Abstract
The purpose of this study was to investigate the inhibitory effect of baicalein on the proliferation of cervical carcinoma cells and stimulate cervical carcinoma cells with baicalein. MTT method was used to observe cell proliferation. Flow cytometry was used to observe cell cycle, and gene technology was used to observe the expression of corresponding genes at the level of gene and protein. β-catenin activity was assessed using Western blot and ChIP. Baicalein suppressed cervical carcinoma cell HeLa proliferation by enhancing the activity of caspase-3. Baicalein blocked cell cycle at G0/G1 stage by inhibiting the expression of some genes. At the same time, it can prevent the nuclear translocation of β-catenin and inhibit the activity of Wnt. When the Wnt signaling pathway is increased, the proliferation of HeLa cells is inhibited, and apoptosis is promoted in this way. In conclusion, it indicated that baicalein inhibits cervical carcinoma progression by targeting CCND1 via Wnt/β-catenin signaling pathway.
Introduction
Cervical carcinoma is the second most common malignancy in women with the morbidity only after mammary carcinoma around the world [Citation1]. Currently, surgery and chemoradiotherapy are the most commonly used methods to treat cervical carcinoma. Its largest drawback is that healthy cells are also killed, which may cause serious complications. In recent years, numerous researches investigated the potential anticarcinoma properties of natural products, which are considered as non-toxic and thus may have fewer side effects [Citation2]. The potential of natural products from medicinal plants used in the prevention and treatment of carcinoma has been recognized by the scientific community. Traditional Chinese medicine and its active ingredients can be selected in carcinoma treatment via a variety of mechanisms, including inducing apoptosis [Citation3], inhibiting telomerase activity [Citation4], suppressing angiogenesis, improving immune functions and cytotoxicity. The majority of studies aimed to shrink or eliminate carcinoma cells.
Baicalein is a flavonoid derived from the root of Scutellaria baicalensis, exhibiting various biological activities, such as antimicrobial, antitumor, anti-inflammation and anti-ischemia properties [Citation5]. Baicalein shows a significant anticarcinoma effect both as a single or combined treatment. It is found that the infection of baicalein was associated with the inhibition of numerous types of malignant tumor (for instance, the gastric carcinoma, bladder carcinoma, mammary carcinoma, melanoma/skin carcinoma, colorectal carcinoma, pancreatic carcinoma and multiple myeloma) [Citation6]. The crucial mechanisms of the baicalein consist of restraining specific cyclin-dependent kinase (CDK) or cyclin to block cell cycle, scavenging oxidized free radicals and suppressing MAPK, AKT, or mTOR. Apoptosis was induced by activation of caspase-9/-3 and attenuating invasion and metastasis of tumors by downregulating MMP-2/9 expression [Citation6]. Nevertheless, the role of baicalein on cervical carcinoma and the mechanisms still needs further investigation.
CCND1 belongs to the cyclin family, which is associated with the regulation of cell cycle. During cell cycle, CCND1 forms a complex with other associated cyclins and regulates the activation of cyclin-dependent kinases (CDKs) – CDK4 and CDK6, whose activity is associated with G1/S transition [Citation7]. In addition, the expression of CCND1 is regulated positively by Rb [Citation8] and negatively by P27 [Citation9]. Previous studies found that CCND1 plays an oncogene role in multiple cancers, such as lung cancer [Citation9], breast cancer [Citation10], hepatocellular carcinoma [Citation11] and renal cell carcinoma [Citation12]. CCND1 is a potential therapeutic target of cervical carcinoma [Citation8].
Wnt is a secreted glycoprotein family with various roles in the development of tumors, such as cell cycle, invasion and infiltration. Wnt signaling prevents β-catenin degradation, accelerate the translocation of chromosomes in the nucleus of carcinoma cells and precisely act on the promoter of the downstream gene of LEF/TCF complex [Citation13,Citation14]. Moreover, previous researches reported that Wnt signaling pathway is extremely important in the invasion of carcinoma cell through its targets which, downstream, including Rb, Cyclin D1, and MMP-2 [Citation15–17]. However, the impact of Baicalein in cervical carcinoma and the Wnt signaling pathway has not yet been elucidated. We proposed that baicalein may play a role in inducing cervical carcinoma apoptosis by targeting CCND1 via Wnt/β-catenin signaling pathway.
Materials and methods
Clinical samples
Cervical carcinoma tissues were obtained by surgery on patients in the Affiliated TCM Hospital of South West Medical University from 2014 to 2017. The cervical carcinoma was diagnosis based on WHO pathological diagnostic criteria. Normal cervical samples were collected from the adjacent normal tissue. Samples include 10 cervical carcinoma tissues and 10 pairs of normal samples were compared. All subjects had signed informed consent.
Cell line
The human normal cervical epithelial cell line HUCEC, and human cervical carcinoma cell lines HeLa, MS751, SN12C, CaSki, C-33A and KBV1 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) medium supplement by 10% fetal bovine serum (FBS; Hyclone), 50 U/ml penicillin, and 250 µg/ml streptomycin at 37 °C in a humidified incubator containing 5% CO2. Baicalein purchased from China Institute of pharmaceutical, and biological products were dissolved in dimethyl sulfoxide (DMSO) at 50 mol/l.
Cell transfection
The cells were transfected with Lipofectamine®2000 (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) according to the manual. For the gene expression analyses, cells were seeded in 6-well plates and transfected with 1 μg plasmid DNA per well; 50 ng CCND1 or CCND1 siRNA, 300 ng pDUO-hMD-2/CD14 adjusted with pcDNA3.1 to 1 μg. The total plasmid load was adjusted to 1 μg plasmid DNA with pcDNA3.1.
MTT
MTT was used to determine cell viability. Cells were inoculated into 96-well plates, with about 1 × 103 cells per well (BD Biosciences, Bedford, MA, USA). MTT (5 mg/ml, 20 μl) (Sigma-Aldrich, St. Louis, MO, USA) was added to each micropore, place the Petri dish in a 37 degree Celsius incubator, incubate in the presence of 5% carbon dioxide and remove it after 4 h. Formamide was dissolved with dimethyl sulfoxide (DMSO; Genview, Beijing, China). Absorption was measured with a micro flat-panel reader (BioRad 680, Hercules, CA, USA) with the wavelength at 570 nm.
Cell cycle
HeLa cells were collected and washed with PBS twice. Take 1 ml 70% ethanol, pre-cool it to 4 °C, then add cells to it and incubate overnight. After that, PBS was used to wash the cells, and the cells were treated with 100 mg/l RNase at 37 °C for 30 min. Then, the cells were stained by 50 mg/l PI at 4 °C for 30 min avoid of light. At the end of the period, the HeLa cells were determined using flow cytometry. The primary result of sub-G1 stage, G0/G1 stage, S stage and G2/M stage was analyzed using cell cycle BD™ LSRII flow cytometry system (BD Bioscience, Franklin Lakes, USA). All the experiments were repeated for three times.
Colony formation assay
HeLa cells were placed into 6-well Ultra Low Cluster plate (Corning) at 500 cells/well and were maintained in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) for 10–12 days. After that, the HeLa cell spheres characterized as tight, spherical and non-adherent masses >50 µm in diameter was counted by microscope (Olympus, Tokyo, Japan). Sphere formation efficiency = colonies/input cells × 100%.
ChIP
ChIP was performed as described previously [Citation18]. HeLa cells were cross-linked with 1% formaldehyde for 10 min and quenched with glycine (125 mM). After centrifugation, these particles were re-suspended in SDS buffer (0.1%). Next, ultrasound was used to break these cells into fragments of about 200–600 bp. Immunohistochemistry of β-catenin was carried out using chip-level protein G magnetic beads while the IgG as negative control. The qPCR was performed with tiled primers.
TOP flash/FOP flash reporter
In order to estimate the Wnt/β-catenin activity, β-catenin/TCF were transfected according to according to the instructions (Millipore Billerica, MA, USA), 1 × 104 cells were transiently transfected with either 2 μg of pTOPflash or pFOPflash plasmids (TCF Reporter Plasmid/mutant, inactive TCF binding site, Millipore) and pSV40-Renilla plasmid (0.5 μg, Promega) as an internal control by use of Lipofectamine 2000 (Invitrogen). After 48 h, Strictly, according to the instructions, both Renilla and Firefly luciferase were analyzed using Dual-Glo Luciferase Assay System (Promega), with a Glomax luminometer (Promega).
Real-time PCR
According to manufacturer’s instructions, total RNA was extracted from the neutrophils by Trizol and reverse transcribed to cDNA. The primers were designed using PrimerPremier 6.0 software and synthetized using Invitrogen. Real-time PCR was performed at 55 °C for 1 min, followed by 35 cycles of 92 °C for 30 s, 58 °C for 45 s, and 72 °C for 35 s. GAPDH was selected as internal reference. The relative expression of mRNA was calculated by 2−△Ct method.
Western blot
After different treatments, the cells were lysed on ice and quantified using BCA method. The isolated proteins were electrophoresed using 10% SDS-PAGE at 60 V for 5 h. The gel was transferred to PVDF membrane by semi-dry transfer method at 100 mA for 1.5 h. After blocked by 5% skim milk for 2 h, the membrane was incubated in primary antibody at 4 °C overnight. After incubated in secondary antibody avoid of light for 30 min, the membrane was imaged using chemiluminescence reagent for 1 min and analyzed using image processing system software and Quantity one software. The experiment was repeated for four times (n = 4).
Statistical analysis
Statistical analysis was applied on SPSS software 16.0 (SPSS Inc., Chicago, IL, USA). All data were presented as the mean ± standard deviation and compared by t-test. A p value of <0.05 was considered as statistical significance. All experiments were repeated at least three times.
Results
CCND1 was upregulated in human cervical carcinoma tissues and cell lines
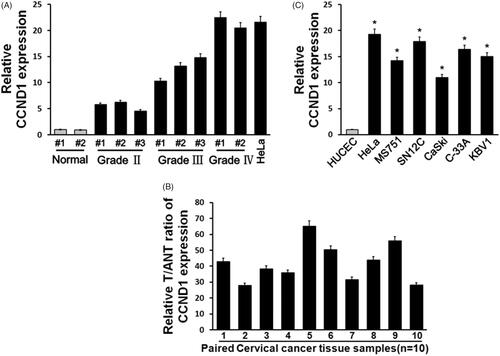
To identify CCND1 expression in human cervical carcinoma, we compared CCND1 level in human cervical carcinoma tissues in different clinical stages. It was showed that CCND1 significantly upregulated in cervical carcinoma tissues compared with healthy control with grade dependence (p < .05, ). Next, we tested 10 pair of cervical carcinoma tissues and relative adjacent controls by real-time PCR. It was demonstrated that CCND1 level in cervical carcinoma tissues was obviously higher than the control (p < .05, ). Moreover, we selected six cervical carcinoma cell lines and observed that CCND1 level was markedly elevated compared with normal cervical epithelial cell line HUCEC (p < .05, ).
Figure 1. CCND1 expression in cervical carcinoma tissues and cell lines. (A) CCND1 expression in cervical carcinoma tissues and normal cervical tissues in different grades. (B) CCND1 expression in paired cervical carcinoma tissues and normal tissues. (C) CCND1 expression in cervical carcinoma cell lines and normal HUCECs. *p < .05 vs control.
Baicalein restrained HeLa cell proliferation induced by CCND1 overexpression
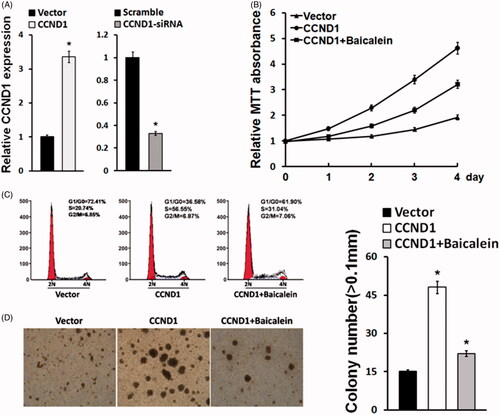
Since CCND1 was upregulated in cervical carcinoma tissues and cell lines, we speculated that CCND1 may play a crucial role in cervical carcinoma. We applied lentivirus for transfection to enhance or decline CCND1 expression in cervical carcinoma cell line HeLa cells, which showed the relatively highest level of CCND1 among different cervical carcinoma cell lines. Real-time PCR confirmed that CCND1 mRNA level in HeLa cells was changed (). Moreover, we tested the influence of CCND1 and Baicalein on cell behavior. MTT assay demonstrated that CCND1 facilitated HeLa cell proliferation, whereas Baicalein obviously restrained the impact of CCND1 on HeLa cell viability (). Flow cytometry revealed that CCND1 promoted HeLa cells in S stage while Baicalein arrested cell cycle (). Colony formation assay showed that CCND1 apparently enhanced sphere formation compared with control while Baicalein attenuated its influence (). It indicated that CCND1 may affect HeLa cells proliferation, cell cycle and stemness.
Figure 2. Baicalein reduced proliferation and apoptosis-related gene expression upregulated by CCND1. (A) CCND1 expression in HeLa cells after CCND1 or CCND1 siRNA transfection. (B) The results of cell cycle detection by flow cytometry. (C) HeLa cell proliferation detected using MTT assay. (D) Cell colony formation after CCND1 transfection or baicalein treatment. *p < .05 vs control.
Baicalein reduced proliferation and apoptosis-related gene expression upregulated by CCND1
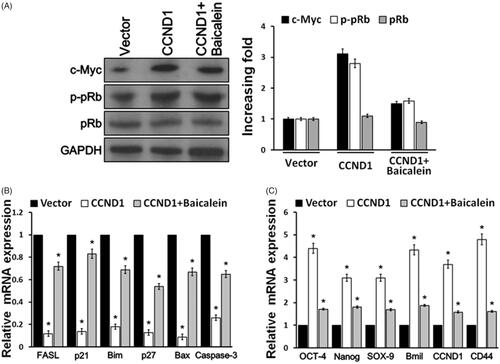
Furthermore, we tested Rb phosphorylation, apoptosis-related gene and stemness-related gene expressions in order to investigate the mechanism of CCND1 and baicalein on cervical cell behavior. Western blot exhibited that CCND1 overexpression significantly upregulated c-Myc expression and pRb phosphorylation, whereas baicalein reduced their levels enhanced by CCND1 in HeLa cells (). Next, real-time PCR demonstrated that apoptotic factor FASL, p21, Bim, p27, Bax and Caspase-3 levels were obviously decreased by CCND1 transfection, while their mRNA expressions were enhanced by baicalein (). Furthermore, we explored the influence of CCND1 on cell stemness-related factors. It was found that OCT4, Nanog, SOX-9, Bmil and CD44 levels were markedly elevated induced by CCND1 overexpression. On the other hand, baicalein apparently declined their levels compared with CCND1 transfection group ().
Figure 3. Baicalein reduced proliferation and apoptosis-related gene expression upregulated by CCND1. (A) Western blot was used to detect the expression of c-Myc and pRb in Hela cells. (B) Real-time PCR detection of apoptosis-related gene expression in HeLa cells. (C) Real-time PCR detection of stemness-related gene expressions in HeLa cells. *p < .05 vs control.
Baicalein targeted CCND1 through Wnt signaling pathway
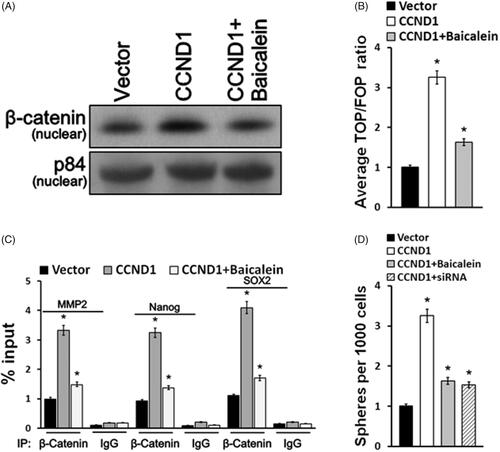
Wnt signaling pathway played a critical role in cervical carcinoma development. For this purpose, we intended to investigate whether baicalein affects CCND1 by regulating Wnt signaling pathway activity. First, Western blot results confirmed that nuclear β-catenin expression upregulated in CCND1 transfection group while baicalein reduced its nuclear translocation induced CCND1 (). TOP/FOP ratio was significantly enhanced in HeLa cells after CCND1 overexpression while it downregulated after baicalein treatment (). Furthermore, the ChIP assay was applied to evaluate the activity of Wnt signaling pathway and was demonstrated that CCND1 can accelerate the β-catenin of nuclear binding to the Nanog’s promoter region, MMP2 and SOX2, and facilitating cervical carcinoma cell colony formation (). Baicalein effectively blocked the β-catenin of nuclear binding with the Nanog’s promoter region, MMP2 and SOX2, and suppressing cervical carcinoma cell colony formation induced by CCND1, which was similar to CCND1 siRNA transfection ().
Figure 4. Baicalein targeted CCND1 via Wnt signaling pathway. (A) The expression of β-catenin in nucleus was detected using Western blotting. (B) TOP/FOP ratio of Wnt signaling pathway activity in HeLA cells was measured. (C) Sox 2, Nanog and MMP-2 levels were detected in HeLA cells. (D) Cells treated with baicalein or transfected with CCND1/CCND1 siRNA formed colonies. *p < .05 vs control.
Discussion
One of the main strategies of anticarcinoma drug is to block carcinoma cell’s growth. The results of MTT suggested baicalein can inhibit the overexpression of CCND1, thereby reducing the induction of HeLa cell growth. This effect is time-dependent. The morphology and function of apoptotic cells have changed. This kind of New Year's greeting may affect the cycle of apoptotic cells. These results suggest that baicalein can induce apoptosis by activating caspase-3 and 9 [Citation6]. Hence, HeLa cells were used to detect the expression of CCNd1 and its changes under the action of baicalein. Caspase-3 was cleaved from procaspase-3 and poly (ADP-ribose) polymerase that was cut at Asp216-Gly217 once activated, In this way, DNA between nucleosomes divides and cells apoptosis [Citation19]. We can learn from the results that baicalein obviously enhanced caspase-3 activity diminished by CCND1. Moreover, in the detection of FASL and Bax expression, baicalein has been verified to cause apoptosis in cervical carcinoma cells.
Cell cycle regulation is regulated many factors, such as CDKs, Bim, p21 and the RB gene. A variety of studies illustrated the relevance of cell cycle dysregulation in several types of human carcinoma [Citation20]. In spite of many reports about Baicalein ability in blocking cell cycle at checkpoints, few researches have inquired into the mechanism of baicalein in the cervical carcinoma cell cycle. Recent research showed that HeLa cell can be regulated by baicalein in the distribution of the cell cycle, especially by increasing the proportion of cells in the G0/G1 stage while reducing the proportion of cells in the S stage.
In this study, baicalein was used to treat HeLa cell cycle proteins D1, p21 and Rb, and their expression levels were detected before and after treatment. The aim was to further explore the changes of cell cycle under the action of baicalein. Cyclin D1, as a critical regulator of the G1 checkpoint, was mainly expressed in the early stage of G1. Then, cyclin D1/CDK4/6 forms a complex, which is regulated by Rb phosphorylation [Citation21]. It has been suggested that Rb expression and phosphorylation play a key regulatory role in cell cycle [Citation22]. It has been proved by experiments that the overexpression of d1, Bim and p21 were due to the overexpression of CCND1 and baicalein can reduce it. However, baicalein treatment can reduce Rb phosphorylation, suggesting that it may cause cell arrest in G1 phase. However, it puts forward requirements for studying the mechanism of baicalein affecting cell cycle.
Increasing evidence suggests that abnormal Wnt/β-catenin signaling pathways may cause carcinomas including endometrial carcinoma [Citation23]. The Wnt/β-catenin signaling cascade can be utilized to target carcinoma cells and has a strong potential for carcinoma treatment. In this signaling pathway, β-catenin is mainly used as a mediator to achieve carcinogenic effect. More and more studies suggest that if we want to inhibit the signal activity of Wnt/β-catenin, we can achieve the goal by reducing the level of β-catenin and utilizing the upstream effect of targeting [Citation24,Citation25]. In addition, in endometrial carcinoma and other malignant tumors, gene mutation of β-catenin can be found, so that it cannot play a normal role in Wnt/β-catenin signaling cascade upstream effectors. This principle is used to treat carcinoma with inhibitors [Citation26]. On the other hand, we also found a better way to inhibit Wnt/β-catenin signaling activity, that is, to apply targeted β-catenin/TCF protein complexes. In fact, in many studies on small molecules, this signaling activity has been proved to be successfully suppressed [Citation27]. The canonical Wnt/β-catenin signaling pathway has been extensively characterized as a primary driver of cell growth and proliferation [Citation28]. During G1, Wnt signaling induces the transition to the S-phase by active transcription of c-Myc, promoting passage through the restriction point [Citation29]. c-MYC upregulates expression of cyclin D1 and represses expression of CDK4 inhibitors p21 and p27 [Citation30]. Cyclin D1 binds to and activates CDK4, which phosphorylates and inactivates RB, promoting passage through the restriction point. This study suggests that baicalein may be a potential inhibitory target since it can negatively regulate Wnt/β-catenin signaling. We have found several evidences that baicalein can inhibit the transcriptional activity of β-catenin. It can be seen from experiments that the transcriptional activity of β-catenin is significantly inhibited by baicalein. These evidences can serve as a theoretical basis, on which we propose a hypothesis that baicalein may destroy the transcriptional function of β-catenin, which is induced by CCND1. For a long time, researchers have believed that Wnt signaling pathway can regulate the occurrence and development of endometrial carcinoma. In agreement, baicalein can also inhibit the nuclear translocation of β-catenin, and combine with the promoters of Sox-2, MMP-2 and Nanog genes. The results further indicate that baicalin can specifically affect some genes, which can regulate the growth and development of endometrial carcinoma cells. Taken together, baicalein represented a key regulator for suppressing cervical carcinoma cell proliferation by its ability to targeting Wnt signaling pathway to restrain CCND1.
Flavonoids have been explored for their possible functions of hormone-dependent carcinoma because its molecular structure is very similar to steroid. Baicalein belongs to the anthocyanin family and has been proved to have estrogen-mediated effects by many studies [Citation31]. Baicalein and other extracts have been previously observed to arrest the cell cycle during the S and G2/M-stages in MCF-7 human mammary carcinoma cells by suppressing 17β-estradiol-induced transactivation of estrogen receptor α [Citation32]. Baicalein interferes with 17β-estradiol (E2). E2 can induce a novel G protein-coupled estrogen receptor-related signaling pathway. When this pathway is inhibited, E2-induced carcinoma cell migration and invasion will also be inhibited [Citation33]. Baicalein can regulate the NF-ĸB pathway as well as the estrogen-like activity of this pathway, thus inhibiting the production of inflammatory cytokines, especially the verification induced by lipopolysaccharide. These results indicate that baicalein has a theoretical basis for preventing inflammation-related diseases. However, the inhibition of baicalein on cervical carcinoma cells and its effect on estrogen-like activity remain to be further studied.
In conclusion, CCND1 can induce the proliferation of HeLa cells while baicalein can inhibit the expression of CCND1 in a time-dependent manner. Baicalein is presumed to inhibit the expression of CCND1 by blocking Wnt signaling pathway and cell cycle. The results show that baicalein has the potential to be an ideal drug for the treatment of cervical carcinoma. However, the molecular mechanism of this anti-carcinoma effect needs further study to elucidate.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Di Domenico F, Foppoli C, Coccia R, et al. Antioxidants in cervical cancer: chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta. 2012;1822:737–747.
- Peng Y, Guo C, Yang Y, et al. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol Med Rep. 2015;11:2129–2134.
- Mandal SK, Biswas R, Bhattacharyya SS, et al. Lycopodine from Lycopodium clavatum extract inhibits proliferation of HeLa cells through induction of apoptosis via caspase-3 activation. Eur J Pharmacol. 2010;626:115–122.
- Park SE, Yoo HS, Jin CY, et al. Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem Toxicol. 2009;47:1667–1675.
- Tsang PW, Chau KY, Yang HP. Baicalein exhibits inhibitory effect on the energy-dependent efflux pump activity in non-albicans Candida fungi. J Chemother. 2015;27:61–62.
- Liu H, Dong Y, Gao Y, et al. The fascinating effects of baicalein on cancer: a review. IJMS. 2016;17:1681.
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20.
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566.
- Li N, Zeng J, Sun F, et al. p27 inhibits CDK6/CCND1 complex formation resulting in cell cycle arrest and inhibition of cell proliferation. Cell Cycle. 2018;17:2335–2348.
- Yang L, Ye F, Bao L, et al. Somatic alterations of TP53, ERBB2, PIK3CA and CCND1 are associated with chemosensitivity for breast cancers. Cancer Sci. 2019;110:1389–1400.
- Chan AW, Zhang Z, Chong CC, et al. Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma. J Pathol. 2019. [Epub ahead of print]. doi: 10.1002/path.5313
- Karim S, Al-Maghrabi JA, Farsi HM, et al. Cyclin D1 as a therapeutic target of renal cell carcinoma – a combined transcriptomics, tissue microarray and molecular docking study from the Kingdom of Saudi Arabia. BMC Cancer. 2016;16:741.
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239.
- Murad LB, da Silva Nogueira P, de Araujo WM, et al. Docosahexaenoic acid promotes cell cycle arrest and decreases proliferation through WNT/β-catenin modulation in colorectal cancer cells exposed to γ-radiation. Biofactors. 2019;45:24–34.
- Planutiene M, Planutis K, Holcombe RF. Lymphoid enhancer-binding factor 1, a representative of vertebrate-specific Lef1/Tcf1 sub-family, is a Wnt-beta-catenin pathway target gene in human endothelial cells which regulates matrix metalloproteinase-2 expression and promotes endothelial cell invasion. Vasc Cell. 2011;3:28.
- Mao D, Qiao L, Lu H, et al. B-cell translocation gene 3 overexpression inhibits proliferation and invasion of colorectal cancer SW480 cells via Wnt/β-catenin signaling pathway. Neoplasma. 2016;63:705–716.
- Gore AJ, Deitz SL, Palam LR, et al. Pancreatic cancer-associated retinoblastoma 1 dysfunction enables TGF-β to promote proliferation. J Clin Invest. 2014;124:338–352.
- Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–748.
- Lyakhovich A, Surralles J. Constitutive activation of caspase-3 and Poly ADP ribose polymerase cleavage in fanconi anemia cells. Mol Cancer Res. 2010;8:46–56.
- Wachtel M, Schafer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat Rev. 2010;36:318–327.
- Caldon CE, Sutherland RL, Musgrove E. Cell cycle proteins in epithelial cell differentiation: implications for breast cancer. Cell Cycle. 2010;9:1918–1928.
- Broceno C, Wilkie S, Mittnacht S. RB activation defect in tumor cell lines. Proc Natl Acad Sci USA. 2002;99:14200–14205.
- Dellinger TH, Planutis K, Tewari KS, et al. Role of canonical Wnt signaling in endometrial carcinogenesis. Exp Rev Anticancer Ther. 2012;12:51–62.
- Gurney A, Axelrod F, Bond CJ, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–11722.
- Chan DW, Mak CS, Leung TH, et al. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–1556.
- Machin P, Catasus L, Pons C, et al. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33:206–212.
- Sukhdeo K, Mani M, Zhang Y, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA. 2007;104:7516–7521.
- Rasmussen ML, Ortolano NA, Romero-Morales AI, et al. Wnt signaling and its impact on mitochondrial and cell cycle dynamics in pluripotent stem cells. Genes (Basel). 2018;9:109.
- Vadlakonda L, Pasupuleti M, Pallu R. Role of PI3K-AKT-mTOR and Wnt signaling pathways in transition of G1-S phase of cell cycle in cancer cells. Front Oncol. 2013;3:85.
- Matsu-Ura T, Moore SR, Hong CI. WNT takes two to tango: molecular links between the circadian clock and the cell cycle in adult stem cells. J Biol Rhythms. 2018;33:5–14.
- Zhu JT, Choi RC, Chu GK, et al. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death. J Agric Food Chem. 2007;55:2438–2445.
- Wang CZ, Li XL, Wang QF, et al. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17:63–68.
- Shang D, Li Z, Zhu Z, et al. Baicalein suppresses 17-beta-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol Rep. 2015;33:2077–2085.
