;12:8214–8223.
- Shi Q, Wang J, Wang XL, et al. Comparative analysis of vascular endothelial cell activation by TNF-alpha and LPS in humans and baboons. Cell Biochem Biophys. 2004;40:289–303.
- Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231–244.
- Khodabandehlou K, Masehi-Lano JJ, Poon C, et al. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (Maywood). 2017;242:799–812.
- Smith BA, Smith BD. Biomarkers and molecular probes for cell death imaging and targeted therapeutics. Bioconjugate Chem. 2012;23:1989–2006.
- Jeong WJ, Bu J, Kubiatowicz LJ, et al. Peptide-nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 2018;5:38.
- Mlinar LB, Chung EJ, Wonder EA, et al. Active targeting of early and mid-stage atherosclerotic plaques using self-assembled peptide amphiphile micelles. Biomaterials. 2014;35:8678–8686.
- Su T, Wang YB, Han D, et al. Multimodality imaging of angiogenesis in a rabbit atherosclerotic model by GEBP11 peptide targeted nanoparticles. Theranostics. 2017;7:4791–4804.
- Cloft HJ, Lynn MJ, Feldmann E, et al. Risk of cerebral angiography in patients with symptomatic intracranial atherosclerotic stenosis. Cerebrovasc Dis. 2011;31:588–591.
- Willinsky RA, Taylor SM, terBrugge K, et al. Neuroradiology neurologic complications of cerebral angiography prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–528.
- Achenbach S, Raggi P. Imaging of coronary atherosclerosis by computed tomography. Eur Heart J. 2010;31:1442–1448.
- Moghbel M, Al-Zaghal A, Werner TJ, et al. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–497.
- Vithanarachchi S, Allen M. Strategies for target-specific contrast agents for magnetic resonance imaging. Cmi. 2012;1:12–25.
- Clappers N, Brouwer MA, Verheugt FW. Antiplatelet treatment for coronary heart disease. Heart. 2007;93:258–265.
- Libby MAP. Lipid lowering therapy in atherosclerosis. Semin Vasc Med. 2004;4:257–367.
- Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Ijn. 2017;12:7291–7309.
- Araujo LA, Addor F, Campos PM. Use of silicon for skin and hair care: an approach of chemical forms available and efficacy. An Bras Dermatol. 2016;91:331–335.
- Robberecht H, Van Dyck K, Bosscher D, et al. Silicon in foods: content and bioavailability. Int J Food Prop. 2008;11:638–645.