Abstract
Objective
To investigate the regulatory mechanism of miR-204 on proliferation, apoptosis and autophagy of cervical cancer cells.
Methods
The expression of miR-204 in cervical cancer tissues and cell lines was detected by real-time PCR. Cell viability and apoptosis were detected by MTT and flow cytometry, respectively. Protein expression of Bcl-2, Bax, Caspase-3 and LC3I/II in C33A cells after transfection was detected by Western blot assay. The interaction between miR-204 and ATF2 was verified by Targetscan online prediction, dual-luciferase assay and Western blot.
Results
The expression of miR-204 was significantly down-regulated in cervical cancer tissues and cell lines (p < .05). Cell viability was suppressed while apoptosis was enhanced in C33A cells transfected with miR-204. In addition, overexpression of miR-204 reduced protein expression of Bcl-2 and LC3I/II and elevated protein expression of Bax and Caspase-3 in C33A cells (p < .05). The results of Targetscan online prediction, dual-luciferase assay and Western blot indicted that miR-204 could regulate the expression of ATF2. Cell viability was increased (p < .05) and the expression of ATF2 and LC3I/II was down-regulated (p < .05) in C33A cells after silencing of ATF2. Ectopic expression of ATF2 could restore miR-204 mediated inhibition on proliferation of C33A cells.
Conclusions
miR-204 could inhibit proliferation and autophagy and induce apoptosis of cervical cancer cells by targeting ATF2, representing promising target for cervical cancer treatment.
Introduction
Cervical cancer is a common gynaecological malignancy that seriously threatened women's health. There are about 530,000 new cases of cervical cancer every year, with the death toll of 270,000. It ranked the 4th cancer related death in female malignant tumours [Citation1]. According to statistics, about 85% of cervical cancer cases in the world occurred in underdeveloped or developing countries. In 2013, the number of new cases and deaths of cervical cancer in China was 100,000 and 26,000, respectively, and the age of onset was younger [Citation2]. Currently, surgery, chemotherapy and radiotherapy are the main treatments for cervical cancer. Although the overall survival rate has been improved to a certain extent, postoperative recurrence, lymphatic metastasis and toxic side effects on surrounding normal tissues vitiated the therapeutic outcomes. At present, the research of targeted therapy in the clinical treatment of cervical cancer has been widely concerned by medical workers [Citation3].
MicroRNA (microRNA) is an endogenous non-coding RNA molecule that widely exists in eukaryotes. It can complementary target mRNA 3′-UTR to inhibit or degrade its translation process, and regulate the expression of many genes at the post-transcriptional level [Citation4]. It has been reported that differentially expression of microRNAs are the cause of many cancers. As tumour suppressor or oncogene, microRNAs have become new therapeutic tools and targets that have broad clinical application prospects [Citation5]. Studies [Citation6,Citation7] have shown that the expression of miR-204 is down-regulated in breast cancer, gastric cancer and other tumour tissues, and it can regulate the proliferation and apoptosis of cancer cells by targeting related genes. In this study, we examined the expression of miR-204 in cervical cancer tissues and cell lines, and the effects of overexpression of miR-204 on the proliferation, apoptosis and autophagy of cervical cancer cells to explore the biological mechanism of miR-204 in the development of cervical cancer.
Materials and methods
Clinical sample
From March 2017 to June 2018, 31 patients with cervical cancer, aged 39–63 years old, who were diagnosed by pathology in the Fourth Affiliated Hospital of Harbin Medical University were selected. The tumour tissues and corresponding adjacent tissues (more than 5 cm away from the lesion) were removed surgically. All the tissues were quickly put into liquid nitrogen after excision and stored in the refrigerator at –80 °C. The experiment was approved by the Medical Ethics Committee. All patients did not receive radiotherapy, chemotherapy and other related treatment before surgery and signed informed consent.
Materials
End/E6E7 human endocervical cells, human cervical cancer cell lines CaSKi, Hela, C33A, HCC94 and SiHa were purchased from ATCC (Manassas, VA); foetal bovine serum, RPMI-1640 medium, trypsin, MTT and DMSO were purchased from Sigma (St. Louis, MO); Annexin V-FITC/PI apoptosis detection kit was purchased from Thermo Fisher Scientific Co., Ltd. (Waltham, MA); Lipofectamine 2000 transfection reagent was purchased from Invitrogen (Carlsbad, CA); miR-204 primer was purchased from TaqMan; Rabbit anti-human Bax polyclonal antibody, rabbit anti-human Bcl-2 polyclonal antibody, rabbit anti-human Caspase-3 polyclonal antibody, rabbit anti-human β-actin polyclonal antibody, rabbit anti-human ATF2 polyclonal antibody, rabbit anti-human LC3I/II polyclonal antibody was purchased from Proteintech Group (Rosemont, IL); control mimics, miR-204 mimics, control inhibitors, miR-204 inhibitors were purchased from Invitrogen Company (Carlsbad, CA); PVDF membrane, dual luciferase reporter gene detection kit, ECL luminescent liquid was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China); ATF2 wild type and mutant vector were purchased from Beijing Benyuan Zhengyang Gene Co., Ltd. (Beijing, China); cell culture incubator and microplate reader were purchased from Thermo Company (Waltham, MA); flow cytometer were purchased from Miltenyi Biotec Company (Bergisch Gladbach, Germany).
Cell culture and grouping
All the cells were cultured in RPMI-1640 medium containing 10% foetal bovine serum at 37 °C and 5% CO2 saturated humidity. The logarithmic phase cells were collected for qRT-PCR detection. C33A cells with confluence about 40% were collected, digested with trypsin and suspended with DMEM medium. The cells were seeded into 24-well plate with a density of 1 × 105 cells per well and cultured for 24 h. According to the instruction of Lipofectamine 2000, control mimics, miR-204 mimics, control inhibitors, miR-204 inhibitors, control siRNA, ATF2 siRNA, miR-204 mimics and control mimics, miR-204 mimics and ATF2 mimics were transfected into C33A cells and marked as miR-con group, miR-204 group, anti-con group, anti-miR-204 group, si-con group, si-ATF2 group, miR-204 + Ctrol group and miR-204 + ATF2 group.
Real-time PCR
Appropriate amount of cervical cancer tissues and adjacent tissues, End/E6E7, CaSKi, Hela, C33A/HCC94, SiHa cells and cells in NC groups, microRNA-con group and microRNA-204 group were collected. Total RNA was extracted by Trizol method. The RNA was reverse transcribed into cDNA according to the TaKaRa reverse transcription kit instructions, and the reaction system was prepared in the dark using the TaKaRa fluorescence quantification kit and subjected to PCR amplification. Each sample was repeated three times, and the relative expression of miR-204 was calculated by using the beta-actin as the internal reference and the F = 2–Delta Ct method. Beta-actin primer: upstream: 5′-TGGATCAGCAAGCAGCAGGAGTA-3′, downstream: 5′-TCGGCCACAT TGTGTGAACTTT-3′.
MTT assay for cell viability
The cells in the NC group, miR-con group, miR-204 group, si-con group, si-ATF2 group, miR-204 + Ctrol group and miR-204 + ATF2 group were digested and resuspended. Suitable amount of cell suspension was seeded on 96-well plate and cultured for 0 h, 24 h, 48 h, 72 h and 96 h at 37 °C 5% CO2. Then, 20 μL MTT (5 mg/mL) was added for 4 h. Afterward, 150 μL DMSO was added. The shock reaction was lasted for 10 min. The absorbance (A) at 490 nm was measured by microplate reader.
Apoptosis detection
The cells of NC group, microwave-con group, microwave-204 group, si-con group, si-ATF2 group, microwave-204 + Ctrol group and microwave-204 + ATF2 group were digested with trypsin and collected by centrifugation at 4 °C and 300×g after washing with PBS. Five microlitres of Annexin V-FITC and 10 μL of PI were sequentially added using Annexin V-FITC/PI apoptosis assay kit, gently mixed, incubated at room temperature for 15 min in the dark, and apoptosis was detected by flow cytometry.
Western blot
The cells of NC group, miR-con group, miR-204 group, anti-con group, anti-miR-204 group, si-con group, si-ATF2 group, miR-204 + Ctrol group and miR-204 + ATF2 group were subjected to ultrasonic pulverization, and RIPA lysate containing protease inhibitor was added and lysed on ice for 5 min, centrifuged at 4 °C, 12,000×g for 15 min, and the supernatant was collected. After taking protein samples for SDS-PAGE electrophoresis, the protein was transferred to PVDF membrane and blocked for 2 h by 5% skimmed milk powder. Rabbit anti-human Bax polyclonal antibody (1:5000), rabbit anti-human Bcl-2 polyclonal antibody (1:1000), rabbit anti-human Caspase-3 polyclonal antibody (1:500), rabbit anti-human LC3I/II polyclonal antibody (1:1000), rabbit anti-human ATF2 polyclonal antibody (1:500) and rabbit anti-human beta-actin polyclonal antibody (1:1000) were added and incubated at 4 °C overnight. The membrane was rinsed with PBST for three times, and incubated with HRP labelled secondary antibody was for 2 h at room temperature. After rinsing with PBST for three times (10 min/time), the ECL kit was illuminated and each sample was repeated three times.
Dual luciferase reporter gene assay
Targetscan online prediction found that miR-204 binding sites existed on ATF2 3′-UTR. To further verify whether ATF2 is a target gene for miR-204, wild-type ATF2 3′-UTR-WT (containing ATF2 3′-UTR fragment) and mutant ATF2 3′-UTR-MUT (mutant of ATF2 3′-UTR fragment) vectors were co-transfected into C33A cells with control mimics and miR-204 mimics according to Lipofectamine 2000 instructions. The activity of luciferase was detected 48 h after culture, and relative fluorescence intensity = firefly fluorescence intensity/sea kidney fluorescence intensity.
Statistical analysis
Data analysis was performed using GraphPad Prism 7 (La Jolla, CA, USA) and SPSS 19.0 (Chicago, IL, USA). The experimental data were expressed as mean standard deviation (x ± s). The data were compared between the two groups by t test. The data among groups were compared by one-way ANOVA. p < .05 was statistically significant.
Results
Expression of miR-204 in cervical cancer tissues and cells
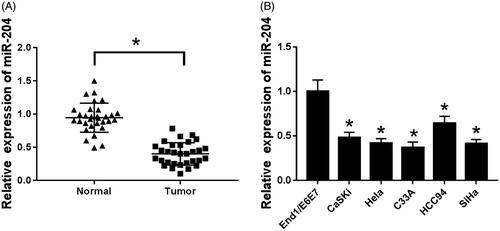
By detecting the expression of miR-204 in cervical cancer tissues and their cell lines, the expression of miR-204 was significantly down-regulated in cervical cancer tissues and their cell lines compared with normal cervical tissues and End/E6E7 human endometrial cells (p < .05). Among the cell lines used in the experiment, the expression level of miR-204 was the lowest in C33A cells, therefore, C33A cells were selected for subsequent experiments ().
Overexpression of miR-204 inhibits proliferation of C33A cells
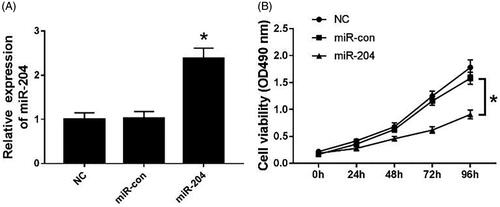
MiR-204 mimics was transfected into C33A cells, and cell viability was measured by MTT assay. The results are shown in . The expression level of miR-204 in the miR-204 group was significantly higher than that in the NC group and the miR-con group (p < .05) (). Besides, cell viability was suppressed in C33A cells transfected with miR-204 mimics compared with the NC group and the miR-con group (p < .05) ().
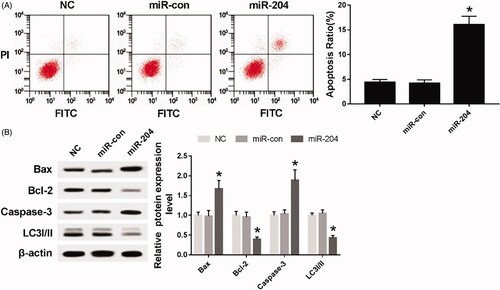
Overexpression of miR-204 inhibits autophagy and induces apoptosis in C33A cells
As can be seen from , compared with NC group and the miR-con group, the apoptosis rate was significantly increased after transfection of miR-204 mimics to C33A cells (p < .05). The expressions of apoptosis-related proteins Bax and Caspase-3 were up-regulated (p < .05), while the expression of apoptosis-related protein Bcl-2 and autophagy-related protein LC3I/II were down-regulated (p < .05).
Figure 3. Overexpression of miR-204 inhibits autophagy and induces apoptosis in C33A cells. (A) The detection of apoptosis rate in each group; (B) the expression of apoptosis-related proteins Bcl-2, Bax, Caspase-3 and autophagy-related protein LC3I/II in each group. Compared with NC group and miR-con group, *p < .05.
MiR-204 targets and regulates ATF2 expression
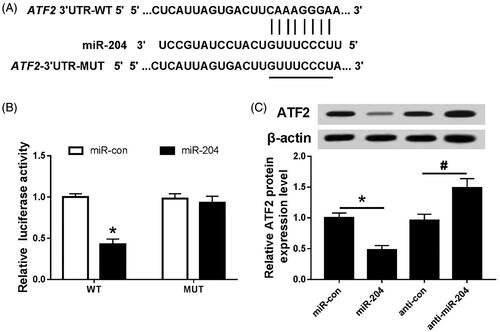
The binding site of miR-204 was found on the ATF2 3′-UTR by Targetscan prediction (); the dual luciferase reporter system and Western blot assay were used to further verify the targeting relationship between miR-204 and ATF2. The results are shown in : the luciferase activity of the cells was decreased after co-transfection of miR-204 mimics with ATF2 3′-UTR wild-type plasmid (p < .05). However, there was no significant change in luciferase activity after co-transfection with ATF2 3′-UTR mutant plasmid. Moreover, up-regulation of miR-204 inhibited ATF2 protein expression (p < .05), and down-regulation of miR-204 promoted the expression of ATF2 protein (p < .05).
Effect of silencing ATF2 on proliferation, autophagy and apoptosis of C33A cells
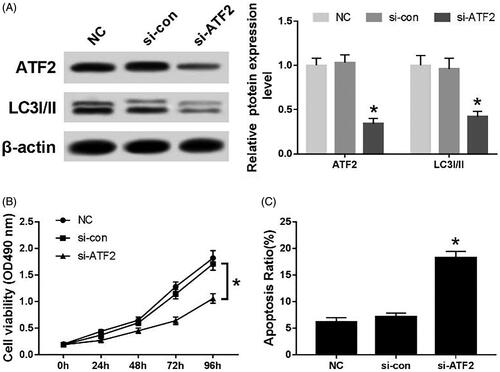
After transfection of ATF2 siRNA into C33A cells, the expression of ATF2 and LC3I/II protein was down-regulated (p < .05) (). The cell viability of si-ATF2 transfection group was significantly lower than that of the NC group and the si-con group (p < .05) (). Conversely, the apoptotic rate was significantly higher in si-ATF2 transfection group than that of the NC group and the si-con group (p < .05) ().
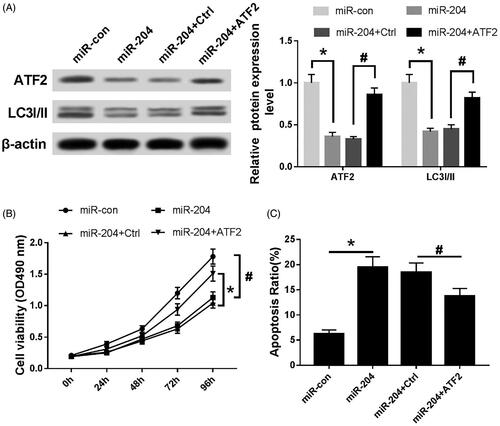
Effects of miR-204 and ATF2 on proliferation, autophagy and apoptosis of C33A cells
As can be seen from , the expression of ATF2 and LC3I/II was down-regulated (p < .05), the cell viability was significantly decreased (p < .05), and the apoptosis rate was significantly increased (p < .05) after transfection of miR-204 mimics into C33A; after co-transfection of miR-204 mimics and ATF-2 into C33A cells, the expression of ATF-2 and LC-3I/II protein and cell viability were increased to some extent (p < .05), and the apoptosis rate was decreased (p < .05).
Figure 6. Effects of miR-204 and ATF2 on proliferation, autophagy and apoptosis of C33A cells. (A) The expression of ATF2 LC3I/II protein of each group, (B) MTT assay was utilized to detect cell viability and (C) the apoptosis rate of each group was evaluated by flow cytometry. Compared with miR-con group, *p < .05; compared with miR-204 + Ctrl group, #p < .05.
Discussion
The incidence and mortality of cervical cancer in developed countries such as the United States have decreased to a certain extent, while in urban areas of China, the incidence of cervical cancer is on the rise [Citation8,Citation9]. The occurrence of cervical cancer is related to various factors such as public health, residential area, education level and human papillomavirus (HPV) vaccine coverage [Citation10–12]. Among them, HPV infection is a major risk factor for cervical cancer. Most HPV infections are transient, and the body will spontaneously clear. In some cases, persistent infection will lead to the formation of intraepithelial neoplasia, which eventually leads to cervical cancer [Citation13]. HPV vaccination and early screening can effectively reduce the incidence of cervical cancer, but because the early symptoms of cervical cancer are not obvious, most patients are in the late stage of treatment and difficult to cure [Citation14].
MicroRNA plays an indispensable role in biological processes such as cell cycle, neuronal differentiation, immune regulation and cell metabolism, and is abnormally expressed in various human lesion tissues, which is closely related to the occurrence and development of diseases [Citation15]. A number of studies [Citation16,Citation17] have confirmed that microRNAs are abnormally expressed in a variety of human tumour tissues and participate in the development of tumours, such as: miR-320 was down-regulated in colorectal cancer tissues and microRNAs-222 was up-regulated in liver cancer tissues, and they were related to cancer cell growth, lymph node metastasis, tumour stage and prognosis. MiR-204 is located on human chromosome 9 and can regulate cell proliferation and differentiation through specific binding with target genes. There was evidence [Citation18,Citation19] that the expression of miR-204 was down-regulated in thyroid and gastric cancer tissues, and overexpression of miR-204 can inhibit cell proliferation and metastasis. In cervical cancer, it has been reported that the expression of miR-204 in primary cervical cancer tissue was significantly down-regulated, and its ectopic expression can inhibit the migration and invasion of cancer cells in vitro. Real-time PCR showed that the expression of microRNA204 was significantly down-regulated in 31 cervical cancer tissues and cell lines (p < .05), which was consistent with the above conclusion [Citation20]. Overexpression of miR-204 in C33A cells inhibits cell proliferation and autophagy and induces apoptosis. Targetscan online prediction, double luciferase reporter gene and Western blot experiments showed that microRNAs-204 could target ATF2 expression.
Activating transcription factor 2 (ATF2) belongs to the alkaline leucine zipper transcription factor family. ATF2 is localized on human chromosome 2q32, plays an inhibitory or promoting role in tumours and participates in cell stress, DNA damage and other processes [Citation21]. Activation of ATF2 is mainly through phosphorylation modification after stress stimulation. Phosphorylated ATF2 binds to specific sequence of promoter region of target gene such as cell cycle molecule, adhesion molecule and apoptosis-related molecule, thereby activating target gene expression. Wu et al. [Citation22] found that ATF2 in renal cell carcinoma can regulate the expression of cell cycle protein B1, cell cycle protein D1, vimentin expression, thereby regulating cell proliferation and metastasis. In addition, previous studies [Citation23] have confirmed that miR-204 can specifically inhibit the expression of ATF2 protein and regulate the proliferation, migration and invasion of human brain keratinocytoma tumour cells. In this study, after transfecting ATF2 siRNA into C33A cells, the expression level of autophagy-related protein LC3I/II was significantly decreased (p < .05), cell viability decreased (p < .05) and apoptosis rate increased (p < .05). When miR-204 mimics and ATF2 siRNA were co-transfected into C33A cells, the inhibitory effect of miR-204 on proliferation and autophagy of C33A cells and its promotion of apoptosis were attenuated.
In conclusion, the expression of miR-204 is low in cervical cancer tissues and cells, and it can inhibit the proliferation and autophagy of cervical cancer cells and induce apoptosis by targeting ATF2. This study is expected to provide a new target for early screening and clinical treatment of cervical cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Small W Jr, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer. 2017;123:2404–2412.
- Song B, Ding C, Chen W, et al. Incidence and mortality of cervical cancer in China, 2013. Chin J Cancer Res. 2017;29:471–476.
- Crafton SM, Salani R. Beyond chemotherapy: an overview and review of targeted therapy in cervical cancer. Clin Ther. 2016;38:449–458.
- Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med. 2016;14:143–153.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222.
- Wang X, Qiu W, Zhang G, et al. MicroRNA-204 targets JAK2 in breast cancer and induces cell apoptosis through the STAT3/BCl-2/survivin pathway. Int J Clin Exp Pathol. 2015;8:5017–5025.
- Zhang B, Yin Y, Hu Y, et al. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med Oncol. 2015;32:331–338.
- Lewis DR, Chen HS, Cockburn MG, et al. Early estimates of SEER cancer incidence, 2014. Cancer. 2017;123:2524–2534.
- Wang J, Bai Z, Wang Z, et al. Comparison of secular trends in cervical cancer mortality in China and the United States: an age-period-cohort analysis. Int J Environ Res Public Health. 2016;13:1–16.
- Melan K, Janky E, Macni J, et al. Epidemiology and survival of cervical cancer in the French West-Indies: data from the Martinique Cancer Registry (2002–2011). Glob Health Action. 2017;10:1–8.
- Vincerževskienė I, Jasilionis D, Austys D, et al. Education predicts cervical cancer survival: a Lithuanian cohort study. Eur J Public Health. 2017;27:421–424.
- Lowy DR. HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions. J Clin Invest. 2016;126:5–11.
- Wardak S. Human papillomavirus (HPV) and cervical cancer. Med Dosw Mikrobiol. 2016;68:73–84.
- Yang DY, Bracken K. Update on the new 9-valent vaccine for human papillomavirus prevention. Can Fam Physician. 2016;62:399–402.
- Christopher AF, Kaur RP, Kaur G, et al. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7:68–74.
- Vishnubalaji R, Hamam R, Yue S, et al. MicroRNA-320 suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1. Oncotarget. 2016;7:35789–35802.
- Liu Z, Sun J, Liu B, et al. miRNA-222 promotes liver cancer cell proliferation, migration and invasion and inhibits apoptosis by targeting BBC3. Int J Mol Med. 2018;42:141–148.
- Wu ZY, Wang SM, Chen ZH, et al. MiR-204 regulates HMGA2 expression and inhibits cell proliferation in human thyroid cancer. Cancer Biomark. 2015;15:535–542.
- Liu Z, Long J, Du R, et al. miR-204 regulates the EMT by targeting snai1 to suppress the invasion and migration of gastric cancer. Tumor Biol. 2016;37:1–9.
- Shu L, Zhang Z, Cai Y. MicroRNA-204 inhibits cell migration and invasion in human cervical cancer by regulating transcription factor 12. Oncol Lett. 2018;15:161–166.
- Bollmann A, Husser D. ATF2: a transcription factor that elicits oncogenic or tumor suppressor activities. Cell Cycle. 2008;7:2341–2345.
- Wu DS, Chen C, Wu ZJ, et al. ATF2 predicts poor prognosis and promotes malignant phenotypes in renal cell carcinoma. J Exp Clin Cancer Res. 2016;35:1–11.
- Song S, Fajol A, Tu X, et al. miR-204 suppresses the development and progression of human glioblastoma by targeting ATF2. Oncotarget. 2016;7:70058–70065.