Abstract
Our previous studies have revealed that a dominant mutation in vacuolar protein sorting 4B (VPS4B), a member of the AAA ATPase family, causes dentin dysplasia type I. The purpose of the present study was to investigate the roles of VPS4B in human dental pulp stem cells (hDPSCs) and to elucidate the underlying molecular mechanisms. In this study, we found that VPS4B was highly expressed in the dental pulp cells of the mouse molar tooth germ, and the expression of VPS4B increased significantly during the odontoblastic differentiation of hDPSCs. VPS4B downregulation inhibited the proliferation, migration, and odontoblastic differentiation of hDPSCs. Moreover, treatment with lithium chloride, an agonist of the Wnt-β-catenin signalling pathway, partially reversed the VPS4B knockdown-driven suppression of proliferation and of odontoblastic differentiation of hDPSCs. Collectively, our findings indicate that VPS4B, via Wnt-β-catenin signalling, acts as a regulator of the proliferation and differentiation of hDPSCs. Our results suggest potential therapeutic avenues for dentin formation and regenerative endodontics in patients with dentin dysplasia type I.
Introduction
VPS4B is mapped to chromosome 18 (18q21.33) and encodes a member of the AAA ATPase family [Citation1]. The VPS4B protein plays essential roles in multiple cellular processes, including the formation of multivesicular bodies, virus budding, the abscission step of cytokinesis, and degradation of various membrane receptors [Citation2,Citation3]. VPS4B is an essential component of the endosomal sorting complexes required for the transport (ESCRT) machinery that mediates the final abscission step of cytokinesis in eukaryotes and many archaeal species [Citation4]. Some studies have shown that VPS4B can regulate the progression of non–small cell lung cancer [Citation5] and is involved in hepatocellular carcinoma [Citation6] and in the apoptosis of chondrocytes and intestinal epithelial cells during osteoarthritis and Crohn’s disease, respectively [Citation7,Citation8]. Furthermore, VPS4B has been identified as a putative tumour suppressor in breast cancer because VPS4B promotes degradation of epidermal growth factor receptor, EGFR [Citation9]. However, the role and mechanism of action of VPS4B in the development of other cell types, particularly stem cells, remain mostly unknown.
Recently, we reported a dominant splicing mutation in the VPS4B gene that is associated with dentin dysplasia type I (DD-I) in a Chinese family [Citation10]. The family members carrying the VPS4B mutation were all found to have tooth defects, including obliterated pulp chambers, abnormally mineralized dentin and short blunted roots. An experimental knockdown of Vsp4b expression in zebrafish reduced the number of teeth and also shortened the length of the teeth, thereby mimicking the DD-I phenotype. Moreover, a Vps4b knockout mouse model has proven that complete loss of VPS4B in mice results in placental death during the early stages of embryonic development, whereas Vsp4b+/– (heterozygous) mice feature a widened predentin zone [Citation11]. Because these studies have highlighted defects in dentin and pulp canal obliteration in both DD-I patients and animal models, we hypothesized that VPS4B deficiency is associated with imperfect dentinogenesis and might play a differential role in the disruption of the development of this mineralized tissue.
It has been reported that human dental pulp contains undifferentiated mesenchymal cells termed dental pulp stem cells (DPSCs), which play an essential part in dentin formation and regeneration [Citation12]. Human DPSCs (hDPSCs) can differentiate into odontoblasts that secrete a mineralized matrix with the mineral and molecular characteristics of dentin. Furthermore, normal differentiation of hDPSCs is essential for dentin development and formation [Citation13]. Therefore, hDPSCs represent a valuable model to investigate odontoblastic differentiation and to explore the mechanisms by which VPS4B affects the function of such cells.
The Wnt signalling pathway substantially participates in multiple cellular processes, including cell migration, proliferation, and differentiation [Citation14,Citation15]. Canonical Wnt signals are transduced via the intracellular mediator β-catenin. Of note, genetic studies have revealed that constitutive stabilization of β-catenin in dental pulp leads to excessive dentin formation, whereas inactivation of β-catenin in odontoblasts disrupts root odontoblast differentiation [Citation16,Citation17]. Moreover, recent studies established a link between the functions of VPS4B and the Wnt signalling pathway. For example, VPS4B can stimulate Wnt5a signalling via charged multivesicular body protein 4 b (CHMP4B), a subunit of the ESCRT-III complex. Indeed, CHMP4B binds to VPS4B to regulate cytokinesis and has also been reported to activate the β-catenin-independent Wnt pathway, which is involved in cytokinesis [Citation18]. Besides, our previous study indicates that a knockdown of VPS4B in human gingival fibroblasts is accompanied by a significant decrease in the amounts of β-catenin mRNA. These findings represent important clues to the mechanisms via which VPS4B can regulate the odontogenic differentiation of hDPSCs.
Therefore, the aim of this study was to uncover the precise functions of VPS4B in the proliferation and migration of hDPSCs and in the mineralization by hDPSCs. We hypothesized that VPS4B modulates the proliferation and odontogenic differentiation of hDPSCs via the Wnt-β-catenin signalling pathway. This line of investigation should expand our understanding of the clinical significance of VPS4B and uncover potential therapeutic avenues for treating such diseases as DD-I.
Materials and methods
Animals and immunohistochemical staining
All experiments involving laboratory animals were approved by (and were conducted under the supervision of) the Ethics Committee of Nanfang Hospital. Adult C57BL/6J mice were housed and mated overnight at a specific pathogen-free animal facility. The day of birth was designated as postnatal day (PN) 0 and tooth development was examined in the mice on PN1, PN3, PN5 and PN7. Immunostaining was performed as described previously [Citation19]. Monoclonal antibodies against VPS4B (Santa Cruz Biotechnology, Dallas, TX, USA) were used at a dilution of 1:300. The tissue sections were developed in 3,3′-diaminobenzidine and counterstained with hematoxylin. Images were captured by means of a fluorescence microscope (Olympus, Japan).
Cultivation of hDPSCs and their odontoblastic differentiation
Extracted healthy wisdom teeth were collected from 18- to 20-year-old individuals following protocols approved by the Ethics Committee of Southern Medical University and Nanfang Hospital of Stomatology. Isolation of hDPSCs was performed as described elsewhere [Citation20]. The cells were cultured at 37 °C in a humidified atmosphere containing 5% of CO2 in high-glucose Dulbecco’s Modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% of fetal bovine serum (FBS; Gibco), and 1% of a penicillin/streptomycin solution (Gibco), hereafter referred to as the growth medium (GM). For odontoblastic differentiation experiments, the cells were cultured in an odontogenic medium (OM), consisting of DMEM, 10% of FBS, 50 µg/mL ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA), 5 mM β-glycerophosphate (Sigma-Aldrich), and 10 nM dexamethasone (Sigma-Aldrich). Moreover, to determine whether VPS4B inhibited odontoblastic differentiation via the Wnt-β-catenin signalling pathway, hDPSCs were cultured first in the GM and then in the OM supplemented with lithium chloride (LiCl; Sigma-Aldrich): an agonist of the Wnt-β-catenin signalling pathway.
Construction of a VPS4B short hairpin RNA (shRNA)-expressing lentivirus and cell infection
The VPS4B-specific oligoribonucleotide shRNA-Vps4b and control oligoribonucleotide shRNA-Ctrl were designed and synthesized using Obi-O Technology (Shanghai, China). The hDPSCs were seeded in 12-well plates at a density of 105 cells/well and grown for 24 h. They were approximately 70% confluent at the time of infection. The cells were infected at a multiplicity of infection (MOI) of 10 in the presence of 5 µg/mL polybrene for 10 h at 37 °C and 5% CO2. The knockdown efficiency was evaluated using fluorescence microscopy, reverse-transcription quantitative polymerase chain reaction (RT-qPCR), and western blot analysis.
The cell proliferation assay and flow-cytometric analysis
The impact of the VPS4B knockdown on the proliferation of hDPSCs was examined using the Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan). Briefly, hDPSCs transduced with either the shRNA-Vps4b or shRNA-Ctrl lentivirus were seeded in four 96-well plates at a density of 3 × 103 cells/well and then cultured overnight. Cell proliferation was then examined on days 1, 3, 5 and 7. The CCK-8 reagent (10 µL) was added into each well, and the cells were incubated for 2 h at 37 °C in the dark. Absorbance at 450 nm was measured on a microplate reader (PerkinElmer, Shanghai, China) to determine the number of live cells in each well.hDPSCs were transduced with either the shRNA-Vps4b or shRNA-Ctrl lentivirus and cultured for 72 h. After centrifugation, the cells were harvested by trypsinization, washed with ice-cold phosphate-buffered saline (PBS), and fixed for 48 h at 4 °C with 70% ethanol. The fixed cells were then rinsed twice with PBS, stained by means of the Cell Cycle Kit (BestBio, Shanghai, China), and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Tokyo, Japan).
Wound healing and the transwell migration assay
For wound healing evaluation, hDPSCs were seeded in 6-well plates and grown at 37 °C until 100% confluence. The cell monolayers were scratched with a 1-ml sterile pipette tip to create a wound. Then, the cell monolayers were washed three times with PBS and were cultured for additional 24 h in 2 ml of the serum-free GM per well. The migration of the cells across the wound line was assessed using bright-field microscopy.
The Transwell migration assay was performed in 24-well plates fitted with membranes of 8 μm pore size (BD Falcon, New York, NY). Briefly, approximately 5 × 104 cells in 200 µL of the serum-free GM were plated in the upper chamber. The lower chambers were filled with the GM, and the plates were incubated for 24 h. The noninvading cells on the upper surface of the well were scraped off with a cotton swab, and the invading cells on the lower surface were stained for 10 min at room temperature with 4% crystal violet and counted under a bright-field microscope.
Alizarin red S (ARS) and alkaline phosphatase (ALP) staining
For ARS staining, hDPSCs were cultured in 12-well plates containing 1 ml of the GM per well. When the cells were 70% confluent, the GM was replaced with the OM to induce the odontogenic differentiation. After 14 days, the induced cells were fixed for 15 min at room temperature in 4% paraformaldehyde and then stained for 30 min with 2% ARS (Beyotime Biotech, Shanghai, China).
For ALP staining, the cells were grown under similar conditions, and the induced cells were analyzed after 7 days. ALP staining was performed using the NBT/BCIP Staining Kit (Beyotime Biotech, Shanghai, China).
RT-qPCR
Using the TRIzol Reagent (Invitrogen, New York, NY, USA), total RNA was extracted from hDPSCs harvested at various time points after treatment. Total RNA was reverse-transcribed using the Prime Script First Strand cDNA Synthesis Kit (TaKaRa Biotechnology, Dalian, China), and RT-qPCR was carried out with SYBR Premix DimerEraser (TaKaRa) on a LightCycler 480 (Roche, Indianapolis, IN, USA). Gene expression was quantified using the 2–ΔΔCt method and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. The sequences of the primer pairs used for quantitation of dentin sialophosphoprotein (DSPP), bone sialoprotein (BSP), ALP, VPS4B, and GAPDH mRNA expression are listed in .
Table 1. Primer sequences used for RT-qPCR.
Western blot analysis
Cells were harvested and then lysed in RIPA buffer (Beyotime, Nanjing, China) supplemented with protease inhibitors. The cell lysates were cleared by centrifugation at 12,000 × g for 10 min at 4 °C. Total-protein samples were separated using SDS-PAGE in a 10% gel and then transferred to a PVDF membrane (Amersham, Little Chalfont, UK) at 200 mA for 2–3 h. The membranes were blocked for 1 h with 5% skim milk and incubated overnight at 4 °C with primary antibodies: anti-VPS4B (1:1000; Santa Cruz Biotechnology), anti-DSPP (1:1000; Santa Cruz Biotechnology), anti-osteopontin (OPN; 1:1000; Wanleibio, Shenyang, China), anti–cyclin A (1:500; Bioworld Technology, Louis Park, USA), anti-PCNA (1:500; Bioworld Technology), anti–β-catenin (1:1000; ABclonal Technology, Wuhan, China), anti–histone H3.1 (1:500; ABclonal Technology), anti–α-tubulin (1:5000; Abcam, MA, USA) and anti-GAPDH (1:2000; Santa Cruz Biotechnology) antibodies. The next day, the membranes were incubated for 1 h at 37 °C with the corresponding secondary antibodies (Proteintech, China), and the immunoreactive proteins were visualized with the ECL Kit (Beyotime Biotech, Shanghai, China) according to the manufacturer’s instructions.
Statistical analyses
Results are presented as means ± standard deviation (SD) of at least three independent biological replicates. Biological replicates were analyzed as at least three technical replicates per experimental point. The significance of differences was determined using one-way analysis of variance. The observed differences were considered statistically significant at p values < .05.
Results
Localization of the VPS4B protein during mouse tooth development
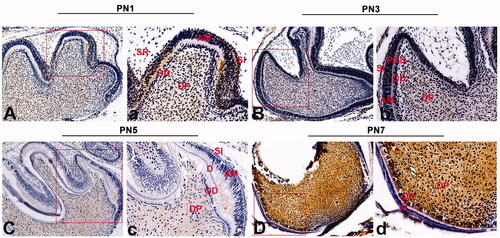
To examine the expression and localization of VPS4B, we first performed immunohistochemical staining of the front sections of a murine first mandibular molar on PN1, PN3, PN5 and PN7 (). We found that during dentin development (from PN1 to PN7), VPS4B was highly expressed in the dental pulp cells near odontoblasts. At the late bell stage (PN1), VPS4B was also mildly expressed in the stratum intermedium and odontoblasts and weakly expressed in the stellate reticulum (). At the stage of hard-tissue formation and mineralization (from PN3 to PN5), VPS4B was consistently expressed in dental pulp cells, preodontoblasts and the stratum intermedium (). In contrast, the cells of the stellate reticulum stained only weakly while the odontoblasts tested negative for VPS4B (no staining; ). When dentin was formed (PN7), VPS4B was intensely expressed in dental pulp cells ().
Figure 1. Immunohistochemical staining of VPS4B in postnatal mouse tooth germs. The tissue sections were counterstained with hematoxylin. (A-a) At the late bell stage (PN1), VPS4B was strongly expressed in the DP and SI and expressed less strongly in OD near the DP. (B-b) On PN2, VPS4B was expressed in both the DP and SI. (C-c) On PN5, VPS4B was consistently expressed in the DP and SI, with minimal expression in OD. (D-d) On PN7, VPS4B was highly expressed in the DP and OD. AM: ameloblasts; D: dentin; DP: dental pulp; OD: odontoblasts; POD: preodontoblasts; SR: stellate reticulum; SI: stratum intermedium.
VPS4B expression during the odontoblastic differentiation of hDPSCs
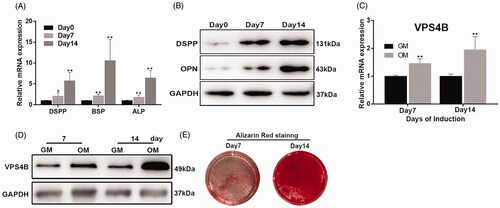
We next assessed the differentiation of hDPSCs grown in either the GM or OM for 7 or 14 days. We found that over time, the cells showed increasing odontogenic differentiation, as evidenced by the increased mRNA expression and protein levels of odontoblast differentiation markers () and ARS staining (). To determine whether VPS4B was differentially expressed and participated in hDPSCs differentiation, we analyzed the changes in the levels of VPS4B mRNA and protein in induced hDPSCs using RT-qPCR and western blotting, respectively. We found that on days 7 and 14 of differentiation, the levels of both VPS4B mRNA and protein were higher as compared to undifferentiated cells ().
Figure 2. Analysis of the mRNA and protein expression of VPS4B and odontoblastic differentiation markers during the odontoblastic differentiation of hDPSCs. (A) Histograms showing the relative mRNA levels of DSPP, BSP, ALP and VPS4B (C) during the induction of differentiation. The mRNA levels were determined using RT-qPCR with GAPDH as a reference for normalization. (B) Representative western blots showing the protein levels of DSPP, OPN and VPS4B (D). (E) ARS staining of cells grown for 7 or 14 days in the OM. Values are mean ± SD of three independent experiments (*p < .05 and **p < .01).
Inhibition of VPS4B mRNA and protein expression with the shRNA-expressing lentivirus
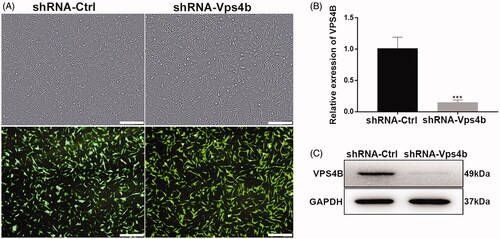
A lentivirus encoding green fluorescent protein (GFP), as an indicator of infection efficiency, and VPS4B-specific shRNA was successfully used to transduce hDPSCs (). Using fluorescence microscopy, we demonstrated that over 90% of the transduced hDPSCs expressed GFP (). Moreover, compared to shRNA-Ctrl lentivirus-transduced cells, the levels of VPS4B mRNA and protein were both significantly lower in the shRNA-Vps4b lentivirus-transduced cells, proving efficient downregulation of VPS4B by the specific shRNA ().
Figure 3. Stable VPS4B downregulation in hDPSCs. Passage-3 hDPSCs were infected with the indicated lentivirus at MOI of 10. (A) Representative bright-field (top) and fluorescence (bottom) microscopy images illustrating the proportion of cells expressing GFP, as an indicator of infection efficiency. (B) Histograms of the relative levels of VPS4B mRNA in transduced hDPSCs. (C) Representative western blots showing the protein level of VPS4B in hDPSCs. Scale bar = 500 μm. The data are mean ± SD of three independent experiments (***p < .001).
VPS4B downregulation inhibits hDPSCs proliferation, migration and odontogenic differentiation
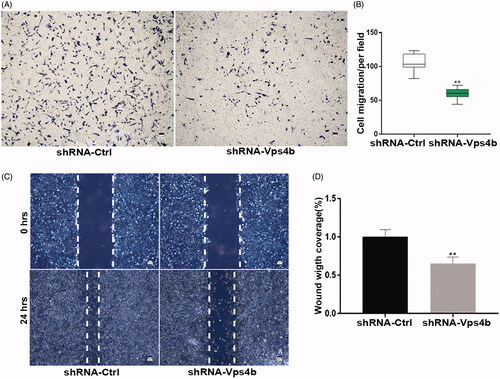
In the CCK-8 assay, we showed that during 7 days, hDPSCs transduced with the shRNA-Vps4b lentivirus proliferated more slowly after than did their counterparts transduced with the control (shRNA-Ctrl) lentivirus (). Furthermore, our flow-cytometric analyses revealed that VPS4B shRNA–mediated downregulation resulted in a concomitant accumulation of cells at the G0–G1 transition of the cell cycle and a decrease in the number of cells in the S phase and at the G2–M transition, as compared to the cells transduced with the shRNA-Ctrl lentivirus (). Moreover, western blot analyses suggested that the protein levels of VPS4B, cyclin A and PCNA were relatively low after 72 h of serum starvation and gradually increased throughout the serum refeeding period (). Consistently with this finding, we observed decreased levels of cyclin A and PCNA after transduction of the shRNA-Vps4b lentivirus (). To further characterize the potential role played by VPS4B in the odontogenic differentiation of hDPSCs, we first examined the effects of VPS4B downregulation on the levels of expression of odontoblast differentiation markers. We found that at 14 days after the induction of differentiation, the levels of mRNA expression of DSPP, BSP and ALP were lower in the cells transduced with the shRNA-Vps4b lentivirus as compared to the cells transduced with the shRNA-Ctrl lentivirus (). Furthermore, our western blot analyses confirmed that after 14 days of odontogenic induction, the protein levels of DSPP and OPN were also decreased by the VPS4B knockdown (). Lastly, ARS staining revealed that VPS4B downregulation inhibited the mineral deposition by DPSCs on day 14 of odontogenic induction, whereas ALP staining on day 7 was significantly weaker in shRNA-Vps4b lentivirus-transduced cells than in shRNA-Ctrl lentivirus-transduced cells (). Finally, our Transwell migration and the wound healing assays revealed that VPS4B downregulation inhibited hDPSCs migration (), indicating that VPS4B is involved in cell migration.
Figure 4. VPS4B downregulation inhibits the proliferation and odontoblastic differentiation of hDPSCs. Where indicated, hDPSCs were transduced with the shRNA-Ctrl or shRNA-Vps4b lentivirus. (A) Cell proliferation was monitored for 7 days using the CCK-8 assay. (B) Flow-cytometric profiles revealing the cell cycle distribution of hDPSCs transduced with the shRNA-Ctrl (left) or shRNA-Vps4b (right) lentivirus. (C) Representative western blots indicating the protein levels of VPS4B, cyclin A, and PCNA after serum starvation (S72h) and refeeding (R8h, R12h and R24h). (D) Representative western blots revealing the levels of cell cycle-related proteins cyclin A and PCNA after VPS4B downregulation. (E) Histograms showing the relative expression of DSPP, BSP, and ALP after VPS4B downregulation in hDPSCs. The mRNA levels were determined using RT-qPCR (with GAPDH as a reference for normalization) after 14 days of induction. (F) Representative western blots showing the protein levels of DSPP and OPN. (G) Representative bright-field microscopy images of ARS (top) and ALP (bottom) staining of hDPSCs at 14 and 7 days after the induction of differentiation, respectively. The data are presented as mean ± SD of three independent experiments (*p < .05 and **p < .01).
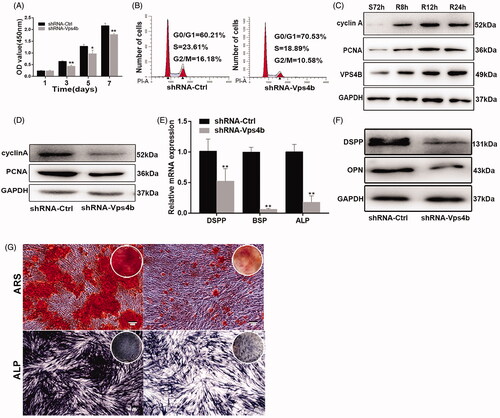
Figure 5. VPS4B downregulation inhibits the migration of hDPSCs. Representative images (A) and a boxplot illustrating the results (B) of Transwell migration assays of hDPSCs with and without VPS4B downregulation. Representative images (C) and quantification (D) of wound healing assays with and without VPS4B downregulation in hDPSCs. The values are mean ± SD of three independent experiments (*p < .05 and **p < .01).
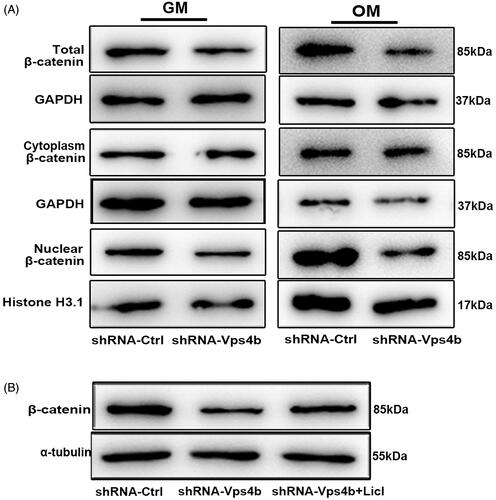
VPS4B downregulation disrupts β-catenin stabilization in hDPSCs
To test whether the Wnt-β-catenin signalling pathway is affected by the VPS4B downregulation in hDPSCs, we examined alterations in this pathway in cells grown in either the GM or OM (). Our western blot analyses showed that compared to shRNA-Ctrl lentivirus-transduced cells, the total level of β-catenin in cells and the accumulation of nuclear β-catenin were both significantly lower in shRNA-Vps4b lentivirus-transduced hDPSCs, regardless of the culture conditions. In contrast, the level of cytoplasmic β-catenin remained constant even after VPS4B downregulation. Our western blot analyses also indicated that the total level of β-catenin increased when shRNA-Vps4b lentivirus-transduced cells were treated with 10 mM LiCl ().
Figure 6. By acting via the Wnt-β-catenin pathway, VPS4B regulates the dentinogenesis driven by hDPSCs. Where indicated, hDPSCs were transduced with the shRNA-Ctrl or shRNA-Vps4b lentivirus and cultured in the presence of 10 mM LiCl. (A) Representative western blots showing the total, cytoplasmic, and nuclear protein levels of β-catenin after VPS4B downregulation in hDPSCs grown in the GM or OM for 14 days. GAPDH served as a loading control for total and cytoplasmic proteins, while histone H3.1 was employed as a loading control for nuclear proteins. (B) Representative western blots showing the relative protein levels of β-catenin after induction of the odontogenic differentiation of hDPSCs.
LiCl partially reverses the inhibitory effects of VPS4B downregulation on hDPSCs proliferation and odontogenic differentiation by activating the Wnt-β-catenin signalling pathway
Because VPS4B is likely to perform crucial functions in hDPSCs and because VPS4B downregulation impaired β-catenin stabilization and nuclear translocation, we decided to examine the effects of LiCl, an inhibitor of glycogen synthase kinase (GSK)-3β. LiCl is known to activate the Wnt-β-catenin signalling pathway (). Concomitantly, analysis of cell proliferation using the CCK-8 assay revealed that shRNA-Vps4b lentivirus-transduced hDPSCs treated with LiCl had a significantly higher proliferation rate (on days 3, 5 and 7) than did shRNA-Vps4b lentivirus-transduced cells (). Furthermore, our analysis of the cell cycle distribution indicated that LiCl treatment of shRNA-Vps4b lentivirus-transduced hDPSCs increased the percentage of cells in the S phase (). However, LiCl treatment did not affect the migration of the shRNA-Vps4b lentivirus-transduced hDPSCs (data not shown).
Figure 7. LiCl partially reverses the inhibitory effects of the VPS4B knockdown on hDPSCs proliferation and odontogenic differentiation by stimulating the Wnt-β-catenin signalling pathway. Where indicated, hDPSCs were transduced with the shRNA-Ctrl or shRNA-Vps4b lentivirus and were cultured in the presence of 10 mM LiCl. (A) Cell proliferation was monitored for 7 days using the CCK-8 assay. (B) Flow-cytometric profiles of the cell cycle distribution. (C) A histogram of the relative expression of DSPP, BSP and ALP in hDPSCs grown in the OM. (D) Representative western blots depicting the relative protein levels of DSPP and OPN after induction of the odontogenic differentiation of hDPSCs. (E) Representative bright-field microscopy images of ARS staining of hDPSCs cultured in the OM for 14 days. The data are presented as mean ± SD of three independent experiments (*p < .05 and **p < .01).
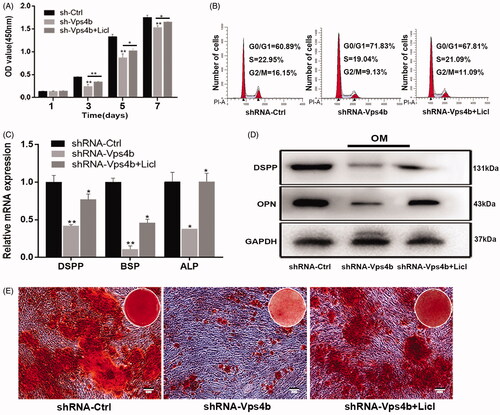
Lastly, we determined whether the activation of the Wnt-β-catenin signalling pathway by LiCl was sufficient to promote the odontoblastic differentiation of hDPSCs after VPS4B downregulation. Using RT-qPCR and western blotting, we found that the mRNA and protein expression of mineralization makers significantly increased when shRNA-Vps4b lentivirus-transduced hDPSCs were treated with LiCl (). By ARS staining, we also demonstrated increased formation of mineralized nodules among LiCl-treated cells (). Thus, our findings indicate that by enhancing the Wnt-β-catenin signalling pathway, LiCl partially reverses the inhibitory effects of the VPS4B knockdown on hDPSCs proliferation and on odontoblastic differentiation.
Discussion
Patients carrying a dominant splicing mutation of VPS4B have distinct clinical features of DD-I, including severe tooth hypermobility and premature exfoliation; short, blunted, and malformed roots; and total obliteration of pulp cavities. Moreover, functional studies conducted on a zebrafish model or in vitro have revealed that VPS4B plays an essential part in tooth development. Given the clinical characteristics of patients with DD-I and the involvement of VPS4B in tooth development, we wondered whether VPS4B takes part in the biological functions of hDPSCs. The findings reported in this study highlight the previously unidentified functions of VPS4B in stem cells.
Dentinogenesis is a complex process that involves the precise and time-dependent orchestration of multiple genetic, molecular, and cellular interactions. During this process, dentin is formed via the progressive proliferation and differentiation of hDPSCs into mature odontoblasts [Citation21]. It is well established that the most critical step for cell proliferation is progression from the G0–G1 transition of the cell cycle to the S phase through the G1–S checkpoint [Citation22]. Therefore, faster G1–S transition results in an increased rate of cell proliferation, which has important implications for dental development and repair. Our findings revealed that shRNA-mediated downregulation of VPS4B markedly reduced the proliferation rate of hDPSCs, with fewer cells reaching the S phase and the G2–M transition of the cell cycle (). Furthermore, our western blot analyses showed that VPS4B downregulation decreased the cellular levels of the essential cell cycle regulators: PCNA and cyclin A (). Moreover, we found that the protein levels of VPS4B, PCNA, and cyclin A concomitantly increased after serum starvation and refeeding (). Of note, other studies have also shown that VPS4B depletion is associated with a marked reduction in the proliferation rate of various cell types including multiple myeloma cells, hepatocellular carcinoma cells, HT-29 colon carcinoma cells, and A549 lung carcinoma cells [Citation5,Citation6,Citation8,Citation23]. Our results are consistent with these findings and show that an impairment of VPS4B function can influence the proliferation of hDPSCs.
Wound-healing processes require the migration of mesenchymal cells to the wound site, followed by their proliferation and differentiation to enable the repair of the damaged tissue [Citation22]. The relation between VPS4B and cell migration has not been studied previously, and our results clearly show that after VPS4B downregulation, the migratory and invasive capabilities of hDPSCs markedly diminished. Collectively, our results strongly indicate that VPS4B is involved in the regulation of hDPSCs proliferation and migration.
To date, the research on the participation of VPS4B in cell functions has mainly focused on tumour cell lines, with few studies conducted on stem cells. The neural-crest-derived mesenchymal progenitors hDPSCs have key stem cell characteristics, including self-renewal and multilineage differentiation, and can form dentin pulp-like complexes. In this study, we examined the role of VPS4B in the odontogenic differentiation of hDPSCs (). To this end, we performed ALP and ARS staining to monitor ALP activity and mineralization, respectively, and we examined the levels of expression of odontoblastic differentiation markers DSPP, BSP, ALP and OPN. DSPP is the primary noncollagenous protein in tooth dentin and has long been regarded as a specific marker of dentinogenesis [Citation24]. BSP is an acidic noncollagenous glycoprotein abundantly expressed in mineralized tissues and performs an essential function in the initial mineralization of bone, dentin and cementum [Citation25]. ALP activation is an early marker of odontoblastic and mineralized-cell differentiation, whereby ALP can induce matrix deposition by generating pyrophosphate [Citation26]. OPN is an extracellular protein that regulates both bone and tooth mineralization [Citation27]. It should be noted that some studies have proved that inactivation of a single osteogenic differentiation gene can reduce cell differentiation and mineralization of the dental tissue. Our experiments with the lentivirus-mediated knockdown of VPS4B clearly showed that VPS4B is required for the normal expression of characteristic osteogenesis genes after the induction of odontogenic differentiation. ALP and ARS staining provided further evidence that VPS4B is involved in the odontogenic differentiation of hDPSCs and therefore may regulate dentin formation during tooth development. However, the detailed molecular mechanisms underlying the VPS4B function during hDPSCs proliferation, migration and differentiation are yet to be elucidated.
The roles of the classic Wnt-β-catenin signalling pathway in the control of cell proliferation, differentiation, migration and apoptosis have been demonstrated in numerous studies [Citation28]. Notably, the nuclear translocation of β-catenin has been reported to play an essential part in cell growth and endothelial-cell proliferation. One study has shown that β-catenin can promote the odontoblastic differentiation of dental pulp cells and the proliferation and odontoblastic differentiation of stem cells derived from the apical papilla [Citation29,Citation30]. Nonetheless, the modulatory function of the Wnt-β-catenin signalling pathway in osteogenic differentiation remains controversial [Citation30,Citation31]. These discrepancies prompted us to investigate whether VPS4B regulates the odontoblastic differentiation of hDPSCs via the canonical Wnt-β-catenin signalling pathway. It has been demonstrated that CHMP4B, a subunit of the ESCRT-III complex, binds to VPS4B to regulate cytokinesis while Wnt5a signalling, which activates the β-catenin–independent pathway, is required for the cytokinesis associated with CHMP4B [Citation32]. Moreover, VPS4B has been proven to upregulate β-catenin, with the overexpression and downregulation of VPS4B in human gingival fibroblasts resulting in increased and decreased levels of β-catenin mRNA, respectively [Citation10]. However, the mechanisms by which VPS4B influences the functions of hDPSCs have remained unclear.
During our experiments, we found that VPS4B downregulation decreased the total cellular level of β-catenin and hindered β-catenin nuclear translocation while the cytoplasmic level of β-catenin remained unchanged (). Meanwhile, we demonstrated that treatment with the GSK-3β inhibitor LiCl partially reverses the inhibition of proliferation and odontogenic differentiation by the VPS4B knockdown and concomitantly increases the total cellular level of β-catenin (). In contrast, LiCl treatment did not affect the inhibition of cell migration resulting from VPS4B downregulation (data not shown). Therefore, it is reasonable to conclude that the Wnt-β-catenin signalling pathway promotes the proliferation and osteogenic differentiation of hDPSCs, but the inhibition of hDPSC migration caused by the VPS4B knockdown cannot be attributed to the downregulation of the Wnt-β-catenin signalling pathway. Although the link between VPS4B and β-catenin nuclear translocation needs to be further investigated, the results presented here clearly indicate that the VPS4B-Wnt-β-catenin signalling axis regulates the proliferation and odontogenic differentiation of hDPSCs. Future studies that test whether VPS4B affects other molecules in the Wnt or other signalling pathways will undoubtedly improve our understanding of the molecular mechanisms underlying VPS4B functions. Moreover, further works expanding our understanding of these mechanisms will provide valuable clues to the development of novel therapeutic strategies against dentin dysplasia caused by VPS4B mutations or by other mutations in the VPS4B regulatory pathway.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Inoue M, Kamikubo H, Kataoka M, et al. Nucleotide-dependent conformational changes and assembly of the AAA ATPase SKD1/VPS4B. Traffic. 2008;9:2180–2189.
- Watanabe R, Lamb RA. Influenza virus budding does not require a functional AAA + ATPase, VPS4. Virus Res. 2010;153:58–63.
- Urata S, Yokosawa H, Yasuda J. Regulation of HTLV-1 Gag budding by Vps4A, Vps4B, and AIP1/Alix. Virol J. 2007;4:66.
- Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–101.
- Liu Y, Lv L, Xue Q, et al. Vacuolar protein sorting 4B, an ATPase protein positively regulates the progression of NSCLC via promoting cell division. Mol Cell Biochem. 2013;381:163–171.
- Jiang D, Hu B, Wei L, et al. High expression of vacuolar protein sorting 4B (VPS4B) is associated with accelerated cell proliferation and poor prognosis in human hepatocellular carcinoma. Pathol Res Pract. 2015;211:240–247.
- Xu L, Zhai L, Ge Q, et al. Vacuolar protein sorting 4B (VPS4B) regulates apoptosis of chondrocytes via p38 mitogen-activated protein kinases (MAPK) in osteoarthritis. Inflammation. 2017;40:1924–1932.
- Zhang D, Wang L, Yan L, et al. Vacuolar protein sorting 4B regulates apoptosis of intestinal epithelial cells via p38 MAPK in Crohn's disease. Exp Mol Pathol. 2015;98:55–64.
- Legent K, Liu HH, Treisman JE. Drosophila Vps4 promotes Epidermal growth factor receptor signaling independently of its role in receptor degradation. Development. 2015;142:1480–1491.
- Liu C, Li Y, Yu Q, et al. A splicing mutation in VPS4B causes dentin dysplasia I. J Med Genet. 2016;53:624–633.
- Hu A, Lu T, Chen D, et al. Vps4b heterozygous mice do not develop tooth defects that replicate human dentin dysplasia I. BMC Genet. 2019;20:7.
- Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630.
- Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151–157.
- Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999.
- Song Y, Liu X, Feng X, et al. NRP1 accelerates odontoblast differentiation of dental pulp stem cells through classical Wnt/beta-catenin signaling. Cell Reprogram. 2017;19:324–330.
- Kim TH, Bae C, Lee JC, et al. beta-Catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013;92:215–221.
- Kim T-H, Lee J-Y, Baek J-A, et al. Constitutive stabilization of ss-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412:549–555.
- Fumoto K, Kikuchi K, Gon H, et al. Wnt5a signaling controls cytokinesis by correctly positioning ESCRT-III at the midbody. J Cell Sci. 2012;125:4822–4832.
- Li H, Ramachandran A, Gao Q, et al. Expression and function of NUMB in odontogenesis. Biomed Res Int. 2013;2013:182965
- Ma D, Yu H, Xu S, et al. Stathmin inhibits proliferation and differentiation of dental pulp stem cells via sonic hedgehog/Gli. J Cell Mol Med. 2018;22:3442–3451.
- Huang XF, Chai Y. Molecular regulatory mechanism of tooth root development. Int J Oral Sci. 2012;4:177–181.
- Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164–1188.
- Tang J, Ji L, Wang Y, et al. Cell adhesion down-regulates the expression of vacuolar protein sorting 4B (VPS4B) and contributes to drug resistance in multiple myeloma cells. Int J Hematol. 2015;102:25–34.
- Porntaveetus T, Nowwarote N, Osathanon T, et al. Compromised alveolar bone cells in a patient with dentinogenesis imperfecta caused by DSPP mutation. Clin Oral Invest. 2019;23:303–313.
- Gordon JA, Tye CE, Sampaio AV, et al. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473.
- Bakopoulou A, Leyhausen G, Volk J, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56:709–721.
- Foster BL, Ao M, Salmon CR, et al. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone. 2018;107:196–207.
- Turashvili G, Bouchal J, Burkadze G, et al. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–223.
- Han N, Zheng Y, Li R, et al. Catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One. 2014;9:e88890. beta
- Wang J, Liu B, Gu S, et al. Effects of Wnt/β-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 2012;45:121–131.
- Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130.
- McCullough J, Fisher RD, Whitby F, et al. GALIX-CHMP4 interactions in the human ESCRT pathway. Proc Natl Acad Sci USA. 2008;105:7687–7691.
