Abstract
Puerarin has been reported to be useful in protection against hypoxia-induced injury. In our current study, we attempted to explore the protective effects of puerarin against hypoxia-caused damages in neural stem cells (NSCs). Additionally, the relative molecular underpinning studies preliminarily proceeded. NSCs were pre-incubated with puerarin before the hypoxic stimulus. MicroRNA-214 (miR-214) inhibitor was transfected into NSCs. Subsequently, the viability of NSCs was assessed by CCK-8 assay. Flow cytometry was employed to detect apoptotic cells after staining. qRT-PCR was performed to quantify miR-214. Western blot was applied for analyzing the expression of apoptosis-relative proteins and regulators. We found that puerarin alleviated hypoxia-induced apoptosis and maintained cell viability. Hypoxia-evoked up-regulation of miR-214 was further enhanced by puerarin. By contrast, miR-214-deficient NSCs showed the reduction in cell viability and the facilitation in apoptosis progress after pre-treatment with puerarin and stimulation in a hypoxia circumstance. Additionally, puerarin restored the phosphorylation of relative regulators, which was originally blunted by hypoxia. However, puerarin did not evidently restore the phosphorylation for response to hypoxia in miR-214-silenced NSCs. In conclusion, puerarin might be applied as a novel agent to ameliorate hypoxia-evoked damages in NSCs. Molecularly, miR-214 might be implicated in the protective roles of puerarin.
Keywords:
Introduction
Hypoxia in newborns contributes to brain damage, which usually leads to neuro-degeneration or neurodevelopmental disabilities and even neonatal deaths [Citation1,Citation2]. In addition to hypothermia, which has been reported to be clinically effective [Citation3], exploration of other therapeutic strategies have been performed to prevent hypoxia-induced brain injury, for instance, employment of pharmacological and hypoxic precondition [Citation4]. Particularly, the anti-hypoxic role of several natural compounds extracted from traditional Chinese medicine has been validated in rat neural stem cells [Citation5,Citation6].
Puerarin is a bioactive isoflavone-C-glucoside (its chemical structure is shown in ), extracted from the dried root of Pueraria labata (Willd.) Ohwi. It has been demonstrated that puerarin exerts a neuroprotective function against cerebral ischemia by prevention of apoptosis [Citation7]. Furthermore, studies have proved that the beneficial effects of puerarin against apoptosis stimulated by environmental insults rely on its anti-inflammatory and anti-oxidative functions [Citation8,Citation9]. Above-mentioned findings prompted us to further probe whether puerarin prevented neural stem cells (NSCs) from hypoxia-induced apoptosis and cell viability loss, since its anti-hypoxic effects on NSCs are still not completely defined. Further, it should be noted that puerarin can mediate the accumulation or suppression of specific mRNAs by regulating microRNAs (miRNA/miR) [Citation10–12]. These miRNAs coding for critical enzymes or signalling regulators are involved in proliferation, differentiation, and apoptosis [Citation10–12]. As a consequence, we considered that puerarin might exhibit an activity through the modulation of miRNA in neural stem cells.
Figure 1. The chemical structure of puerarin (daidzein-8-glucoside). Molecular formula: C21H20O9; Molecular weight: 416.38.
MicroRNA-214 (miR-214) has been reported to play multiple disparate biological functions, such as differentiation [Citation13] and apoptosis [Citation14]. A growing body of studies has confirmed a connection between miR-214 and hypoxia stimuli in these biological progresses [Citation15,Citation16]. Recent studies found miR-214 exhibits a cardio-protective effect in response to hypoxia [Citation17]. Intriguingly, isoflurane-triggered down-regulation of miR-214 leads to an enhanced level of Bax which is identified as a prominent target of miR-214 [Citation18]. Of note, indirect up-regulation of miR-214 contributes to the protective effect on myocardial ischemia/reperfusion lesions [Citation19]. In fact, to date, the available information is limited and does not necessarily imply an unequivocal causal role of puerarin in miR-214. Hence, we investigated whether miR-214 functions as a mediator of puerarin in conferring cytoprotection against hypoxia-induced NSCs injury.
In the present study, we verified that hypoxia-caused reduction in cell viability and exacerbation in apoptosis progress were ameliorated by puerarin pretreatment in NSCs. Additionally, we confirmed that miR-214 functioned as a crucial mediator of puerarin in alleviating hypoxia-induced damages in NSCs. In mechanism, our results implied a critical role of miR-214 in triggering critical signalling transduction cascades, which might be of significance in impeding hypoxia-mediated NSCs injury after pre-incubation with puerarin.
Materials and methods
NSCs collection and treatment
All animal experiments were performed with the approval from the Experimental Animal Care Committee of Linyi Women and Children’s Hospital. NSCs were derived from the hippocampus of E14 rats. Briefly, after terminal anaesthesia, the hippocampus part was immediately dissected, digested with 0.125% trypsin (Pierce, Appleton, WI), and dissociated into single-cell suspensions. The cell suspensions were plated into flasks at a density of 1 × 104 cells/mL with NSCs culture medium, Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F12 (DMEM/F12; 1:1; Sigma-Aldrich, St. Louis, MO) containing 2% B27 (Invitrogen, Carlsbad, CA), 10 ng/mL basic fibroblast growth factor (bFGF; Gibco, Gaithersburg, MD), 100 units/mL penicillin (Gibco) and 100 μg/mL streptomycin (Gibco). Subsequently, NSCs were incubated in a humidified incubator (STEMCEL, Vancouver, BC, Canada) containing 95% air and 5% (v/v) CO2 at 37 °C. Neurospheres were divided into single-cell suspensions and seeded in 96-well plates (1–2 cells per well). The subclonal neurospheres were then digested and passaged again. NSCs were passaged three times to obtain neurospheres. After digestion of the neurospheres, the NSCs were seeded into multi-well plates (5 × 105 cells per mL) for the downstream experimentation. For hypoxia treatment, cell cultures were incubated in a hypoxia chamber filled with an anaerobic gas mixture of 94% N2, 5% CO2, and 1% O2 for 8 h. NSCs were exposed to various concentrations of puerarin (20–100 μM) for 24 h before hypoxia treatment. The puerarin with a purity of 99% was purchased from Sigma-Aldrich.
Cell viability
NSCs were incubated in 96-well plate with a density of 5 × 103 cells per well. To assess cell viability, cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto, Japan) was used in our study. After pre-treatment and stimulation, CCK-8 solution was added to the culture medium and subsequently incubated for 1 h at 37 °C. Then, the absorbance was measured at 450 nm using Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific, Waltham, MA).
Apoptosis assay
Apoptotic cells were observed by flow cytometry after staining. After pre-incubation and stimulation, NSCs were washed with phosphate buffered saline (PBS; Sigma-Aldrich). Next, NSCs were fixed in 70% ethanol. Fixed NSCs were washed twice in PBS, stained in propidium iodide/fluorescein isothiocyanate (PI/FITC)-Annexin V containing 50 μg/mL RNase A (Sigma-Aldrich) and subsequently incubated for 1 h at room temperature in the dark. Apoptotic cells were analyzed with Synergy LX Multi-Mode Reader (BioTek, Winooski, VT).
Quantitative reverse transcription PCR (qRT-PCR)
Trizol reagent (Life Technologies, Carlsbad, CA) was used to extract the total RNA from cells according to the manufacturer’s instructions. The expression of miR-214 was detected with Taqman MicroRNA Reverse Transcription Kit and Taqman Universal Master Mix II with the TaqMan MicroRNA Assay of miR-214 (Applied Biosystems, Foster City, CA). U6 served as a housekeeping gene.
Transfection
To enforce down-regulation of miR-214, miR-214 inhibitor (GenePharma, Shanghai, China) was exploited with NC inhibitor as a negative control. In short, NSCs was grown in 96-well plate in a density of 40,000 cells per well. Then the culture was supplemented with 15 pmol miR-214 inhibitors. Lipofectamine 3000 (Invitrogen) was applied as transfection reagent following the manufacturer’s protocol. The transfection efficiency was identified by qRT-PCR.
Western blotting
Proteins from NSCs were extracted with RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors (Roche Applied Science, Indianapolis). The protein concentration was quantified by BCATM Protein Assay kit (Pierce, Appleton, WI) according to the manufacturer’s instructions. The proteins were separated by sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA) on a transfer system (Bio-Rad, Hercules, CA). The membrane was probed with specific antibodies against caspase-3 (Catalog No. C2087-22A; 1:1000) (USBiological, Salem, Massachusetts), caspase-9 (C2088-13B; 1:1000) (USBiological), phosphotylinosital 3 kinase (PI3K) (orb395437; 1:1000) (Biorbyt, Cambridge, UK), phospho (p) Tyr458-PI3K (orb106105, 1:1000) (Biorbyt), protein kinase B (AKT) (3247; 1 μg/mL) (BioVision, Milpitas, CA), pSer473-AKT (3257; 1 μg/mL) (BioVision), mitogen-activated protein kinase (MEK) and pSer217/221-MEK (M2865-02; 1:1000) (USBiological), extracellular signal-regulated protein kinase (ERK) (5AD13MA, 5 µg/mL) (Invitrogen), pThr202/Tyr204-ERK (PA5-37828, 1:1000) (Invitrogen), and β-actin (4970, 1:1000) (Cell Signalling Technology, CST, Beverly, MA) for overnight at 4 °C. The primary antibodies were prepared with 5% bull serum albumin (BSA; Millipore) following which, the membrane was incubated with the secondary antibody (7074, 1:5000) (CST) for 1 h at room temperature. The PVDF membrane was transferred into a Bio-Rad ChemiDoc XRS system for visualizing the protein bands. The signalling intensity of the protein band was quantified with Image Lab Software (Bio-Rad, Hercules, CA).
Statistical analysis
Statistical analyses were conducted with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). Data were presented as means ± standard deviation (SD). For comparisons between two groups, unpaired or paired two-tailed Student’s t-test was performed. As for multiple comparisons, one-way analysis of variance (ANOVA) was conducted. The results were statistically significant when P-values were less than .05.
Results
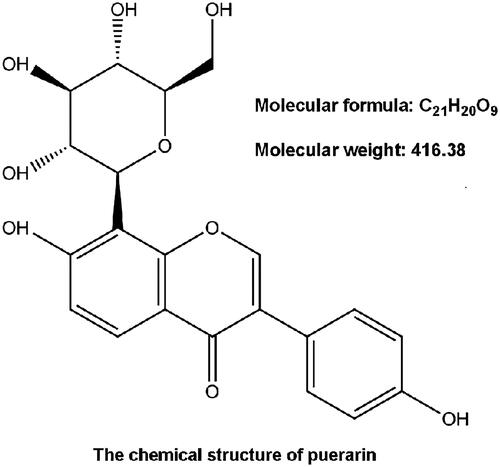
Hypoxia weakened cell viability and induced apoptosis in NSCs
To validate the protective effects of puerarin, we constructed hypoxia-caused injury model in NSCs. Our results showed that the cell viability of NSCs was remarkably (P < .01) decreased by hypoxia (). Besides, apoptosis progress was notably (P < .001) fortified by hypoxia relative to normal control (), with the cleavage of caspase-3 (P < .001) and caspase-9 (P < .001) (). Consequently, we smoothly established the hypoxia-induced insult model in NSCs.
Figure 2. Hypoxia treatment weakened cell viability while facilitating apoptosis progress in NSCs. (A) Cell viability was determined by CCK-8 assay. (B) Apoptotic cells were observed by flow cytometry after stained by Annexin V-FITC/PI. (C) The specificity of antibodies against the indicated proteins was shown by immunoblotting assay. Protein expression was relatively quantified with densitometry with reference to β-actin. NSCs were stimulated with hypoxia for 8 h. Bars were means ± SD of triplicate experiments. **P < .01, ***P < .001. CCK-8: cell counting kit-8; PI/FITC: propidium iodide/fluorescein isothiocyanate; NSCs: neural stem cells; SD: standard deviation.
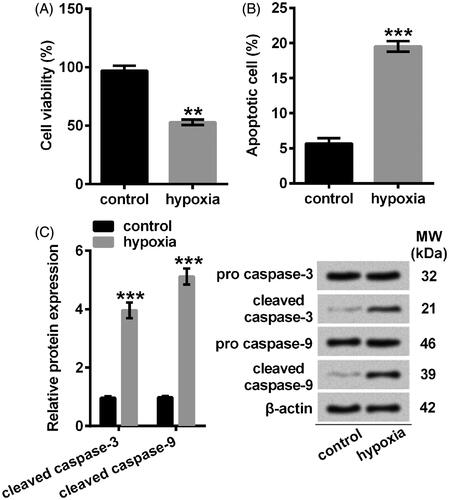
Figure 3. Puerarin maintained cell viability and retarded apoptosis in NSCs against hypoxia. (A) Cell viability was examined by CCK-8 assay. NSCs were pre-incubated with different concentrations of puerarin (20–100 μM) for 24 h. (B) Cell viability was assessed by CCK-8. (C) Apoptotic cells were detected with flow cytometry after staining. (D) Representative Western blotting of total cell lysates immune-blotted and probed with antibodies against the indicated proteins. Protein level was relatively quantified with densitometry with reference to β-actin. NSCs were pretreated with puerarin (60 μM) for 24 h then stimulated with hypoxia for 8 h. Bars were means ± SD of triplicate experiments. nsP > .05, *P < .05, **P < .01 or ***P < .001. CCK-8: cell counting kit-8; PI/FITC: propidium iodide/fluorescein isothiocyanate; NSCs: neural stem cells; SD: standard deviation.
Puerarin alleviated hypoxia-induced decrease in cell viability and acceleration in apoptosis
Next, NSCs were pre-incubated with puerarin before stimulated in hypoxia condition. The results indicated that cell viability was obviously (P < .05) mitigated by puerarin at higher concentrations (80 and 100 μM) while not significantly (P > .05) altered by puerarin at lower concentrations (20–60 μM) relative to control group (). As a consequence, we pre-treated NSCs with 60 μM puerarin in the following studies. To further investigate the protective effects of puerarin against hypoxia-caused insults, NSCs were pre-administrated with puerarin before treated by hypoxia. Our results showed that puerarin distinctly (P < .05) restored the viability of NSCs which was inhibited by hypoxia (). As for apoptosis, its anti-apoptotic functions were observed when NSCs were pretreated with puerarin before stimulated with hypoxia (P < .05) (). Specifically, puerarin definitely suppressed the expression of cleaved caspase-3 and cleaved caspase-9 (P < .01) (). These results indicated that puerarin exerted a protective effect against hypoxia-induced damages in NSCs.
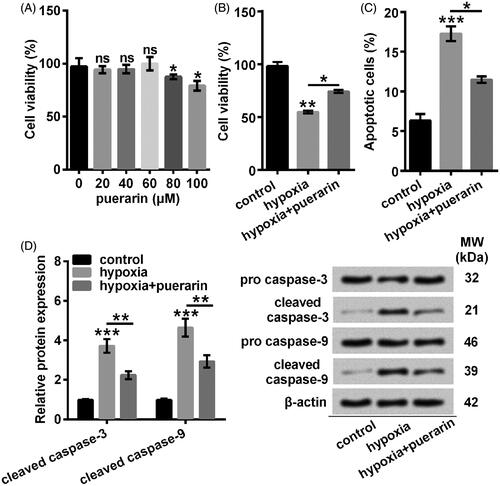
Puerarin further enhanced the expression of miR-214 induced by hypoxia
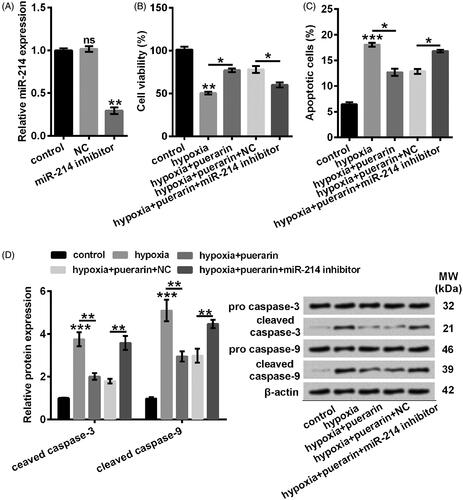
A functional study has indicated that hypoxia drives the expression of miR-214 to modulate a metabolic shift in the heart [Citation20]. Consistently, we found that miR-214 was markedly augmented by hypoxia relative to the control group (P < .05) (). As expected, after pre-incubating with 60 μM puerarin, NSCs exhibited an elevated level of miR-214 (P < .05) (). Consequently, we considered that puerarin further strengthened the expression of miR-214 in the NSCs stimulated with hypoxia.
Figure 4. Hypoxia-induced miR-210 was further up-regulated by puerarin. The expression of miR-214 was quantified by qRT-PCR. NSCs were pretreated with puerarin (60 μM) for 24 h and then stimulated with hypoxia for 8 h. Bars were means ± SD of triplicate experiments. *P < .05. microRNA-214: miR-214; qRT-PCR: quantitative reverse transcription PCR; NSCs: neural stem cells; SD: standard deviation.
miR-214 participated in the protective effects of puerarin in hypoxia-treated NSCs
To address whether miR-214 was associated with the protective effects of puerarin, we established the miR-214-deficient NSCs by transfection with miR-214 inhibitor. As shown in , the expression of miR-214 was efficaciously (P < .01) mitigated by miR-214 inhibitor in NSCs (). Additionally, our results showed that the viability was considerably inhibited in miR-214-silenced NSCs although these cells were pre-incubated with puerarin before stimulated in hypoxia condition, relative to its corresponding negative control (P < .05) (). What’s more, our results indicated that miR-214 inhibitor dramatically reversed the suppressive effects of puerarin on the apoptotic progress (P < .05) (). Besides, puerarin-caused decrease in cleaved caspase-3 and cleaved caspase-9 was notably abolished by miR-214 inhibitor (P < .01) (). Conclusively, puerarin might protect NSCs against hypoxia-evoked damages by up-regulating miR-214.
Figure 5. miR-214 inhibitor reversed the protective effects of puerarin against hypoxia-caused decrease of cell viability and facilitation of apoptosis. (A) qRT-PCR was conducted to detect miR-214. NSCs were transfected with miR-214 inhibitor. (B) Cell viability was detected with CCK-8 assay. (C) Apoptotic cells were observed by flow cytometry after staining. (D) Representative Western blotting of total cell lysates immune-blotted and probed with antibodies against the indicated proteins. Protein level was relatively quantified with densitometry with reference to β-actin. NSCs were transfected with miR-214 inhibitor and then pre-treated with puerarin (60 μM) for 24 h before stimulated with hypoxia for 8 h. nsP > .05, *P < .05, **P < .01 or ***P < .001. NC: negative control; miR-214: microRNA-214; qRT-PCR: quantitative reverse transcription PCR; CCK-8: cell counting kit-8; PI/FITC: propidium iodide/fluorescein isothiocyanate; NSCs: neural stem cells; SD: standard deviation.
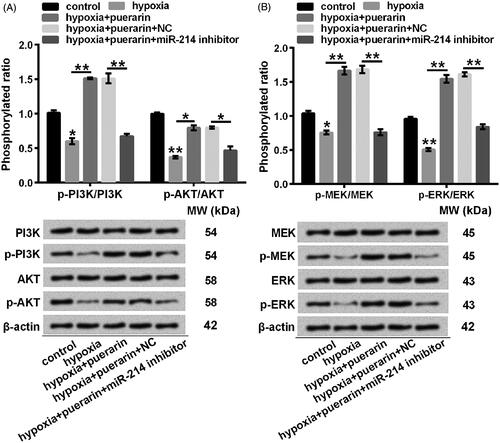
Puerarin might stimulate the activation of PI3K/AKT and MEK/ERK pathways by enhancing miR-214
To explore the plausible mechanisms, changes in phosphorylated expression of associated-regulators were analyzed using Western blotting. The phosphorylation of PI3K and AKT was extremely abated by hypoxia (P < .05 or P < .01), while puerarin distinctly promoted the phosphorylation of PI3K Tyr458 (P < .01) and AKT Ser473 (P < .05). However, the phosphorylated expression of PI3K Tyr458 (P < .01) and AKT Ser473 (P < .05) was abrogated in miR-214 null NSCs (). Analogously, pSer217/221 MEK (P < .05) and pThr202/Tyr204 ERK (P < .01) was remarkably repressed by hypoxia relative to the control. Nevertheless, puerarin conversely aggrandized the phosphorylation of MEK Ser217/221 (P < .01) and ERK Thr202/Tyr204 (P < .01). Moreover, the phosphorylated expression of MEK Ser217/221 (P < .01) and ERK Thr202/Tyr204 (P < .01) was suppressed in miR-214-deficient NSCs, which were pre-treated with puerarin before stimulated by hypoxia (). Summarily, the activation of PI3K/AKT and MEK/ERK signalling pathways might be driven by puerarin by enhancing miR-214.
Figure 6. Puerarin might trigger PI3K/AKT and MEK/ERK signalling pathways by up-regulating miR-214 in hypoxia-treated NSCs. Representative western blotting of total cell lysates immune-blotted and probed with antibodies against (A) p-PI3K, PI3K, p-AKT, AKT, (B) p-MEK, MEK, p-ERK, and ERK. Protein expression was relatively quantified with densitometry with reference to β-actin. NSCs were transfected with miR-214 inhibitor and then pre-treated with puerarin (60 μM) for 24 h before stimulated with hypoxia for 8 h. nsP > .05, *P < .05 or **P < .01. NC: negative control; miR-214: microRNA-214; qRT-PCR: quantitative reverse transcription PCR; p-PI3K: phosphoTyr458-phosphotylinosital 3 kinase; p-AKT: phosphoSer473-protein kinase B; p-MEK: phosphoSer217/221-mitogen-activated protein kinase kinases; p-ERK: phosphoThr202/Tyr204-extracellular signal regulated protein kinase; NSCs: neural stem cells; SD: standard deviation.
Discussion
In our current study, we corroborated that puerarin protected NSCs against hypoxia-elicited cellular lesions. Concretely, puerarin moderated the apoptosis and maintained the viability by aggrandizing miR-214. Through further elevating miR-214, puerarin restored the activation of PI3K/AKT and MEK/ERK cascades.
An antecedent study indicated that NSCs in the subventricular zone are vulnerable to hypoxia/ischemia, appearing the diminishment of apoptotic cells [Citation21]. Consistently, we found that the cell viability was remarkably decreased with the aggravation in apoptosis progress. Because hypoxia often causes irreversible brain damage and potentially results in cognitive and motor dysfunction [Citation21], inhibition of apoptosis plays crucial roles in limiting the extent of brain damages. Actually, it has been revealed that puerarin decreases neuron loss and inhibits cell apoptosis and presents anti-apoptosis and neuro-protection functions in the rat model of acute spinal cord injury [Citation22]. Besides, a recent study proved that puerarin shows a neuroprotective effect in vascular dementia rats by alleviating the expression of hypoxia-inducible factor-1 alpha [Citation23]. Furthermore, puerarin ameliorates the lung and cerebrum injury under the condition of hypobaric hypoxia [Citation24]. Similarly, we found that puerarin moderated the loss of NSCs viability and the apoptosis process insulted by hypoxia.
Since the cleaved products of caspases are critical in the execution of apoptosis after neonatal hypoxia-ischemia or reperfusion [Citation25–27], we investigated the cleavage of caspase-3 and caspase-9 in NSCs pretreated with puerarin before hypoxia stimulus. Our results showed that NSCs were obviously more resistant to hypoxia-induced apoptosis after puerarin pre-incubation with a decline in cleavage of caspase-3 and caspase-9. Similarly, studies proved that puerarin inhibits the accumulation of cleaved caspase-3 [Citation28] and cleaved caspase-9 in brain sections or PC12 cells after cerebral ischemia or H2O2-triggered insults [Citation29,Citation30]. In conclusion, we considered that the inhibitory effects of puerarin on the cleavage of caspases were indispensable for suppressing hypoxia-induced apoptosis in NSCs.
The mechanism underlying puerarin promotes cell viability and attenuates apoptosis may be correlated with its anti-oxidative effects since puerarin has been manifested as a free-radical scavenger [Citation30]. Moreover, the potential mechanism by which puerarin accelerates the proliferation and differentiation might be through modulating the expression of miR [Citation11]. Pharmacological researches revealed a direct association of miR-214 down-regulation with the learning and memory impairment, neuro-degeneration as well as neuronal cell death [Citation18,Citation31]. As a consequence, we hypothesized a possible mechanistic link between miR-214 and the protective effects of puerarin in impeding hypoxia-induced loss of viability and apoptosis process. In fact, the protective role of miR-214 against ischemic/reperfusion injury has been validated in mice [Citation32]. To prove that puerarin-elicited up-regulation of miR-214 exhibited a protective activity, miR-214 was compulsorily silenced in NSCs. As expected, we found miR-214 inhibitor relieved the positive effect on the viability of NSCs and the anti-apoptotic function of puerarin. In a combination with our results, we considered that puerarin exhibited a cytoprotective effect against hypoxia-induced lesions by inducing NSCs to express miR-214.
A temporal increase of phosphorylation in regulators occurs after a short-term hypoxia stimulus [Citation33] like a most important aspect of the protective mechanism, which might not be entirely compatible with its kinase activity [Citation34]. With hypoxia stimulus proceeding further, the dephosphorylation of PI3K, AKT, MEK and ERK occupies the dominant position [Citation33] and the activity of these regulators is actually weakened [Citation34]. Recent studies found that ischemia-induced injury could be attenuated by puerarin, which works on the PI3K/AKT [Citation28] and MEK/ERK [Citation29] signalling pathways. These studies are analogical to our findings, that puerarin promoted the phosphorylation of PI3K, AKT, MEK, and ERK in response to the loss of viability and the apoptosis progress induced by hypoxia. Mechanically, a study found that hypoxia stimulus results in the abundance of miR-214 in cardiomyocytes [Citation20]. miR-214 overexpression subsequently triggers the phosphorylation of AKT in response to ischemia/reperfusion, which is recognized as a protective reaction [Citation35]. Consequently, we hypothesized that puerarin triggered PI3K/AKT and MEK/ERK signalling pathways by up-regulating the expression of miR-214. Expectedly, we identified miR-214 as a functional mediator of puerarin in phosphorylating PI3K, AKT, MEK, and ERK.
Conclusions
Collectively, the protective activity of puerarin was testified in hypoxia-treated NSCs. Further, we presented a protective mechanism of puerarin whereby miR-214 actively facilitated the activation of PI3K/AKT and MEK/ERK signalling pathways.
Disclosure statement
No potential conflict of interest was declared by the authors.
References
- Rodriguez-Alvarez N, Jimenez-Mateos EM, Dunleavy M, et al. Effects of hypoxia-induced neonatal seizures on acute hippocampal injury and later-life seizure susceptibility and anxiety-related behavior in mice. Neurobiol Dis. 2015;83:100–114.
- Askalan R, Gabarin N, Armstrong EA, et al. Mechanisms of neurodegeneration after severe hypoxic-ischemic injury in the neonatal rat brain. Brain Res. 2015;1629:94–103.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. The Coch Database of Syst Rev. 2013;1:Cd003311.
- Parmar J, Jones NM. Hypoxic preconditioning can reduce injury-induced inflammatory processes in the neonatal rat brain. Int J Dev Neurosci. 2015;43:35–42.
- Yan R, Xu H, Fu X. Salidroside protects hypoxia-induced injury by up-regulation of miR-210 in rat neural stem cells. Biomed Pharmacother. 2018;103:1490–1497.
- Zheng Z, Zhao B. Astragalus polysaccharide protects hypoxia-induced injury by up-regulation of miR-138 in rat neural stem cells. Biomed Pharmacother. 2018;102:295–301.
- Xu X, Zhang S, Zhang L, et al. The neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71:585–591.
- Jiang M, Yun Q, Niu G, et al. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson’s disease model. Genet Mol Res. 2016;15:gmr.15047501.
- Cong X, Zhang Q, Li H, et al. Puerarin ameliorates heat stress-induced oxidative damage and apoptosis in bovine Sertoli cells by suppressing ROS production and upregulating Hsp72 expression. Theriogenology 2017;88:215–227.
- Li Z, Xu W, Ren X, et al. Puerarin promotes DUSP1 expression by regulating miR133a3p in breast cancer. Mol Med Rep. 2019;19:205–212.
- Shan Z, Cheng N, Huang R, et al. Puerarin promotes the proliferation and differentiation of MC3T3-E1 cells via microRNA-106b by targeting receptor activator of nuclear factor-kappaB ligand. Exper Therap Med. 2018;15:55–60.
- Zhan XQ, Zeng XW, Zhang YY, et al. Puerarin promotes the viability and differentiation of MC3T3E1 cells by miR204regulated Runx2 upregulation. Mol Med Rep. 2017;16:6262–6268.
- Shu P, Fu H, Zhao X, et al. MicroRNA-214 modulates neural progenitor cell differentiation by targeting quaking during cerebral cortex development. Sci Rep. 2017;7:8014.
- Phatak P, Byrnes KA, Mansour D, et al. Overexpression of miR-214-3p in esophageal squamous cancer cells enhances sensitivity to cisplatin by targeting survivin directly and indirectly through CUG-BP1. Oncogene 2016;35:2087–2097.
- Yang L, Zhang W, Wang Y, et al. Hypoxia-induced miR-214 expression promotes tumour cell proliferation and migration by enhancing the Warburg effect in gastric carcinoma cells. Cancer Lett. 2018;414:44–56.
- Liu H, Yin T, Yan W, et al. Dysregulation of microRNA-214 and PTEN contributes to the pathogenesis of hypoxic pulmonary hypertension. COPD. 2017;12:1781–1791.
- Wan DY, Zhang Z, Yang HH. Cardioprotective effect of miR-214 in myocardial ischemic postconditioning by down-regulation of hypoxia inducible factor 1, alpha subunit inhibitor. Cell Mol Biol. 2015;61:1–6.
- Yan H, Xu T, Zhao H, et al. Isoflurane increases neuronal cell death vulnerability by downregulating miR-214. PloS One. 2013;8:e55276.
- Liu PY, Tian Y, Xu SY. Mediated protective effect of electroacupuncture pretreatment by miR-214 on myocardial ischemia/reperfusion injury. J Ger Cardiol. 2014;11:303–310.
- el Azzouzi H, Leptidis S, Dirkx E, et al. The hypoxia-inducible microRNA cluster miR-199a approximately 214 targets myocardial PPARdelta and impairs mitochondrial fatty acid oxidation. Cell Metabol. 2013;18:341–354.
- Levison SW, Rothstein RP, Romanko MJ, et al. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001;23:234–247.
- Zhang D, Ma G, Hou M, et al. The neuroprotective effect of puerarin in acute spinal cord injury rats. Cell Physiol Biochem. 2016;39:1152–1164.
- Wu H, Wang H, Zhang B, et al. Puerarin decreases hypoxia inducible factor-1 alpha in the hippocampus of vascular dementia rats. Neur Regen Res. 2012;7:421–425.
- Wang C, Yan M, Jiang H, et al. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-kappaB signaling pathway. Int Immunopharmacol. 2016;40:300–309.
- Blomgren K, Zhu C, Wang X, et al. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? The J Biol Chem. 2001;276:10191–10198.
- Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668.
- Khurana P, Ashraf QM, Mishra OP, et al. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochem Res. 2002;27:931–938.
- Tao J, Cui Y, Duan Y, et al. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3beta signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 2017;8:106283–106295.
- Wang N, Zhang Y, Wu L, et al. Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology 2014;79:282–289.
- Cheng Y, Leng W, Zhang J. Protective effect of puerarin against oxidative stress injury of neural cells and related mechanisms. Med Sci Monit. 2016;22:1244–1249.
- Wang J, Zhou M, Wang X, et al. Impact of ketamine on learning and memory function, neuronal apoptosis and its potential association with miR-214 and PTEN in adolescent rats. PloS One. 2014;9:e99855.
- Aurora AB, Mahmoud AI, Luo X, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232.
- Li D, Qu Y, Mao M, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:1903–1913.
- Wang Z, Zhang H, Xu X, et al. bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett. 2012;212:137–146.
- Wang X, Ha T, Hu Y, et al. MicroRNA-214 protects against hypoxia/reoxygenation induced cell damage and myocardial ischemia/reperfusion injury via suppression of PTEN and Bim1 expression. Oncotarget 2016;7:86926–86936.
