?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pseudomonas aeruginosa (P. aeruginosa) is an aerobic gram-negative, non-spore forming, rod-shaped bacterium. It accelerates the decline in lung function and ultimately leads to increased mortality and morbidity rate. Survival and virulence of P. aeruginosa is due to its biofilm formation ability. The main aim of this study was to test the synergistic effect of silver nanoparticles (AgNPs) in combination with Polymyxin B against biofilms of P. aeruginosa. A total of 500 pus aspirations were collected and bacterial pathogens were identified. Biofilm formation was attained using a glass tube method and microtiter plate assay. The minimum inhibitory concentration of Polymyxin B was determined using agar well diffusion method. Silver nanoparticles were synthesized by chemical reduction method followed by determination of their anti-pseudomonal ability separately and in combination with Polymyxin B using microtiter plate assay. Our results showed that 120 out of 500 samples were Pseudomonas positive. The ratio of multidrug-resistant (MDR) in our collected Pseudomonas samples was 83% (25/30). Generally, the minimum inhibitory concentration (MIC) of Polymyxin B was 16 µg/mL and that of AgNPs was null. However, AgNPs showed great synergistic effect in combination with Polymyxin B. Synergistically, the efficacy of Polymyxin B was enhanced four times as compared to unaided Polymyxin B.
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is an aerobic gram-negative, non-spore forming, rod-shaped bacterium, oxidase positive due to its synthesis of indophenol oxidase enzyme. It also produces pyoverdin and pyocyanin pigments which are water soluble. The habitat of P. aeruginosa is soil and water but can be also found on fresh vegetables and fruits. In the laboratory, it produces sweetgrape-like odor. Instead of fermentation, these get energy by oxidizing sugars. Pseudomonas aeruginosa is a nosocomial and community-acquired infectious agent. It can be found in hospital and intensive care units and can persist in some antiseptic solutions used for disinfecting surgical instruments and contact lenses [Citation1]. It infects patients with weak immune system and leads to serious diseases, e.g. urinary tract infections, bacteremia, pneumonia and ulcerative [Citation2]. It is an opportunistic pathogen in cystic fibrosis. It accelerates the decline in lung function and ultimately leads to increased mortality and morbidity rate [Citation3]. The multidrug-resistant (MDR) P. aeruginosa in hospitalized patients leads to increased mortality [Citation4]. It causes nosocomial urinary tract infection (UTI) associated with an indwelling catheter, instrumentation of the urinary system. Community-acquired UTI’s are infrequently caused by P. aeruginosa [Citation5]. It is considered as the leading cause of ventilator-associated pneumonia [Citation6] complications include acute respiratory distress syndrome (ARDS) and septic shock [Citation7].
Survival and virulence of P. aeruginosa is due to its biofilm formation ability that is the formation of microcolonies on the solid surface followed by maturation. Pseudomonas aeruginosa form biofilm by producing and releasing exopolysaccharide alginate. These lipopolysaccharides (LPS) not only help in the formation but also stabilize the structure of P. aeruginosa biofilm. Cell to cell signals of quorum sensing are mandatory for the promotion of rapid biofilm formation. These signals contain the presence of a suitable surface, higher extracellular iron concentration, availability of indole, calcium bile salt and polyamines [Citation8]. The sessile mode of growth in biofilm allows huge numbers of Pseudomonal cells to coexist than free-floating planktonic bacterial populations, which leads to antimicrobial resistance in the case of biofilm [Citation9]. Due to its medical importance, effective methods for controlling its formation and improving patient care are necessary [Citation10]. Upon the usage of unsterilized medical devices and implants, the patients may get opportunistic Pseudomonal infections that may lead to produce biofilms inside the victims’ bodies [Citation11,Citation12]. Biofilms exhibit high cell densities ranging from 108 to 1011 cells g−1 wet weight [Citation13,Citation14].
Resistance of biofilm against antibiotic is as great as 100–1000 folds as compared to planktonic bacteria [Citation15]. Novel antimicrobial strategies are extremely desirable to control the drug resistance of biofilms [Citation16]. Various antibiotics are used against P. aeruginosa, i.e., meropenem, ceftazidime, cefepime, but they are effective up to some extent [Citation17]. Tobramycin used for cystic fibrosis has effective but limited antibacterial activity because of the inability to penetrate the biofilms formed in the lungs [Citation18]. Polymyxin B is recently reintegrated in clinical practice. It has a high affinity for LPS of gram-negative bacteria. It induces aggregation of LPS, thus increases the surface charge. This leads to internalization and binding of Polymyxin B to bacterial phosphatidylglycerol rich membrane causing leakage of cellular contents [Citation19]. It directly interacts withthe lipid-A component of lipopolysaccharide increasing permeability of cell membrane of bacteria [Citation20]. However, plasmid-borne Polymyxin B microbial resistance has been reported [Citation21].
Drug discovery programs, in order to develop new safer and more efficacious new Polymyxin B derivatives to overcome these problems, have got miniature success [Citation20]. Another option is the usage of Polymyxin B in amalgamation with other antimicrobial agents including nanoparticles (NPs). Use of NPs-antibiotic combinations in order to eliminate and prevent bacterial biofilms formed by multidrug-resistant bacteria show great promise [Citation22,Citation23]. Silver nanoparticles are extraordinarily efficient at absorbing, optical, electrical and thermal properties. These are reported as antibacterial agents to treat septic burns and wounds [Citation24]. Silver NP is one of the most attractive inorganic materials due to its wonderful applications in catalysis, biomolecular detection, photography, diagnostics, biosensor and particularly antimicrobial activities. Furthermore, it is environmentally non-threatening in nature [Citation25]. The size of NPs plays a key role as greater prevention rate can be achieved by a smaller size of NPs and high surface to mass ratio. Some studies showed that shape is also a remarkable factor, e.g. rod-shaped NPs have greater destruction effect as compared to spherical shape NPs against biofilms [Citation26]. Biofilm integrity is interfered by NPs through interacting with EPSs [Citation27].
Current study was designed to optimize in vitro production of biofilm by P. aeruginosa followed by determination of the minimum inhibitory concentration (MIC) of Polymyxin B against biofilm produced by P. aeruginosa. Various combinations of Polymyxin B and NP were also fabricated to determine the inhibition of biofilm produced by P. aeruginosa. The ultimate goal was to find out the most effective synergistic concentrations which may permit for the effective clinical use of considerably lower levels of Polymyxin B.
Materials and methods
Collection and identification of isolates
A total of 500 bacterial samples from superficial wounds, scrapes, cuts, incisions and burns were collected using sterile cotton swab without contaminating it with skin commensals. Samples were streaked on McConekey agar, nutrient agar, cetrimide agar and blood agar. The bacterial growth was observed on these media for the colony morphology. For further identification and characterization, these isolates were subjected to microscopic examination, Gram staining, biochemical assays and finally confirmed by 16Sribosomal RNA technique.
Minimum inhibitory concentration assay for Polymyxin B
One molar Polymyxin B sulfate stock solution (w/v) was prepared in distilled water. Seven different working aqueous solutions were prepared from a stock solution in sterilized Eppendorf tubes, i.e. 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7 and 2:8. In the first test tube, 900 µL stock solution and 100 µL of water were mixed. In the second test tube, 800 µL of stock solution and 200 µL of water, in the third, 700 µL of stock solution and 300 µL of distilled water, in the fourth, 600 μl of stock solution and 400 µL of distilled water, in the fifth, 500 µL of stock solution and 500 µL of distilled water, in the sixth, 400 µL of stock solution and 600 µL distilled water, in the seventh tube, 300 µL of stock solution and 700 µL of distilled water was transferred and mixed.
Sterilized nutrient agar was taken in Petri plates while inoculum containing P. aeruginosa was spread on these plates via sterilized cotton swab. Wells were made in agar using 8 mm diameter of cork borer, whereas 100 µg of Polymyxin B was transferred to each well. The plates were then incubated overnight at 37 °C. Next day, the zones of inhibitions were observed around each well followed by measurement in millimeter.
Production and quantification of in vitro biofilm
In case of biofilm formation in a microtiter plate, 1% of log phase cells were added to 198 µl LB broth to microtiter plate wells. As a negative control, 200 µL of LB broth was added to the microtiter plate well without inoculum. The plate was incubated for 24 h at 37 °C. Upon completion of incubation, the plate was turned over and shacked to remove the media. The wells were washed twice and each time water was removed by turning over the plate. Thereafter, 125 µL of 0.05% solution of crystal violet (CV) was added to all wells. The microtiter plates were incubated at room temperature for 10–15 until desiccated. Consequently, visible biofilm formation was determined. For the quantification of biofilm, cell-bound CV was dissolved in 125 µL of 30% acetic acid and transferred to a second sterilized plate followed by incubation at 37 °C for 10–15 min. The second plate was then analyzed on a microtiter plate reader at 600 nm. The optical density (OD) of the microtiter plate was recorded. CFU/mL was measured using the following formula:
In the case of glass tube biofilm synthesis, the Luria–Bertani (LB) broth supplemented with 1% glucose was taken in a test tube. Inoculum of P. aeruginosa was taken with the help of wire loop and added in the 3 mL LB broth taken in the glass tube and incubated at 37 °C overnight. Upon completion of incubation, the glass tube was turned over and shacked to remove the media. The tube was washed twice and the above-mentioned protocol was used from staining with CV.
Preparation and characterization of silver nanoparticles (AgNPs)
One percent (1.5 mL) aqueous solution of silver nitrate was prepared. Briefly, 20 ml trisodium citrate was added to 75 mL of water and mixed for 15 min at 70 °C. The prepared silver nitrate solution was added to the solution followed by the addition of a few drops of 1% sodium borohydride. This mixture was then heated at 70 °C for 60 min and cooled at room temperature [Citation28]. Water was added to the flask to make a total volume up to 100 ml. To check the absorbance of synthesized silver nanoparticles Perkin–Elmer UV-visible spectroscopy lambda 25 was used. The nanoparticles solution was dispersed in double distal water through pipetting. The measurements of particles in solution were done at 395–450 nm wavelength using plastic cuvettes and double distal water was used as a blank.
The nanoparticle surface morphology was examined using TEM. The mean particle size of silver nanoparticles was measured by dynamic laser light scattering using an (ELS-Z, DLS-8000; Otsuka Electronics Co., Osaka, Japan). The mean particle size of the NPs was determined in triplicate and the average values were calculated.
Biofilm inhibition using Polymyxin B and AgNPs combination
LB Broth supplemented with 1% glucose was taken in the test tube. Inoculum of P. aeruginosa was taken with the help of wire loop and suspended in 3 mL LB broth in a test tube and incubated overnight at 37 °C. About 2 µL of log phase cells were added to 198 µL LB broth and transferred to microtiter plate wells. Then, 200 µL of LB broth was added to the microtiter plate well without inoculum for negative control. Incubation of this plate was done for 24 h at 37 °C. Different concentrations of Polymyxin B, i.e. 2 µg, 4 µg, 8 µg, 16 µL, 32 µg, 64 µg, and 128 µg were added to wells in triplicates. The plate was then incubated for 24 h at 37 °C.
One mM AgNPs were used in combination with Polymyxin B (1 µg) at a volume of 2 µL, 4 µL, 8 µL and 16 µL was used separately in 4th, 5th and 6th column of a microtiter plate and in combination with prepared AgNPs (64 µL) in 10th, 11th and 12th column. In column number 7th, 8th and 9th different concentrations of AgNPs were used. The plate was then incubated and after incubation, staining of these plates was done. Then, biofilms inhibition was quantified by measuring and comparing the absorbance of the above tested antibacterial agents using microtiter plate reader at 600 nm.
Results
Nanoparticles preparation and characterization
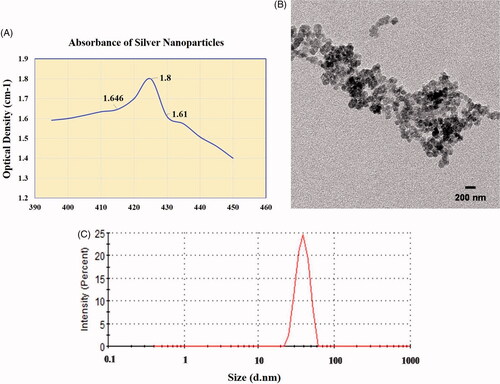
Nanoparticles of silver were prepared by chemical reduction method. The appearance of brown color after the addition of reducing agent indicated the formation of AgNPs. Absorbance of the prepared nanoparticles was increased from 1.591 (at 395 nm wavelength) to 1.8 (425 nm) and then it was decreased until 1.4 (450 nm). The data is depicted in . The transmission electron microscopy shows the formation of small nanoparticles with much lower size . The smaller size of nanoparticles was also confirmed by dynamic light scattering analysis.
Isolation of Pseudomonas aeruginosa
A total of 500 pus samples were collected and processed. Total of 120 isolates was of P. aeruginosa positive. Out of these 120 isolates, 44 cases were of female and 76 cases were of male positive samples, the percentage is given in .
Figure 2. Pseudomonas aeruginosa gender wise distribution in pus samples and growth on plate and glass tube (A) Gender wise distribution of P. aeruginosa in pus samples. (B) Growth of P. aeruginosa on Cetrimide agar. (C) Biofilm formation through glass tube method.
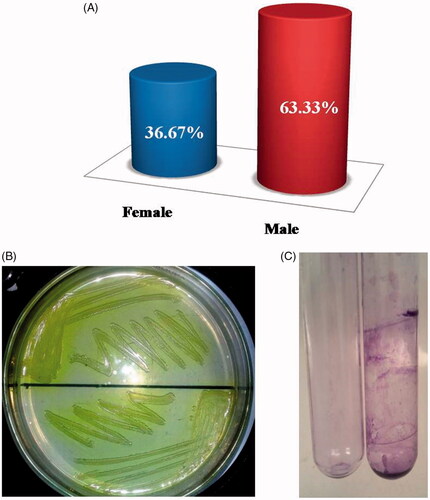
Samples were determined by colony morphology using the standard guidelines. On nutrient agar, smooth, raised, semi-translucent and mucoid colonies were observed. Besides the growth, these isolates also changed the color of nutrient agar to green along with fruity smell . Under the microscope, colonies were observed as pink color gram-negative rods after gram staining. Furthermore, it was also confirmed by the 16S RNA test. Results of all the biochemical tests showed that the isolated specimen have the P. aeruginosa.
Biofilm formation in glass tube
In order to find out the ability of P. aeruginosa to form biofilm in glass tube method was used. Most of the isolates formed biofilms. Some isolates formed strong biofilms on the walls of glass slide when stained with CV dye gave strong purple color and observed through the naked eye and some formed weak biofilms giving a very thin film. These biofilms were compared with negative control having no inoculum shown in .
Minimum inhibitory concentration by agar well diffusion method
In order to find out the minimum inhibitory concentration of Polymyxin B agar well, diffusion assay was performed. Different working concentrations, from one molar stock solution of Polymyxin B were prepared. From each concentration, 100 µl was transferred into different wells, respectively. Maximum zone of inhibition (ZI) was observed using 0.9 mL concentration giving 22 mm ZI. On 0.2 ml, the minimum zone was observed, i.e. 11 mm from these results, we concluded that 0.3 mL (300 µL in 700 µL water) of 1 µg/1 µL Polymyxin B was our MIC ().
Figure 3. Minimum inhibitory concentration of Polymyxin B (A) MIC of Polymyxin B (Agar well diffusion method) (B) MIC of Polymyxin B against P. aeruginosa biofilm.
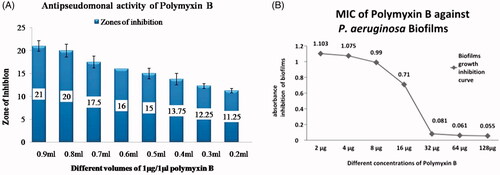
Before using Polymyxin B in combination with AgNPs for inhibition of biofilms of P. aeruginosa, we determined the minimum inhibitory concentration of Polymyxin B in 96 well microtiter plate assay. As shown in (), the absorbance of our sample of biofilms produced by P. aeruginosa were high, i.e. 1.947. When different concentrations were used, the absorbance decreased with increase in the concentration of Polymyxin B. Complete biofilms were inhibited when Polymyxin B in the concentration of 32 µL, 64 µL and 128 µL were used. On 2 µL, the absorbance recorded was 1.103 followed by 4 µL giving absorbance of 1.075, 8 µL giving absorbance of 0.99, 16 µL giving absorbance of 0.71, 32 µL giving absorbance of 0.081, 64 µL giving absorbance of 0.061 and 128 µL giving absorbance of 0.055 (). From the above results, we have concluded that at a concentration of 32, 64 and 128 µL biofilm formation was inhibited, while on 16 µL, very less growth was observed. Therefore, 16 µL was our MIC determined.
Antibacterial activity of silver nanoparticles and its synergistic effect
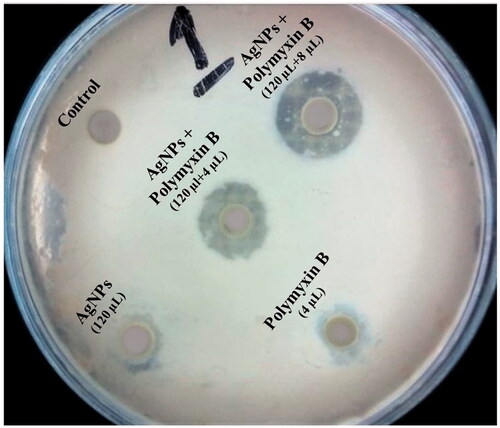
The antibacterial activity of selected standard antibiotic Polymyxin B displayed activity against the test pathogen but increased fold activity was observed when antibiotics was evaluated in combination with silver nanoparticles against the test pathogen ().
The synergistic effect of Polymyxin B and AgNPs inhibited the biofilm formation
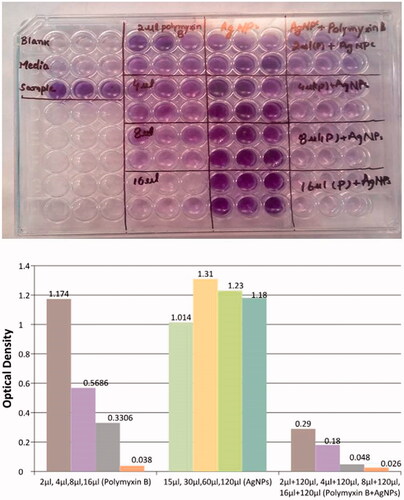
We determined the activity of Polymyxin B, AgNPs separately and Polymyxin B in combination with AgNPs. As shown in different concentrations of AgNPs when used against Biofilm formation. The biofilms were not inhibited even at a higher concentration that was 120 µL. However, when these AgNPs were used in combination with Polymyxin B, they enhanced the anti-biofilm activity of Polymyxin B effectively even at a lower dose of Polymyxin B. Here, it is necessary to compare the activity of Polymyxin B singly and in combination with NPs. As much the absorbance is low, it shows greater inhibition of biofilms formation. Absorbance of 2 µL Polymyxin B was 1.174 and when this concentration was used in combination with NPs, we got absorbance of 0.29 that is very less as compared to single use of Polymyxin B. Same as above, absorbance of Polymyxin B at concentrations of 4 µL, 8 µL, and 16 µL was 0.5686, 0.3306 and 0.038, respectively. The absorbance of Polymyxin B in combination with AgNPs at concentrations of 2 µL Polymyxin B and 120 µL AgNPs, 4 µL Polymyxin B and 120 µL AgNPs, 8 µL Polymyxin B and 120 µL AgNPs and 16 µL Polymyxin B and 120 µL AgNPs were 0.18, 0.048 and 0.026. Thus, from the results, we concluded that AgNPs have no notable effect on inhibition of biofilms but havea strong synergistic effect when used in combination with Polymyxin B.
Discussion
Emerging of MDR bacterial strains is the main issue. Pseudomonas aeruginosa is one of the leading MDR among other bacteria. As these bacteria are resistant to antibiotics, thus for treatment of infections, new strategies are required. Polymyxin B antibiotic in the previous years were used to treat infections but its use was banned due to its nephrotoxicity and neurotoxicity. In the present situation, due to the strong resistance of bacteria towards other antibiotics, Polymyxin B is the last choice of drug to treat infections. Our study is based on the synergistic effect of AgNPs combined with Polymyxin B in order to reduce the dose of Polymyxin B, thus decreasing its toxicity towards the host. It is demonstrated that NPs possesses broad-spectrum antibacterial properties against both gram-positive and gram-negative bacteria [Citation29]. Some studies showed the concentration dependent antimicrobial activity of AgNPs against E. coli and P. aeruginosa is also investigated [Citation30].
Pseudomonas aeruginosa was isolated from pus samples. Total of 500 samples was collected and processed through microbial identification techniques. Out of these samples, 120 samples were P. aeruginosa positive cases. Raytekar et al. [Citation31] also isolated P. aeruginosa from pus samples. The prevalence rate they determined was 20.19%. Andhale et al. [Citation32] observed that 76.66% isolates were from male samples and 23.33% isolates from female. Another study also investigated gender-wise distribution of P. aeruginosa in which 61% were from male samples and 48% from female isolates [Citation33]. Andhale et al. [Citation32] also worked on antibiotic profiling of P. aeruginosa isolated from pus samples. They observed that the percentage of MDR strain was 68.83%, which is near to our observed percentage that was 63.33%.
For finding a minimum inhibitory concentration of Polymyxin B against P. aeruginosa different working concentrations from 1 M solution were prepared and from each concentration, 100 µL were transferred to each well. At a concentration of 0.9 ml, we observed the highest zone of inhibition that was 22 mm while at a concentration of 0.2 mL zone of inhibition was 11 mm. Thus, we concluded that the MIC of Polymyxin B according to observation was 0.3 mL.
We also determined the ability of P. aeruginosa by glass tube method. Most of the isolates formed biofilms. Some isolates formed strong biofilms on the walls of glass slide when stained with crystal violet dye gave strong purple color and observed through naked eye and some formed weak biofilms giving very thin film. Glass tube method was also used by Abidi et al. [Citation34] for the formation of biofilms, their results were similar to that of our findings.
Silver nanoparticles were prepared by chemical reduction method [Citation35]. Mohanty et al. [Citation36] synthesized AgNPs using silver nitrate (AgNO3) and these AgNPs exhibited activity against bacterial biofilms. Furthermore, we determined the activity of Polymyxin B against biofilms. Different concentrations of Polymyxin B were used against P. aeruginosa biofilms. At a concentration of 2 µL, no inhibition was observed because the optical density of 2 µL was similar to that of P. aeruginosa without the addition of Polymyxin B. At concentrations of 4 µL and 8 µL of Polymyxin B, the OD gradually decreased. At a concentration of 16 µL, the biofilms were completely inhibited. Prevention and overcoming of biofilm formation can be achieved by Ag-based NPs [Citation37].
Synergistic effect of AgNPs in combination with Polymyxin B was also investigated in our studies from our experiments we observed that AgNPs separately were not effective in inhibition of biofilms but when used in combination with Polymyxin B, these NPs interestingly enhanced the activity of biofilms inhibition. A similar study carried out by Fayaz et al. [Citation38] suggests that the combination of silver nanoparticles and antibiotics resulted in increased activity against human pathogens. Using Polymyxin B separately, biofilms were inhibited at a concentration of 16 µL. While on the other hand, biofilms formation was completely inhibited at a concentration of 4 µL when NPs were combined with it. These results showed that AgNPs have a strong synergistic effect when used in combination with Polymyxin B.
Conclusion
This study of the antibacterial activity of AgNPs combined with antibiotic clearly confirmed the existence of a synergistic effect resulting from the combination of these two antimicrobial agents. From the current study, we can conclude that the ratio of Pseudomonas in all our collected samples (500 samples) was 24%. The ratio of MDR in our collected Pseudomonas was 83% (25/30). Polymyxin B is outstandingly efficacious against Pseudomonas. Polymyxin B can inhibit the formation of biofilm produced by Pseudomonas. Generally, the minimum inhibitory concentration of Polymyxin B was 16 µg/mL, while AgNPs originally were totally ineffective at this much lower concentration of 16 µg/mL. However, silver nanoparticles showed a great synergistic effect in combination with Polymyxin B. Synergistically, the efficacy of Polymyxin B was enhanced four times as compared to Polymyxin B alone. From the current work, it can be recommended that blind antibiotic therapy should be discouraged. High concentration of Polymyxin B is not recommended, however, AgNPs can augment its effect in low concentration. Therefore, it can be used in combination with silver nanoparticles against biofilm produced by Pseudomonas. In addition, the observed decrease in minimum inhibitory antibiotic concentration substantially reduces any adverse effects of antibiotics currently used in medical practice. Altogether, combining antibiotics with AgNPs provides one potential approach to an effective fight against the unresolved problem of increasing resistance of pathogenic bacteria against traditional antibiotics.
Data availability
All the required data, which is related to current study, are embedded in this manuscript.
Disclosure statement
The authors declare that they have no conflict of interest regarding this publication.
References
- Wilson LA, Schlitzer RL, Ahearn DG. Pseudomonas corneal ulcers associated with soft contact-lens wear. Am J Ophthalmol. 1981;92:546–554.
- Sadikot RT, Blackwell TS, Christman JW, et al. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223.
- Courtney J, Bradley J, Mccaughan J, et al. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:525–532.
- Nathwani D, Raman G, Sulham K, et al. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3:32.
- Salman M, Ali A, Haque A. A novel multiplex PCR for detection of Pseudomonas aeruginosa: a major cause of wound infections. Pak J Med Sci. 2013;29:957.
- Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892.
- Yasuda H, Ajiki Y, Koga T, et al. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob Agents Chemother. 1993;37:1749–1755.
- Japoni A, Farshad S, Alborzi A. Pseudomonas aeruginosa: burn infection, treatment and antibacterial resistance. Iran Red Crescent Med J. 2009;3:244–253.
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193.
- Rewatkar A, Wadher B. Staphylococcus aureus and Pseudomonas aeruginosa-biofilm formation methods. IOSR-JPBS. 2013;8:36–40.
- DeVos WM. Microbial biofilms and the human intestinal microbiome. NPJ Biofilms Microbiomes. 2015;1:15005.
- Shirtliff M, Leid JG. The role of biofilms in device-related infections. Vol. 2. Springer-Verlag Berlin Heidelberg: Springer; 2009. p. 1–44.
- Balzer M, Witt N, Flemming HC, et al. Faecal indicator bacteria in river biofilms. Water Sci Technol. 2010;61:1105–1111.
- Morgan-Sagastume F, Larsen P, Nielsen JL, et al. Characterization of the loosely attached fraction of activated sludge bacteria. Water Res. 2008;42:843–854.
- Percival SL, Malic S, Cruz H, et al. Introduction to biofilms. In: Percival SL, editor. Biofilms and veterinary medicine. Springer, Berlin, Heidelberg: Springer Press; 2011. p. 41–68.
- Marcinkiewicz J, Strus M, Pasich E. Antibiotic resistance: a “dark side” of biofilm associated chronic infections. Pol Arch Med Wewn. 2013;123:309–313.
- Sader HS, Farrell DJ, Castanheira M, et al. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011–12). J Antimicrob Chemother. 2014;69:2713–2722.
- Deacon J, Abdelghany SM, Quinn DJ, et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa. Microbiol Rev. 2002;15:194–222.
- Domingues MM, Inácio RG, Raimundo JM, et al. Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems. Biopolymers. 2012;98:338–344.
- Velkov T, Roberts KD, Thompson PE, et al. Polymyxins: a new hope in combating Gram-negative superbugs? Future Med Chem. 2016;8:1017–1025.
- Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168.
- de la Fuente-Núñez C, Reffuveille F, Mansour SC, et al. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015;22:196–205.
- Reffuveille F, de la Fuente-Núñez C, Mansour S, et al. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother. 2014;58:5363–5371.
- Ravishankar Rai V, Jamuna Bai A. Nanoparticles and their potential application as antimicrobials. Science against microbial pathogens,” communicating current research and technological advances. In: Méndez-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances. 2011, p. 197–209. FORMATEX 2011.
- Hussain JI, Kumar S, Hashmi AA, et al. Silver nanoparticles: preparation, characterization, and kinetics. AML. 2011;2:188–194.
- Slomberg DL, Lu Y, Broadnax AD, et al. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl Mater Interfaces. 2013;5:9322–9329.
- Su HL, Chou CC, Hung DJ, et al. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30:5979–5987.
- Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. Journal of Nanomaterials. 2015;16:53.
- Khan Z, Al-Thabaiti SA, Obaid AY, et al. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf B Biointerfaces. 2011;82:513–517.
- Ramalingam B, Parandhaman T, Das SK. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl Mater Interfaces. 2016;8:4963–4976.
- Raytekar NA, Choudhari MR, Das S. Antibiotic profiling of Pseudomonas aeruginosa isolates from pus sample of rural tertiary care hospital of Western Maharashtra, Loni, India. Int J Res Med Sci. 2017;5:3076–3081.
- Andhale J, Misra R, Gandham N, et al. Incidence of Pseudomonas aeruginosa with special reference to drug resistance and biofilm formation from clinical samples in tertiary care hospital. J Pharm Biomed Sci. 2016;6:387–391.
- Sharma S, Srivastava P. Resistance of antimicrobial in Pseudomonas aeruginosa. Intjcurrmicrobiolappsci. 2016;5:121–128.
- Abidi SH, Sherwani SK, Siddiqui TR, et al. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol. 2013;13:57.
- Wan G, Ruan L, Yin Y, et al. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. IJN. 2016;11:3789.
- Mohanty S, Mishra S, Jena P, et al. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8:916–924.
- Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 2013;60:523–530.
- Fayaz AM, Balaji K, Girilal M, et al. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6:103–109.