Abstract
Hepatocellular carcinoma is the most common liver cancer among different types of cancers. Cordyceps Militaris mushroom species traditionally used as an alternative medicine in china for centuries. Gold nanoparticles plays vital role in the development of the anticancer drugs. In our research, we investigated the gold nanoparticles with C. Militaris on the hepatocellular carcinoma HepG2 cells. The synthesized gold nanoparticles stability and integrity was studied at different time intervals. The gold nanoparticles potentially halt the growth of the HepG2 cells at the IC50 concentration between 10 μg and 12.5 μg/ml. The HR-TEM and XRD revealed the size and shape of the synthesized gold nanoparticles. The size of the gold nanoparticles was about 15 20 nm and the shape of gold nanoparticles was face-center-cubic structure. The FT-IR results proved that the gold nanoparticles contain hydroxyl and alkynes groups. The gold nanoparticles extract develops ROS and cause damage to the mitochondrial membrane potential in the hepatocellular carcinoma HepG2 cells. The gold nanoparticles extract tends to initiate the apoptosis by activating the Bax, Bid, caspases and inhibits the activation anti-apoptotic bcl-2 in the HepG2 cells. Our results concluded that the gold nanoparticles with C. Militaris would be an efficient chemotherapeutic drug against the hepatocellular carcinoma cells.
Introduction
Liver cancer ranks 50th of all cancer types in the year 2012, worldwide. Overall, it accounts for 9.1% of all cancer deaths. Almost 83% of liver cancer was diagnosed in underdeveloped nations [Citation1]. Globally, 50% of liver cancer new cases and deaths were registered in China [Citation2]. More than 75–90% of liver cancer is hepatocellular carcinomas (HCCs) [Citation3]. Liver cancer incidence is increasing faster than that for any other cancer in humans [Citation4]. The development of liver cancer occurs due to the risk factors such as excess alcohol consumption, cigarette smoking, obesity, hepatitis B and hepatitis C viruses [Citation5]. Surgery, radiation therapy and chemotherapy were followed as a treatment in the cancer patients. These treatments cause serious adverse effects in cancer patients. So, alternative medicines from natural products were used in the cancer treatment to avoid the side effects [Citation6,Citation7]. Cordyceps is one of the emerging herbals in cancer treatment due to its anticancer activity [Citation8]. Cordyceps militaris is a naturally occurring mushroom used as a traditional medicine in Eastern part of Asia [Citation9]. It is widely used in the treatment of hyperlipidaemia, hyperglycaemia, renal dysfunctions and liver diseases [Citation10]. Numerous bioactive compounds such as ergosterol, polysaccharides and cordycepin present in the C. militaris. These bioactive compounds possessed anti-inflammatory and anti-cancer properties on several cancer cell lines [Citation11,Citation12].
Caspases are a family of protease enzymes, which play major roles in the events of apoptosis. Specifically, the initiators caspase-8 and caspase-9 trigger the executioner caspase-3 by cleavage activation, leading to the proteolysis of poly(ADP-ribose) polymerase (PARP) and apoptosis through impairment of DNA repair [Citation13]. Here, we show that treatment of HepG2 cells with gold nanoparticles with C. militaris extract induced caspase-3 activation to enhance apoptosis. Cordyceps militaris crude extract promotes apoptosis on breast cancer and human premyelocytic leukaemia cells by activating the caspase-linked mitochondrial pathway [Citation14,Citation15]. Gold nanoparticles efficiently used in the development of anticancer drugs against several cancers [Citation16,Citation17]. Chemical methods are being followed in the synthesis of gold nanoparticles, which are toxic to the environment as well as expensive, additionally, these synthesized nanoparticles may have serious adverse effects in humans and animals [Citation18]. To minimize the adverse effects the green method/biological method was followed to synthesize the nanoparticles with the plant, bacteria and fungi extracts [Citation19]. In our study, synthesized gold nanoparticles with C. militaris extract were used to identify its efficacy on the HCC (HepG2) cells.
Materials and methods
Chemicals and reagents
HCC HepG2 cells were from American Type Culture Collection (Manassas, VA) and 3-(4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) from Sigma-Aldrich (St. Louis, MO). DMEM and fetal bovine serum (FBS) were from GIBCO (Grand Island, NY). Caspase-3, -8 and -9 enzyme kits were from Cell Signaling (Beverly, MA). All chemicals, reagents and media were purchased and used at analytical grade.
Biomass of C. militaris preparation
Cordyceps militaris was cultured in the potato dextrose agar slant for a week at 25 °C and the cultured slants were stored at 4 °C. Cordyceps militaris was aseptically grown in 100 ml culture flasks with PDA broth at 25 °C for a week in a rotary shaker. Then the cultured mycelia were incubated at 25 °C without shaking for 4 weeks. The C. militaris was harvested and filtered via sterilized double gauze. Biomass (20 g) was sonicated in an individual flask with 200 ml of ultra-pure water for 30 min. The crude cell filtrate was washed and separated at 3000 rpm for 10 min using centrifugation technique. The crude cell filtrate was collected and stored for subsequent analysis.
Synthesis and purification of gold nanoparticles
An aliquot of 4 ml of 10 mM HAuCl4·3H2O solution was added to 36 ml of cell filtrate in the fresh round-bottomed flask. The mixture was kept until the colour changed from light to dark brown. A flask only contains cell lysate was used as control. The extract concentrations were varied between 5 and 50% by volume. The gold nanoparticles were subjected for centrifugation at 10,000 rpm for 15 min for thrice to remove the supernatant, and then the gold nanoparticles were freeze-dried using lyophilizer. The powdered samples were used for further investigations.
Characterization of gold nanoparticles
The gold nanoparticles were assessed using Shimadzu UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan) in the range of 300–700 nm. The cell lysate was mixed with the gold chloride solution. The colour was changed from pale yellow to dark brown. This indicates the formation of gold nanoparticles. The optical density was observed at different time intervals (24 h, 48 h, 5th day, 15th day and 30th day) in both control and test samples. The shape and size of gold nanoparticles were assessed using high-resolution transmission electron microscopy at 40× magnification (Nikon, Tokyo, Japan). The freeze-dried gold nanoparticles were used for X-ray diffraction technique. The powdered gold nanoparticles were fixed with Potassium Bromide disk to analyze functional groups using Fourier transform infrared (FT-IR) spectrometer.
In vitro cytotoxicity study (MTT assay)
The HCC (HepG2) cells were sub-cultured to determine the cytotoxicity of the gold nanoparticles by MTT assay. The HepG2 cells were grown in T-flask with Dulbecco Modified Eagles Medium (DMEM) possess 10% FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin and 4 mM L-glutamine. The cancer cells were incubated at 37 °C in a 5% CO2 for 48 h. Then the cells were seeded (3 × 105 per well) in 0.2 ml of DMEM medium/well in 48-well plates for 24 h. Then the non-adherent HepG2 cells were removed by washing with serum-free DMEM medium. The serum-free medium with varying concentrations of gold nanoparticles (2.5 µg to 15 µg/ml) was added to the adherent cells in the 96-well plates. A negative control containing serum-free medium with DMSO was also evaluated. After 24 h of treatment, the microwell plates were incubated with 20 µl MTT solution (5 mg/ml) for 6 h at 37 °C. The formazan blue crystals were dissolved with 100 µl/well of dimethylsulfoxide (DMSO). The optical density of the formazan dye was measured at 570 nm (model make). The percentage of cell viability was determined using optical density values of test and control samples. The IC50 was assessed by plotting the graph.
Assessment of apoptosis by AO/EtBr staining method
The HCC (HepG2) cells were treated with (10 and 12.5 µg/ml) of gold nanoparticles for 24 h. The HepG2 cells were washed with phosphate-buffered saline (PBS) thrice. A negative control containing serum-free medium with DMSO was also evaluated. Ten microlitres of acridine orange/ethidium bromide solution (100 μg/ml of acridine orange and 100 μg/ml of ethidium bromide in PBS) was added to every well and incubated for 10 min. Then the plates were washed with PBS solution. The images were recorded using a fluorescence microscope at 40× magnification (Nikon, Tokyo, Japan).
Mitochondrial membrane potential (MMP) by rhodamine-123 staining method
The HCC (HepG2) cells were treated with (10 µg and 12.5 µg/ml) of gold nanoparticles for 24 h. The HCC cells were washed with PBS thrice. A negative control containing serum-free medium with DMSO was also evaluated. And 0.5 ml of rhodamine-123 solution (5 μg/ml rhodamine-123 in PBS) was added to every well and incubated at 37 °C for 30 min. Then the plates were washed with PBS solution. The images were recorded using a fluorescence microscope at 40× magnification (Nikon, Tokyo, Japan).
Assessment of intracellular reactive oxygen species by dichlorofluorescein diacetate staining method
The HCC (HepG2) cells were treated with (10 and 12.5 µg/ml) of gold nanoparticles for 24 h. The HepG2 cells were washed with PBS thrice. A negative control containing serum-free medium with DMSO was also evaluated. Then 0.5 ml of 20 μM DCF-DA solution was added to every well and incubated for 30 min. Then the plates were washed with PBS solution. The images were recorded using a fluorescence microscope at 40× magnification (Nikon, Tokyo, Japan).
Apoptosis induction was quantified by colorimetric assay kit method
The HCC (HepG2) cells were treated with gold nanoparticles for 24 h. The initiation of apoptosis by the gold nanoparticles at varying concentrations (10 µg and 12.5 µg/ml) was assessed by quantifying the caspase-3, caspase-8 and caspase-9 activities on HepG2 cells using colorimetric assay kit. The procedure was followed according to the guidelines provided by the manufacturer. The data were compiled and expressed as fold change and percentage.
The gene expression of caspase-9, caspase-3, Bax, Bcl-2 and Bid was analyzed by real-time PCR
The HCC (HepG2) cells were treated with (10 and 12.5 µg/ml) of gold nanoparticles for 24 h. The gene expression of caspase-9, caspase-3, Bax, Bcl-2 and Bid in HepG2 cells were quantified using one step SYBR Green real-time PCR kit (Thermo Fisher Scientific, Waltham, MA). β-Actin used as an internal control housekeeping gene to normalize the gene expression values. The RNA was extracted and quantified using the kit. The quality of the RNA was assessed by using nanodrop spectrophotometer at 260 nm. The primers of Bax, Bcl-2, Bid, caspase-3 and caspase-9 were designed using the software. The procedure was followed using the manufacturer’s guidelines. Data analysis was performed using relative intensity of targeted genes. The forward and reverse primers of caspase-9, caspase-3, Bax, Bcl-2 and Bid are given in .
Table 1. The forward and reverse primers of caspase-9, caspase-3, Bax, Bcl-2 and Bid.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) and the group Mean ± SD was compared with Duncan multiple range tests using SPSS 21 software version (SPSS, Chicago, IL). *p ≤ .05 and #p ≤ .01 were considered to be statistically significant.
Results
Analysis of gold nanoparticles using UV-spectrophotometer
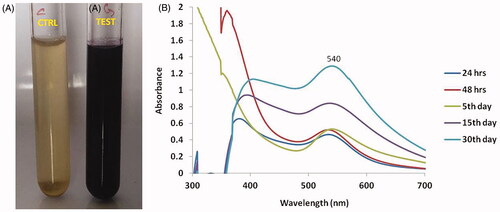
illustrates that the C. militaris extract was in pale yellow before it reacts with the gold nanoparticles. Then the extract colour was turned into deep brown after the reaction with gold nanoparticles. illustrates that the gold nanoparticles produced the peak at the 540 nm. The peak was gradually inclined along with the different reaction time (24 h, 48 h, 5th day, 15th day and 30th day). This proves the stability and integrity of gold nanoparticles over the days.
Figure 1. Synthesis and UV–visible spectrum absorption pattern of gold nanoparticles synthesized from C. militaris. (A) Represents that the C. militaris extract alone (pale yellow colour) and Gold nanoparticles with C. militaris extract (deep brown colour). (B) Represents that the gold nanoparticles with C. militaris extract develop the peak value at 540 nm.
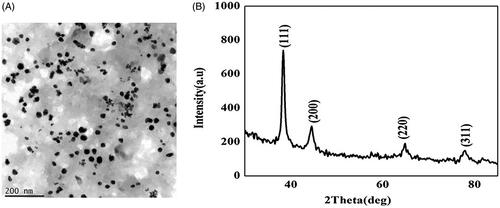
Analysis of gold nanoparticles using HR-TEM
illustrates the size and shape of synthesized gold nanoparticles were assessed by high-resolution TEM. The size of the synthesized gold nanoparticles was about 15–25 nm in diameter. The shape of the synthesized gold nanoparticles was spherical in nature.
Analysis of gold nanoparticles using XRD analysis
illustrates that the synthesized gold nanoparticles possess four Bragg’s diffraction peaks located at 2θ angles: 38.27, 44.65, 64.71 and 77.23 corresponded to (111), (200), (220), (311) confirms having face-centre-cubic structure [Citation20].
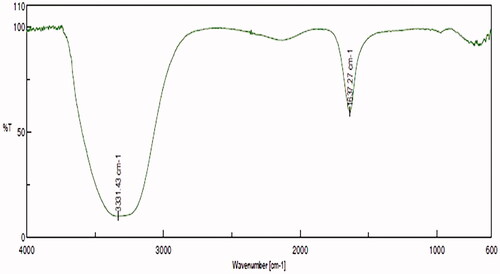
Analysis of gold nanoparticles using FT-IR
shows that the absorption bands at 3331.43 and 1637.27 cm−1 confirms the presence of secondary metabolites capping with the synthesized gold nanoparticles. The peak at 3331.41 confirms the presence of O–H stretch of polyphenols and the peak at 1637.27 confirms the presence of –C=C– stretch of alkynes.
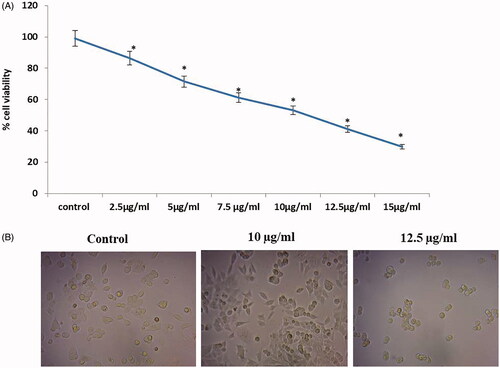
Cytotoxicity effect of gold nanoparticles from C. militaris on HepG2 cell line
illustrates the cytotoxic effect of gold nanoparticles from C. militaris on HepG2 cell line. The 100% viability of the HepG2 cells was observed in the untreated cells. The viability of the HepG2 cells was reduced in the treatment with varying concentration of AuNPs. After 24 h of treatment, the inhibitory concentration (IC50) of gold nanoparticles was found to be 10 and 12.5 µg/ml, respectively. The synthesized gold nanoparticles showed the anti-cancer effect on HepG2 cell line.
Figure 4. The cytotoxicity of gold nanoparticles synthesized from C. militaris was determined in HepG2 cells by MTT. (A) 50% cell viability of the HepG2 cells were observed in the 10 and 12.5 µg/ml concentration treated with AuNPs C. militaris. (B) Morphological analysis of AuNPs C. militaris treated HepG2 cells.
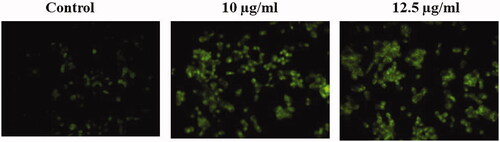
Effect of gold nanoparticles from C. militaris induces apoptotic morphological changes in HepG2 cells
illustrates that the untreated cells showed the absence of apoptosis by observing green fluorescence staining. The gold nanoparticles treatment (10 and 12.5 µg/ml) significantly increased the apoptotic cells by observing orange fluorescence staining in HepG2 cells. The apoptosis occurs in HepG2 cells due to the effective treatment of gold nanoparticles with C. militaris extract.
Figure 5. Effect of gold nanoparticles from C. militaris induces apoptotic morphological changes in HepG2 cells. The untreated cells showed the absence of apoptosis by observing green fluorescence staining. 10 µg and 12.5 µg/ml illustrates that the gold nanoparticles treatment significantly increased the apoptotic cells by observing orange fluorescence staining in HepG2 cells.
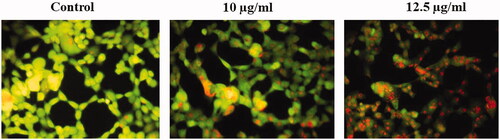
Effect of gold nanoparticles from C. militaris on the mitochondrial membrane potential (ΔΨm) of HepG2 cells
illustrates that the intensity of green fluorescence was found to be high in the untreated cells with high membrane potential. The intensity of green fluorescence was declined in the HepG2 cells treated with synthesized gold nanoparticles at the concentration of 10 and 12.5 µg/ml, respectively. The integrity of the mitochondrial membrane potential was maintained in the untreated cells. Likewise, the integrity of the mitochondrial membrane potential was disrupted in the AuNPs treated HepG2 cells. The effective concentration of gold nanoparticles was found to be 12.5 µg/ml.
Figure 6. Represents that the mitochondrial membrane permeability was determined in untreated and treated HepG2 cells with AuNPs. The mitochondrial membrane permeability was stable (strong fluorescence) in untreated HepG2 cells. The mitochondrial membrane permeability was damaged (weak fluorescence) in HepG2 cells treated with 10 µg/ml AuNPs. 10 µg/ml AuNPs represents that the mitochondrial membrane permeability was damaged more (weaker fluorescence) in HepG2 cells.
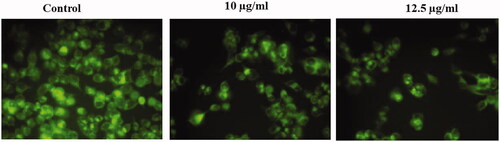
Cordyceps militaris gold nanoparticles induced ROS generation in HepG2 cells
illustrates that the intensity of green fluorescence was found to be decreased in the untreated cells. The intensity of green fluorescence was inclined in the HepG2 cells treated with synthesized gold nanoparticles at the concentration of 10 and 12.5 µg/ml, respectively. The gold nanoparticles treatment (12.5 µg/ml) increased the reactive oxygen species (ROS) generation in HepG2 cells were found to be effective.
Figure 7. Represents the status of the ROS in untreated and treated HepG2 cells with AuNPs C. militaris. The ROS induction was not observed in the untreated cells. The mild ROS induction was observed in HepG2 cells treated with 10 µg/ml AuNPs. The strong ROS induction was observed in HepG2 cells treated with 12.5 µg/ml AuNPs.
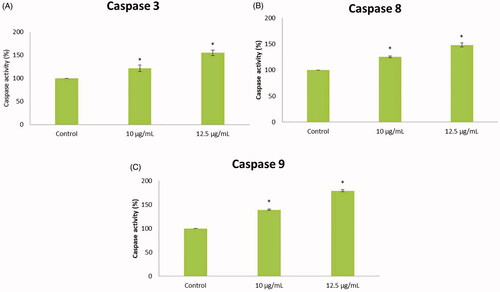
Gold nanoparticles from C. militaris induces caspase dependent apoptosis in HepG2 cells
In this study, the apoptosis was induced in the HepG2 cells treated with synthesized gold nanoparticles at varying concentrations (10 and 12.5 µg/ml) when compared with untreated cells. illustrates that the apoptotic caspase-3, -8 and -9 enzyme activities were markedly increased respectively in the treated groups, when compared with untreated cancer cells. The caspase-3 activity was markedly (1.2- and 1.5-fold change) increased in the gold nanoparticles (10 and 12.5 µg/ml) treated groups, when compared with untreated cancer cells. The caspase-8 activity was markedly (1.25- and 1.45-fold change) increased in the gold nanoparticles (10 and 12.5 µg/ml) treated groups, when compared with untreated cancer cells. The caspase-9 activity was significantly (1.4- and 1.5-fold change) increased in the gold nanoparticles (10 and 12.5 µg/ml) treated groups, when compared with untreated cancer cells. Our data proved that the synthesized gold nanoparticles induce apoptosis in the HCC cells.
Figure 8. The enzymatic activities of caspases were determined in untreated and treated HepG2 cells with AuNPs C. militaris. (A) Represents that the caspase 3 was increased in the AuNPs treated cells when compared with untreated HepG2 cells. (B) Represents that the caspase 8 was increased in the AuNPs treated cells when compared with untreated HepG2 cells. (C) Represents that the caspase 9 was increased in the AuNPs treated cells when compared with untreated HepG2 cells.
Evaluation of the effect gold nanoparticles with C. militaris on the caspase-9, caspase-3, Bax, Bcl-2 and Bid expression level using qPCR
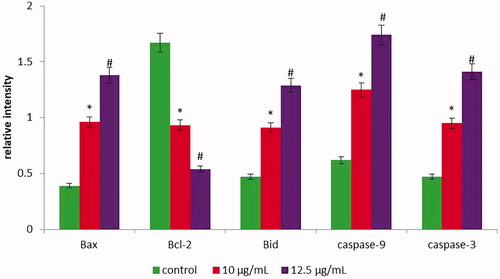
HepG2 cells were cultured and treated with gold nanoparticles with C. militaris (10 and 12.5 µg/ml). Caspase-9, caspase-3, Bax, Bcl-2 and Bid were examined in HepG2 cells after 24 h of treatment with C. militaris gold nanoparticles. Untreated HepG2 cells were used as a control to determine the relative intensity of the genes. illustrates that the apoptotic genes such as caspase-3, caspase-9, Bax and Bid expression were significantly (*p ≤ .05) increased and anti-apoptotic Bcl-2 was significantly (*p ≤ .05) decreased in the HepG2 cells treated with (10 µg/ml) gold nanoparticles with C. militaris, when compared with untreated HepG2 cells. Likewise, the apoptotic genes such as caspase-3, caspase-9, Bax and Bid expression were significantly (#p ≤ .01) increased and anti-apoptotic Bcl-2 was significantly (#p ≤ .01) decreased in the HepG2 cells treated with (12.5 µg/ml) gold nanoparticles with C. militaris when compared with untreated HepG2 cells.
Figure 9. The gene expression of caspase-9, caspase-3, Bax, Bcl-2 and Bid were determined in untreated and treated HepG2 cells with AuNPs C. militaris using q-PCR. The AuNPs (10 µg/ml) treatment significantly (*p ≤ .05) increased the mRNA expression of caspase-3, caspase-9, Bax, Bid and decreased the m-RNA expression of Bcl-2 in HepG2 cells, when compared to untreated cells. The AuNPs (12.5 µg/ml) treatment significantly (#p ≤ .01) increased the mRNA expression of caspase-3, caspase-9, Bax, Bid and decreased the m-RNA expression of Bcl-2 in HepG2 cells. Values were expressed as mean ± SD.
Discussion
Cancer is an escalating global health concern and it requires the development of natural based anti-cancerous therapeutics. Numerous natural extracts have been identified for anticancer properties on cancer cells. The research using mushrooms were extensive and it exhibits a broad spectrum of pharmacological activities [Citation21]. Traditional herbal medicines are potent and alternative for cancer treatment [Citation22]. In this study, we investigated the anticancer effects of gold nanoparticles with C. militaris extract against the HCC (HepG2) cells. The anticancer effects of C. militaris have been reported in very few research studies. The exact anti-cancer mechanism of C. militaris was not clear and established [Citation23–25]. In our research, the synthesized gold nanoparticles with C. militaris were used to study the appropriate mechanisms that induce the apoptosis in the HCC (HepG2) cells. Cordyceps species possess anti-inflammatory, antioxidant and anticancer activity. Cordyceps sinensis species halt the growth of leukaemia, melanoma, and lung cancer cells [Citation26–28]. Rao et al. [Citation23] proved that the C. militaris halt the exponential growth of HepG2 liver HCC cells.
Cordyceps militaris can trigger cancer cell apoptosis in caspase-linked pathways. Activation of caspases modulates the regulation of bid, which induces apoptosis in human liver cancer (HepG2) cells [Citation29]. Cordyceps militaris extract treatment also declined the human bladder carcinoma cells (T24 cells) survival, by activating the caspase-dependent pathways [Citation30]. Previous reports proved that the C. militaris have anticancer activity against several cancer cell lines including leukaemia, lung carcinoma, melanoma and liver carcinoma. Our data proved that the gold nanoparticles with C. militaris stability were maintained at the different time intervals (24 h, 48 h, 5th day, 15th day and 30th day). Wang et al. [Citation31] successfully synthesized the silver nanoparticles using C. militaris extract. Based on the previous article, we successfully synthesized the gold nanoparticles using C. militaris extracts. Likewise, the shape and size of the gold nanoparticles were identified using XRD and HR-TEM. Tsai et al. [Citation32] studied the functional groups present in the extract of the C. militaris using FT-IR. This data support us to evaluate the functional groups present in the gold nanoparticles with C. militaris using FT-IR. Our data revealed the presence of the hydroxy and alkynes groups.
Cordyceps militaris treatment decreased the cell viability of the HepG2 and MCF-7 cancer cells [Citation33]. Our data revealed that the gold nanoparticles with C. militaris having cytotoxic effects on the HCC cells. Our results concluded that the IC50 dosage of the gold nanoparticles with C. militaris was about 10 µg and 12.5 µg/ml, respectively. Song et al. proved that the C. militaris treatment disrupts the mitochondrial membrane potential of the HepG2 cells. Likewise, our study reveals that the AuNPs treatment damaged the integrity of the mitochondrial membrane potential of the HepG2 cells. Both the drug concentration effectively generates the ROS in the HCC cells. Lee et al. [Citation22] proved that the C. militaris treatment induces caspases mediated apoptosis in the human colorectal carcinoma RKO cells.
Cordyceps militaris extract treatment induces apoptosis in glioblastoma and leukaemia cells through caspase and Bcl-2/Bax-mediated pathways [Citation34]. Our results proved that the synthesized gold nanoparticles with the C. militaris activates that the caspases dependent pathway which initiates the apoptosis in the HCC (HepG2) cells. Cordyceps militaris treatment at varying concentrations (10 µg and 12.5 µg/ml) initiates the activation of apoptotic Bax, Bid and inhibits the anti-apoptotic Bcl-2 in HCC cancer cells.
Conclusion
In conclusion, our research presented the first proof of gold nanoparticles with C. militaris on HCC (HepG2) cells. We further demonstrate that the C. militaris might exert its anti-cancer effect on HCC cells by activating the gene expression of Bax, Bid, caspase 3 and caspase 9. These genes inhibit the activation of anti-apoptotic Bcl-2, which is responsible for cancer cell survival. The caspase-linked mitochondrial pathway was activated by the treatment with AuNPs induces cytotoxicity to the HepG2 cells. These findings provide pharmacological proof that the gold nanoparticles with C. militaris are expected to be inducing apoptosis in HepG2 cells. However, further in vivo studies are needed the anticancer activity of biosynthesized AuNPs alone or combined with further natural compounds to examine which may overcome side effects and it could be a novel approach for the development of anticancer therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–386.
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579.
- Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368.
- Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124:2785–2800.
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable factors in the United States in 2014. CA Cancer J Clin. 2018;68:31–54.
- Yoon SY, Park SJ, Park YJ. The anticancer properties of cordycepin and their underlying mechanisms. Int J Mol Sci. 2018;19:3027.
- Ismail E, Saqer A, Assirey E, et al. Successful green synthesis of gold nanoparticles using a Corchorus olitorius extract and their antiproliferative effect in cancer cells. Int J Mol Sci. 2018;19:2612.
- Tuli HS, Sharma AK, Sandhu SS, et al. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869.
- Zhou X, Gong Z, Su Y, et al. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–291.
- Kim DJ, Kang YH, Kim KK, et al. Increased glucose metabolism and alpha glucosidase inhibition in Codyceps militaris water extracts treated HepG2 cells. Nutr Res Pract. 2017;11:180.
- Lee SY, Debnath T, Kim SK, et al. Anti-cancer effect and apoptosis induction of cordycepin through DR3 pathway in the human colonic cancer cell HT-29. Food Chem Toxicol. 2013;60:439–447.
- Aramwit P, Porasuphatana S, Srichana T, et al. Toxicity evaluation of cordycepin and its delivery system for sustained in vitro anti-lung cancer activity. Nanoscale Res Lett. 2015;10:152.
- Li Y, Ke Y, Zou H, et al. Gold nano particles synthesized from Strychni semen and its anticancer activity in cholangiocarcinoma cell (KMCH-1). Artif Cells Nanomed Biotechnol. 2019;47:1610–1616.
- Lee H, Kim YJ, Kim HW, et al. Induction of apoptosis by Cordyceps militaris through activation of caspase-3 in leukemia HL-60 cells. Biol Pharm Bull. 2006;29:670–674.
- Jin CY, Kim GY, Choi YH. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 cells. J Microbiol Biotechnol. 2008;18:1997–2003.
- Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85:101–113.
- Lee DH, Kim HH, Cho HJ, et al. Cordycepin-enriched WIB801C from Cordyceps militaris inhibits collagen-induced [Ca2+]i mobilization via cAMP-dependent phosphorylation of inositol 1,4,5-trisphosphate receptor in human platelets. Biomol Ther. 2014;22:223–231.
- Shankar SS, Ahmad A, Pasricha R, et al. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13:1822–1826.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262.
- Krishnaraj C, Muthukumaran P, Ramachandran R, et al. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep. 2014;4:42–49.
- Madhavi J, Anand S, Singh KS, et al. Anticancer antibacterial and antioxidant activities of Cordyceps militaris. IJEB. 2019;57:15–20.
- Lee HH, Lee S, Lee K, et al. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. Daru. 2015;23:35.
- Rao YK, Fang SH, Wu WS, et al. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator’s production and human cancer cell proliferation. J Ethnopharmacol. 2010;131:363–367.
- Lee J, Chatterjee DK, Lee MH, et al. Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett. 2014;347:46–53.
- Gu YX, Wang ZS, Li SX, et al. Effect of multiple factors on accumulation of nucleosides and bases in Cordyceps militaris. Food Chem. 2007;102:1304–1309.
- Thakur A, Hui R, Hongyan Z, et al. Pro-apoptotic effects of Paecilomyces hepiali, a Cordyceps sinensis extract on human lung adenocarcinoma A549 cells in vitro. J Can Res Ther. 2011;7:421–426.
- Park C, Hong S, Lee J. Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis. Oncol Rep. 2005;13:1211–1216.
- Wu JY, Zhang QX, Leung PH. Inhibitory effects of ethyl acetate extract of Cordyceps sinensismycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine. 2007;14:43–49.
- Shao LW, Huang LH, Yan S, et al. Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signalling pathways. Oncol Lett. 2016;12:995–1000.
- Cao HL, Liu ZJ, Chang Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumour Biol. 2017;39. doi:10.1177/1010428317706915
- Wang L, Liu CC, Wang YY, et al. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr Appl Phys. 2016;16:969–973.
- Tsai CH, Yen YH, Yang JP. Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J Foods Drug Anal. 2015;23:63–70.
- Song J, Wang Y, Teng M, et al. Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells. Mol Med Rep. 2016;13:5132–5140.
- Yang CH, Kao YH, Huang KS, et al. Cordyceps militaris and mycelial fermentation induced apoptosis and autophagy of human glioblastoma cells. Cell Death Dis. 2012;3:e431.