Abstract
Colorectal cancer (CRC) is one of the most common digestive cancers leading to deaths worldwide. In this study, we aimed to investigate the diagnostic value of miR-663 in CRC. The expression of miR-663 was detected by quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). The association between miR-663 and clinical parameters of subjects was evaluated by chi-square test. Additionally, ROC (receiver operating characteristic) analysis was performed to evaluate the diagnostic role of miR-663 in CRC. The expression of miR-663 in CRC patients was significantly upregulated compared with benign colorectal disease patients and healthy controls (p < .01). Besides, the expression of miR-663 was significantly associated with tumour differentiation, invasion, lymph node metastasis and TNM stage (p < .05). The cutoff value of miR-663 was 1.31, and the corresponding sensitivity and specificity were 83.1% and 73.8%, respectively. In ROC analysis, the area under the curve (AUC) was 0.806, which indicated that miR-663 could act as an independent diagnostic biomarker for CRC. In conclusion, miR-663 was up-regulated in CRC patients and may be an effective biomarker for CRC diagnosis.
Introduction
Colorectal cancer (CRC), also known as colon cancer, bowel cancer or rectal cancer, occurs in colon or rectum. It is one of the most common malignancies worldwide, with an estimated incidence of more than 1.2 million cases globally and 608,000 deaths annually [Citation1]. High death rate results from abnormal growth of cells, which have the ability to invade or spread to other parts of the body through blood and lymph systems. About 35% of CRC patients are often in stage IV when diagnosed, among whom the 5-year survival rate is less than 10% [Citation2,Citation3]. Thus, it is necessary to find out specific and sensitive biomarkers for early CRC diagnosis, thus providing the patients with timely treatment.
MicroRNAs (miRNAs), small and noncoding RNAs (20–22 nucleotides), exert regulatory influences on gene expression by targeting sequences located in the 3’-untranslated region of mRNAs, and by inhibiting the translation or degradation [Citation4–6]. Besides, miRNAs participate in cell proliferation, migration, differentiation and apoptosis [Citation7,Citation8]. Increasing evidence have displayed that miRNAs act as oncogenes or tumour suppressors in the pathogenesis of various cancers [Citation9]. So miRNAs showed great potential to function as cancer biomarkers.
Similarly, miR-663 may function as either oncogenes or tumour suppressors in malignant progression. It can serve as an oncogene in many cancers such as non-small cell lung cancer [Citation10], lung cancer [Citation11], prostate cancer [Citation12] and nasopharyngeal carcinoma [Citation13], while acting as a suppressor against gastric cancer [Citation14] and glioblastoma [Citation15]. Currently, few studies have focused on the diagnostic value of miR-663 in CRC.
In this study, we aimed to detect the expression level of miR-663 in CRC patients, its relationship with clinical characteristics, and its diagnostic value in the disease.
Materials and methods
Patients and sample collection
CRC patients were recruited who were diagnosed in the General Hospital of the PLA Rocket Force. The present study was authorized by the Ethics Committee of the above hospital. Each of the 126 CRC patients, benign colorectal disease patients and healthy individuals were enrolled in this study. CRC cases contained 81 females and 45 males with an average age of 59.02 ± 9.43. Benign colorectal disease group and healthy controls were frequency-matched with CRC group in age and gender. All subjects were Chinese Han population without blood relationship and had signed written informed consents before collecting samples.
After fasting for 8–10 h, 10 ml peripheral blood of each participant was extracted in the morning, and coagulated for 1 h at room temperature. The serum was then separated through centrifugation. Serum samples with low levels of haemolysis (haemoglobin, 0.1 g/l) were aliquoted and stored at −80 °C until use.
RNA extraction and quantitative real-time reverse transcriptase- polymerase chain reaction (qRT-PCR)
Total RNA was isolated from serum samples using the mirVana miRNA Isolation Kit (Ambion, Austin, TX). Then, 0.05 μg of total RNA was reverse-transcribed with Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The expression level of miR-663 was detected by qRT-PCR adopting Platinum SYBR Green aPCR SuperMix-UDG reagent (Invitrogen, Renfrew, Scotland) in the Applied Biosystems 7900 Fast Real-time PCR system under optimal conditions. Total RNA samples would be employed only when they reached an OD A260/A280 ratio close to 2.0, which indicated that RNA is pure. The relative expression level of miR-663 was normalized to U6 and calculated using the 2−ΔΔCt method.
Statistical analysis
Statistical analysis was conducted with SPSS version 13.0 (SPSS Inc., Chicago, IL) and Graphpad prism 5. The difference in the expression level of miR-663 was pairwise analyzed between CRC cases, colorectal adenomas patients and healthy controls through the t-test. The relationship between clinical factors and miR-663 expression was estimated by chi-square test. Receiver operating characteristic (ROC) curve was established to assess diagnostic value of miR-663 in CRC. p-values of less than .05 were considered statistically significant.
Results
Expression level of miR-663
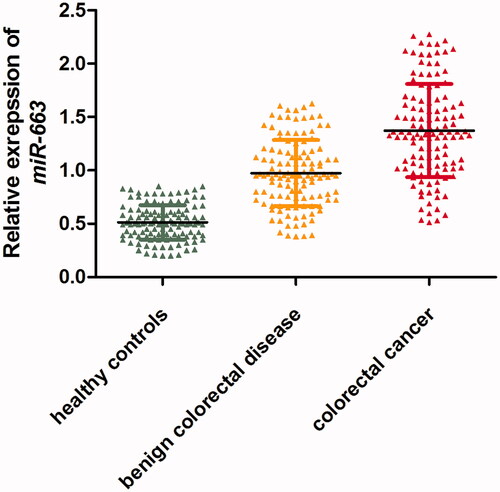
As shown in , the level of miR-663 was significantly higher in patients with CRC than in benign colorectal disease group and healthy controls (p < .01). Meanwhile, the level of miR-663 was higher in benign colorectal disease cases than in healthy controls (p < .01).
Relationship between miR-663 and clinicopathological characteristics of CRC
Clinical information on age, gender, differentiation, invasion, lymph node metastasis, and TNM stage was recorded in a questionnaire. As for the association of clinical factors with miR-663, the results were shown in . The outcome indicated that differentiation, invasion, lymph node metastasis, and TNM stage were significantly associated with the expression level of miR-663 (p = .021, .006, .005 and .001).
Table 1. The relationship between clinical factors and expression level of miR-663.
Diagnostic value of miR-663 in CRC
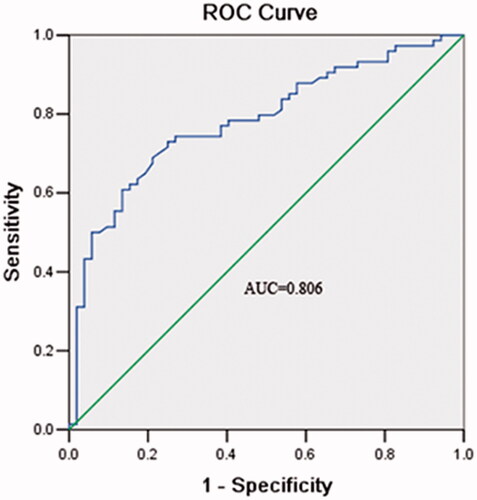
ROC curve was established to estimate the value of miR-663 in CRC diagnosis. The analysis suggested that the serum level of miR-663 was a potential biomarker in differentiating CRC patients from benign cases and healthy controls, with an area under curve (AUC) of 0.806. The cutoff value for miR-663 was 1.31, accompanied by a sensitivity and specificity of 83.1 and 73.8%, respectively ().
Discussion
CRC is one of the most frequent malignant neoplasms [Citation16] and the second leading cause of cancer-related deaths in developed countries [Citation17]. It is urgent to improve CRC diagnosis level for its prevention and better prognosis. Currently, the most common and efficient method in CRC diagnostics is endoscopy, but this invasive method brings pain and other adverse effects. Screening examination adopting tumor markers and intervention for early stages of CRC may significantly decrease the mortality rate of patients [Citation16]. Moreover, tumour markers in blood, urine and body [Citation16] are non-invasive and bring less pain than traditional diagnosis methods.
MiRNAs show great potential to act as effective biomarkers for various cancers. They present in human plasma in a stable form, which protects them from being digested due to endogenous RNase activity [Citation18]. Over the last decade, it has become clear that aberrant miRNA expression plays a functional role in the initiation and progression of CRC [Citation19]. To date, many miRNAs have been detected to be aberrantly expressed in CRC, such as miR-301 [Citation20], miR-182 [Citation21], miR-196b [Citation22], miR-21 [Citation23], miR-601 [Citation24], miR-760 [Citation24] and miR-29a [Citation25].
MiR-663 is a member of the miRNA family and also reported to be involved in many important pathological processes, especially in cancers. MiRNA-663 plays an important regulatory role in ovarian cancer and can promote the progression of ovarian cancer cells through targeting TUSC2 [Citation26]. Reportedly, miR-663 affected apoptosis by controlling mitochondrial outer membrane permeabilization (MOMP) through the expression of two novel direct targets PUMA/BBC3 and BTG2 in NSCLC, and acted as an oncogene in NSCLC [Citation27]. Besides, miR-663 can promote the proliferation of prostate cancer cells, and its expression was closely related to pathological grade and clinical stage of the malignancy, which suggested that it was possibly an effective predictor for the progression of prostate cancer [Citation28]. Studies have shown that miR-663 promoted the proliferation and cell cycle progression of nasopharyngeal carcinoma cells through directly targeting CDKN2A, suggesting that miR-663 may be an effective target in treating cancer [Citation29]. Therefore, miR-663 might be an important regulator during the development and progression of human cancers and may be a candidate biomarker for human cancers.
To the best of our knowledge, there has been no research on the expression level of miR-663 in CRC patients. Since miR-663 acts as a tumour promoter or suppressor in an organ-specific fashion [Citation13,Citation14,Citation30], its effects on the malignant progression of tumours are controversial [Citation31]. In the present study, the level of miR-663 was higher in CRC patients than in colorectal adenomas cases and healthy controls. The result indicated that miR-663 acted as a tumour promoter in CRC. Further exploration through ROC curve suggested that miR-663 held fine diagnostic value in CRC. It is worth mentioning that this was the first study showing the clinical value of the miR-663 expression in CRC.
In conclusion, our findings provided convincing evidence for the first time demonstrating the upregulation of miR-663, which might serve as a novel molecular marker for the diagnosis of CRC, and its expression level was influenced by clinical stages, infiltration degree and distant metastasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917.
- Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol. 2007;13:3806–3815.
- Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896–903.
- Baker AH, van Rooij E. miRNA overexpression induces cardiomyocyte proliferation in vivo. Mol Ther. 2013;21:497–498.
- Valencia-Sanchez MA, Liu J, Hannon GJ, et al. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524.
- Zhang Z, Zheng W, Hai J. MicroRNA-148b expression is decreased in hepatocellular carcinoma and associated with prognosis. Med Oncol. 2014;31:984.
- Wang L, Chang L, Li Z, et al. miR-99a and -99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol. 2014;31:934.
- Feng Y, Kang Y, He Y, et al. microRNA-99a acts as a tumor suppressor and is down-regulated in bladder cancer. BMC Urol. 2014;14:50.
- Cheng AM, Byrom MW, Shelton J, et al. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297.
- Zhang Y, Zhou X, Xu X, et al. Waltonitone induces apoptosis through mir-663-induced Bcl-2 downregulation in non-small cell lung cancer. Tumour Biol. 2015;36:871–876.
- Liu ZY, Zhang GL, Wang MM, et al. MicroRNA-663 targets TGFB1 and regulates lung cancer proliferation. Asian Pac J Cancer Prev. 2011;12:2819–2823.
- Jiao L, Deng Z, Xu C, et al. miR-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J Cell Physiol. 2014;229:834–844.
- Yi C, Wang Q, Wang L, et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene 2012;31:4421–4433.
- Pan J, Hu H, Zhou Z, et al. Tumor-suppressive mir-663 gene induces mitotic catastrophe growth arrest in human gastric cancer cells. Oncol Rep. 2010;24:105–112.
- Shi Y, Chen C, Zhang X, et al. Primate-specific miR-663 functions as a tumor suppressor by targeting PIK3CD and predicts the prognosis of human glioblastoma. Clin Cancer Res. 2014;20:1803–1813.
- Swiderska M, Choromańska B, Dąbrowska E, et al. The diagnostics of colorectal cancer. Contemp Oncol (Pozn). 2014;18:1–6.
- Zhang A, Sun H, Yan G, et al. Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett. 2014;345:17–20.
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
- Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–252.
- Zhang W, Zhang T, Jin R, et al. MicroRNA-301a promotes migration and invasion by targeting TGFBR2 in human colorectal cancer. J Exp Clin Cancer Res. 2014;33:780.
- Liu H, Du L, Wen Z, et al. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. Int J Colorectal Dis. 2013;28:697–703.
- Ge J, Chen Z, Li R, et al. Upregulation of microRNA-196a and microRNA-196b cooperatively correlate with aggressive progression and unfavorable prognosis in patients with colorectal cancer. Cancer Cell Int. 2014;14:128.
- Faltejskova P, Besse A, Sevcikova S, et al. Clinical correlations of miR-21 expression in colorectal cancer patients and effects of its inhibition on DLD1 colon cancer cells. Int J Colorectal Dis. 2012;27:1401–1408.
- Wang Q, Huang Z, Ni S, et al. Plasma miR-601 and miR-760 are novel biomarkers for the early detection of colorectal cancer. PLoS One. 2012;7:e44398.
- Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126.
- Xie HH, Huan WT, Han JQ, et al. MicroRNA-663 facilitates the growth, migration and invasion of ovarian cancer cell by inhibiting TUSC2. Biol Res. 2019;52:18.
- Fiori ME, Villanova L, Barbini C, et al. miR-663 sustains NSCLC by inhibiting mitochondrial outer membrane permeabilization (MOMP) through PUMA/BBC3 and BTG2. Cell Death Dis. 2018;9:49.
- Wang S, Liu J, Li C, et al. Research of the effect of miR-663 on the proliferation of prostate cancer cells, and the correlations of miR-663 with pathological grade and clinical stage. J Buon. 2017;22:1011–1016.
- Liang S, Zhang N, Deng Y, et al. miR-663 promotes NPC cell proliferation by directly targeting CDKN2A. Mol Med Rep. 2017;16:4863–4870.
- Hu H, Li S, Cui X, et al. The overexpression of hypomethylated miR-663 induces chemotherapy resistance in human breast cancer cells by targeting heparin sulfate proteoglycan 2 (HSPG2). J Biol Chem. 2013;288:10973–10985.
- Tili E, Michaille JJ, Adair B, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010;31:1561–1566.