Abstract
To evaluate the long-term biological and clinical results of anterior cervical corpectomy and fusion (ACCF) with a synthesized nano-graphene oxide (nano-GO) loaded hydroxyapatite/polyamide (HAp/PA) strut in the implantation treatment of cervical reconstruction. Bio-ceramic Hydroxyapatite (HAp) combined with suitable polymer matrix is one of the furthermost naturally occurring biomaterial form for hard tissues and dental regeneration therapies due to their bone-like chemical compositional structure and biocompatibility similarity to native bone of the human body. In the present investigation, the development of nano-GO loaded HAp/PA composite strut for anterior cervical reform and fusion properties after corpectomy is studied. Forty patients who suffered from first or second level ACCF, treated with nano-GO loaded HAp/PA strut, were investigated. At final follow-up period, the fusion rate was 99%, and the subsidence value of the prepared strut was 5%. The results of cell viability and proliferation analyses indicated that the prepared nanocomposites did not exhibit non-cytotoxicity with the human cells. In summary, the satisfactory consequences in this research work designated that the nano-GO loaded HAp/PA strut was an active implant in the treatment of cervical reconstruction. Furthermore, the osteoconductive and osseointegration properties of the prepared struts need to be analyzed and optimized for future bio-medical usages.
Introduction
The surgical therapies of the cervical corpectomy and fusion are exceedingly mutable including different approaches such as methods of anterior, posterior and including combination treatment of anterior–posterior and posterior–anterior strategies [Citation1–3]. Among these fixation approaches, only the anterior method is recommended for the close/open reduction, instrumental fusion, discectomy and specifically for intervertebral disc herniation [Citation4,Citation5]. The treatment of anterior cervical corpectomy and fusion has been considered as the most common and fruitful approach for the multi-level therapies of cervical stenosis with spinal cord compression. Though satisfactory decompression abilities of the cervical canal have been attained with methods of cervical reconstruction and corpectomy, a fusion of the anterior cervical plate is tremendously hard and adjustable. Though, the previously reported results from clinical and biochemical analyses have stated the low stability properties of anterior plate fixation and hardware failure of anterior plating, which was needed to improve the stability and biological properties of anterior instrumental fusion [Citation6–9]. Previously, different approaches to bone grafting have been established for the successful cervical spine reconstruction and fusion. Classically, the isolated tricortical bone struts from iliac crest have been used mostly and achieved excellent fusion rate of ∼85% as per the clinical trials. Nonetheless, it would have troubled a patient due to the problems of supplementary surgery for bone graft gathering, donor site morbidity, postoperative pain and importantly long postoperative period. Hence, to avoid these troubles, the artificially prepared titanium mesh cage containing autologous bone has been developed and applied predominantly for cervical reconstruction and fusion after corpectomy, which has achieved high fusion rate of ∼95%. Consequently, the researches for substitute approaches have been continued by bio-medicinal researchers in clinical institutes. Artificial bone graft materials are good as bone implantation alternates in that they can evade the problems of donor site morbidity, less biocompatibility and risk of microbial infections [Citation2,Citation10–13].
Bio-ceramic Hydroxyapatite (HAp) is one of the furthermost naturally occurring ceramic form for hard tissues and dental regeneration therapies due to their bone-like chemical compositional structure and biocompatibility similarity to the native bone of the human body [Citation14–17]. Nevertheless, because of some mechanical and stability deficiency of HAp in the pure form that are comparable to native bone tissues, the usage of HAp materials is limited in implantation therapies [Citation18,Citation19]. The synthesized HAp bio-ceramic is challenged with an essentially greater dissolution rate in an in vivo biological environmental condition and also have low corrosion resistance properties in acidic conditions [Citation20–22]. Furthermore, HAp material has deprived thermal stability, which results in its easy decomposition and conversion into other inorganic phases due to its objectionable and debauched dissolution ability in vivo. The chief problem of HAp materials is its informal brittle nature in the load-bearing therapeutic applications [Citation23–25]. The incorporation of supporting bioactive materials have solved these types of problems and also improved stability and bioactivity. The bioactive materials like biopolymer (gelatin, chitosan, cellulose, etc.,) and carbon forms (graphene oxide (GO), carbon nanotube, etc.) have been used to improve its bioactivity and stability in the biomedical applications [Citation23,Citation24,Citation26–29]. Particularly, graphene oxide has been applied in abundant biomedical areas, such as drug delivery, bone regeneration, biosensing and bioimaging. The synthesized GO has excellent properties of biocompatibility, large surface to volume ratio and excellent dispersity nature in aqueous medium and also have outstanding optical and electrical properties [Citation28,Citation30–32]. In addition, the substitution of GO nanomaterial with HAp material could improve the cell attachment abilities and corrosion protection of Titanium implanting substrate in aqueous SBF medium [Citation33,Citation34]. In the current investigation, the development and investigation of nano-GO loaded hydroxyapatite/polyamide (HAp/PA) composite strut for anterior cervical reform and fusion properties after corpectomy. The chief aim of this report was to evaluate the biological activity, in vivo safety and efficiency of this novel composite strut and deliberate its main advantages and inadequacies, confidently leading to progress of a facile approach for cervical reconstruction and fusion.
Materials and methods
Synthesis of GO
The nano-GO was synthesized under a modified Hummers method by using commercially obtained graphite powder [Citation35,Citation36]. The appropriate amount (2 g) of graphite powder was dissolved into the cold natured concentrated sulfuric acid (98%) with the addition of NaNO3 under an ice bath. The above mixture was stirred for 30 min under magnetic stirring and then KMnO4 (4 g) was added progressively within 10 min at 20 °C. The reaction was continued for 1 h and then the reaction temperature was increased to 35 °C for 30 min. After that, the deionized (DI) water (50 ml) was gradually poured into the reaction mixture to keep the reaction at 98 °C for another 30 min. The processed reaction mixture was diluted again by using DI water (200 ml) with the addition of a subsequent amount of H2O2 (30%) for the purpose of eradicating the residual compounds (KMnO4 and MnO2). Then, the mixture was repeatedly washed and filtered thrice under HCl (5%) and DI water, respectively. Finally, the precipitated GO was dried under vacuum oven at 80 °C for12 h and ground with a mortar and pestle to get uniform sized material.
Synthesis of HAp loaded nano-GO/polyamide strut
The HAp loaded nano-GO/polyamide strut was designed and developed under the approved technique of the Institute of Materials Science and Technology, Department of Orthopaedic Surgery, the First Affiliated Hospital of Zhengzhou University, and our department by the State Drug and Food Administration of China. The composite strut was synthesized by the co-precipitation synthesis method by using normal atmospheric pressurized condition, and the weight percentage ratio of the presented compounds – nano-GO, HA and PA – was about 1:4:6. Firstly, the suspensions of 10 mg mL−1 GO was prepared under sonication by 30 min and then aqueous nano-GO/PA polymeric solution was prepared with the addition of GO suspension by liquifying 60 mg of PA in the DI water under magnetically stirring at 70 °C for 3 h. After that, the precursors of hydroxyapatite nanoparticles were added with the above-prepared reaction mixture resulting in the nano-sized HA particles uniformly covering the macromolecular network of the PA polymer and nano-GO composite form, which is similar to the biological interactions that exist among the normal bone components. Additionally, the HA nanocrystalline particles in the n-GO/PA composite has an analogous crystalline nature and also a nano-size similar to the natural bone minerals. The composite strut was intended to be with an appropriate outer diameter of 8–14 mm and also an appropriate inner diameter of 3–8 mm for clinical utilization as per previously reported literature.
Surgical technique
All the patients experienced surgery have received common suitable anesthetic with a right-sided anterior cervical approach. After a satisfactory acquaintance of the lesion segment, a first and second level corpectomy was achieved with the support of the degree of spinal cord compression data through radiological analysis. After cervical corpectomy and occupied decompression of the cervical canal, the end-to-end cartilage end plates were removed as entirely as possible by using cutting burr and curette and carefully implanted the prepared nano-HAp-GO/PA strut occupied with the autogenous bone granules isolated from the resected vertebrae into the decompressed space. The remaining bone granules were placed around the anterior wall of the n-HA/PA strut. Lastly, a titanium anterior cervical plate was used for the internal strut fixation. Blood loss of the patients and operative time in the surgery were noted. All the patients were taught to custom a cervical collar for controlled immobilization during the important prime postoperative weeks.
Statistical analysis
All the obtainable data are stated as mean ± standard deviation (SD) and were studied using Student’s t-test for the calculation of significance level of the data. Differences were measured as statistically significant at p < .05.
Results and discussion
The microscopic analyses (SEM, AFM and TEM) of the nano-HAp particles loaded n-GO/PA composite are shown in . The synthesized HA lamellar crystalline nanoparticles are exhibited in and were covered on the transparent nano-GO sheets () and the layer-like nano-GO/PA composite, which was non-transparent (). The non-transparency of the PA/nano-GO could be due to the uniformly covered PA macromolecular structure by the non-covalent physical absorption onto both sides of nano-GO sheets, and this was to be confirmed by the results of TEM analysis. Additionally, the nano-crystalline rod-like HAp particles were decorated on the surface of the both synthesized transparent nano-GO and the porous scaffold-like nano-GO/PA were displayed by TEM microscopic analysis, and some particles of the crystalline HA accumulated at the ends of the nano-GO sheets. The nanocrystalline HAp particles were exhibited like a typical rod-like shape with a mean diameter of about ∼30 nm and a length of around ∼120 nm onto the nano-GO sheets and nano-GO/PA composite structure, respectively.
Figure 1. Microscopic images of prepared (a) PA (SEM), (b and b1) rGO (SEM) and (c, c1 and c2) HAp loaded nano-GO/PA (SEM) samples.
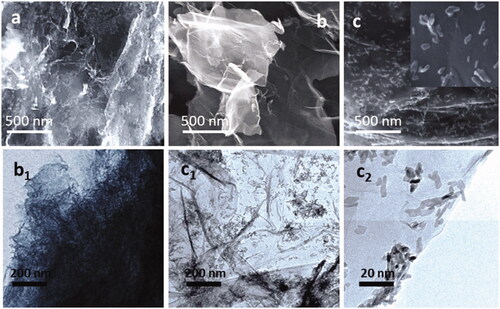
In the present report, HAp particles are dispersed into the nano-GO/PA nanocomposite under ultrasonication before microscopic observation, it could be exhibited that nearly no HA nanoparticles are dispersed outside of the nanocomposite layer, demonstrating a strong physical interaction between the nano-HA particles and the nano-GO/PA nanocomposite. In addition, the greater specific surface area of nano-GO sheets is also highly advantageous and provide a suitable structure for loading ratios of the synthesized nano-HA particles and is supportive for finding an effective macromolecular network within the synthesized biocomposite. The surface structure HAp and nano-GO/PA coatings exhibit the needle-like structure loaded porous atmosphere, which is more favourable for cell attachment and proliferation in the biological in vivo mechanism. Furthermore, the elemental analysis of the HAp loaded nano-GO/PA coating displays the presence of Calcium and Phosphate ratio, assessed as 1.42, which was slightly lower than that of literature ratio of human biological apatite (1.67). Normally, the calcium-deficient apatite phases have been demonstrated to be promising to influence the bone regeneration in vivo.
The phase structure and composition of prepared nano-HAp particles, HAp-GO and HAp-GO/PA nanocomposite were investigated by XRD analysis and displayed in . For GO dispersed PA nanocomposite matrix, the interactions of graphene oxide with PA macromolecular structure was confirmed through rising broad diffraction peaks at 25θ, confirming of PA polymeric molecules on the nano-GO structure. The nano-HAp coating onto the GO/PA nanocomposite was confirmed from the characteristic peaks at ranges of 25.8θ and 32.2θ are presented in the XRD pattern of the nanocomposite. In addition, the disappearance of characteristic GO peaks after nanocomposite formation demonstrated the strong interactions between the crystallographic HAp and GO nanocomposite. The FTIR spectrometer analysis was used to evaluate the surface structure and chemical bonding interactions of HAp particles, GO nanosheets onto the PA polymeric structure as shown in .
Figure 2. Spectroscopic analysis of prepared nanocomposited materials through different analytical methods (a) XRD, (b) Raman spectra and (c) FTIR spectral analysis.
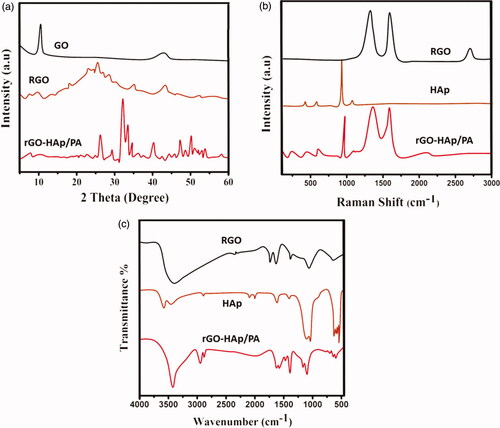
The spectroscopic results were useful to confirm the effective interactions between the nano-GO sheet and PA macromolecular structure, the FTIR spectrum displays a strong characteristics peak at the range 1650 cm−1, which corresponded to the –CO–NH– stretching vibration. This spectral confirmed the effective conjugation of nano-GO sheets onto the PA through amidation. In the spectrum of nanocomposited HAp-GO/PA, the well-defined bands of phosphate and carbonate were observed at the ranges of 1096, 1038, 693 and 1410 cm−1, which confirmed the successful formation of hydroxyapatite onto the nanocomposite coating surface, the carbonate groups presented on the HAp surface due to the atmospheric carbon dioxide when reacted in the apatite process. Furthermore, the broad hydroxyl (OH) stretching vibration of HAp structure appeared at the range of at 3560 cm−1. The FTIR spectroscopic results demonstrated the successful co-deposition of HAp and nano-GO onto the PA nanocomposite coating.
Cell compatibility analysis (MTT assay)
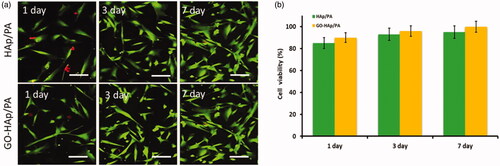
The cell survival and proliferation of the human fibroblast cell line on nano-HAp-GO and nano-HAp/GO-PA nanocomposite scaffolds were evaluated under the technique of MTT assay. As shown in , the cell viability and cell proliferation rate of human fibroblast cells onto the prepared nanocomposite were greatly enhanced by increasing the incubation period (days) comparable to the control. The results of cell viability and proliferation analyses indicated that the prepared nanocomposite did not exhibit non-cytotoxicity with the human cells. Surprisingly, the cell viability percentages of HAp/GO-PA nanocomposite always had greater cell compatibility than that of HAp/PA nanocomposite, specifying the improved biocompatibility of nano-GO layer and its noteworthy role played in enhancing the cell growth and compatibility. In addition, the cell compatibility was visualized by the fluorescence microscopic technique with the AO/EB double staining live-dead assay method. It has already been recognized that AO staining is penetrable to only live cells (green fluorescence colour), while EB staining is penetrable to only dead cells with orange-red colour fluorescence. It can be exhibited from , at different days of culture, the fluorescence visualization has displayed more green colour of fibroblast cells treated with the prepared nanocomposite scaffolds than the red colour, demonstrating the excellent growth and proliferation of the fibroblast cells onto the nanocomposites. Additionally, at increasing incubation days, the cell proliferation and cell numbers were increased, those results are greatly reliable with the results of MTT assay methodic data, thus, further approving the enhanced cytocompatibility of prepared nanocomposite to abilities of cell osteoblasts attachment and healthier proliferation.
Biological analysis of materials
The pre-operative and long-term post-operative (1 and 2-years) surveying evaluated study was attained with the support of 40 patients (20 men and 20 women) with an average age of 47.2 years (∼32–62 years). Twenty patients affected by ACCF were treated by using HAp/PA composite loaded titanium mesh cage and another portion of ACCF suffering patients were treated by using nano-GO sheets dispersed n-HAp/PA nanocomposite coated cages as shown in . The demographics analytical data of treated patients did not exhibit statistically considerable discrepancies with different factors of habits, age and pathogenic particulars and are presented in (p > .05). The clear post-operative investigated data were reported in , which demonstrates that nano-HAp/PA cages have good fusion ability (93%) for the patients, but was little lower than developed nano-HAp-GO/PA (99%). After 2-years post-operative follow-up periods, the developed nano-HAp/GO-PA cages have the outstanding fusion ability and outcome results (∼99%), which is comparable to the fusion ability of the nano-HAp/PA (∼95%). The subsidence analysis results were exhibited in , it can be seen that nano-HAp/PA nanocomposite cages had a subsidence rate of 15% (3/20 patients) after 1-year follow-up and 25% (5/20 patients) after a 2-years follow-up. However, nano-GO sheets dispersed HAp/PA nanocomposite cages had a greater subsidence value, which is the exhibited subsidence ratio of 10 and 5% after 1-year and 2-years follow-up, respectively. The detailed analysis of subsidence evaluations in the interval period of 1 and 2-years post-operative follow-up presented that mean values of SSA, VAS and JOA of the prepared nano-GO loaded HAp/PA nanocomposite was greater and favourable analytic data comparable to the nano-HAp/PA composite. Furthermore, bio-medical researchers found an increased rate of TMC subsidence has been highly associated with more problems of depraved neurological problems, which blindingly disturb the healing benefits of implantation results. The development of biologically active and biocompatible non-metallic composited devices have provided greater benefits and also have been better clinical components with spine surgeons. The enhanced properties of nano-GO sheets loaded with HAp/PA polymeric composited matrices as a superficial orthopaedic material has played an important role in in vivo implantation, due to its favourable bio-mechanical and bioactive properties of nanocrystalline apatite particles and nano-GO with support of elasticity properties of PA polymer, which is very similar to the intrinsic characteristics of natural human bone. The strength values of bending, tensing and compression of nano-GO loaded n-HAp/PA were 98, 76 and 125 MPa, respectively, which is exceedingly similar to the natural human cortical bone. When implanting nano-GO loaded n-HAp/PA composited cages into intervertebral space, which was offered an adequate role in the bone reformation of anterior cervical column. Nevertheless, the value of young’s modulus strength of the nano-GO loaded n-HAp/PA was suggestively lesser (7.8 GPa) than that of bone graft young’s modulus value (∼12 GPa) inside the cage. As per the previous reports, bone tissues have been reforming well in an inadequate value of mechanical stimulus as per the declaration of Wolff’s law. The superior elasticity and mechanical properties of nano-GO loaded n-HAp/PA composite materials into the TMC cages exceedingly promote bone fusion ability of the implantation and regeneration. Additionally, the nano-hydroxyapatite crystalline phase of the implanted cage can be providing that ions exchange mechanism of Ca2+ and PO43− in between the entrenched cages and neighbouring bone tissue to create an active crystal layer onto the surface of the cage for the bony fusion.
Figure 4. (a) Postoperative lateral radiograph image, (b) lateral radiograph image after one year and (c) lateral radiograph image after 2 years.
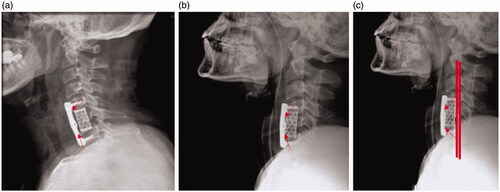
Table 1. Patient demographic details.
Table 2. Clinical and radiographic outcomes.
Conclusions
In this report, we have effectively fabricated the nano-GO loaded HAp/PA cylindrical strut for interbody fusion. The prepared nanocomposite strut has a suitable bio-mechanical property and greater contact angle, which is very similar to the native bone granules and was provided an effective fusion rate with the bony tissues. The biological and physicochemical analysis exhibited a greater biological outcome with the loading of nano-GO into the HAp/PA comparable to the composite in the absence of nano-GO. It is extremely clever to provide suitable mechanical strength and fusion rate greatly similar to the conservative titanium cage and also, chiefly, it has been diminishing subsidence value. We believe that this facile composite material of nano-GO loaded n-HAp/PA has been methodically specified effective results for fusion ability and reconstruction of the ACCF.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang Y, Deng X, Jiang D, et al. Long-term results of anterior cervical corpectomy and fusion with nano-hydroxyapatite/polyamide 66 strut for cervical spondylotic myelopathy. Sci Rep. 2016;6:11.
- Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21:217–220.
- Ghogawala Z. Anterior cervical option to manage degenerative cervical myelopathy. Neurosurg Clin North Am. 2018;29:83–89.
- Zhang Z, Mu Z, Zheng W. Anterior pedicle screw and plate fixation for cervical facet dislocation: case series and technical note. Spine J. 2016;16:123–129.
- Nagaraja S, Palepu V, Peck JH, et al. Impact of screw location and endplate preparation on pullout strength for anterior plates and integrated fixation cages. Spine J. 2015;15:2425–2432.
- Chen JF, Lee ST, Wu CT. A hollow cylindrical PMMA strut for cervical spine reconstruction after cervical multilevel corpectomy. J Spin Disord Techniq. 2010;23:321–327.
- Jang JW, Lee JK, Lee JH, et al. Effect of posterior subsidence on cervical alignment after anterior cervical corpectomy and reconstruction using titanium mesh cages in degenerative cervical disease. J Clin Neurosci. 2014;21:1779–1785.
- Okawa A, Sakai K, Hirai T, et al. Risk factors for early reconstruction failure of multilevel cervical corpectomy with dynamic plate fixation. Spine 2011;36:E582.
- Hussain M, Natarajan RN, Fayyazi AH, et al. Screw angulation affects bone-screw stresses and bone graft load sharing in anterior cervical corpectomy fusion with a rigid screw-plate construct: a finite element model study. Spine J. 2009;9:1016–1023.
- Chen J-F, Wu C-T, Lee S-C, et al. Hollow cylindrical polymethylmethacrylate strut for spinal reconstruction after single-level cervical corpectomy. J Neurosurg. 2006;5:287–293.
- Pickett GE, Duggal N, Theodore N, et al. Anterior cervical corpectomy and fusion accelerates degenerative disease at adjacent vertebral segments. SAS J. 2008;2:23–27.
- Ahn SH, Lee SH, Kim ES, et al. Successful repair of esophageal perforation after anterior cervical fusion for cervical spine fracture. J Clin Neurosci. 2011;18:1374–1380.
- Chou YC, Chen DC, Hsieh WA, et al. Efficacy of anterior cervical fusion: Comparison of titanium cages, polyetheretherketone (PEEK) cages and autogenous bone grafts. J Clin Neurosci. 2008;15:1240–1245.
- Karamian E, Abdellahi M, Khandan A, et al. Introducing the fluorine doped natural hydroxyapatite-titania nanobiocomposite ceramic. J Alloys and Comp. 2016;679:375–383.
- Babaei M, Ghaee A, Nourmohammadi J. Poly (sodium 4-styrene sulfonate)-modified hydroxyapatite nanoparticles in zein-based scaffold as a drug carrier for vancomycin. Mater Sci Eng C.2019;100:874–885.
- Rajesh A, Mangamma G, Sairam TN, et al. Physicochemical properties of nanocomposite: hydroxyapatite in reduced graphene oxide. Mater Sci Eng C. 2017;76:203–210.
- Oyefusi A, Olanipekun O, Neelgund GM, et al. Hydroxyapatite grafted carbon nanotubes and graphene nanosheets: promising bone implant materials. Spectrochimica Acta - Part A: Mol Biomol Spectrosc. 2014;132:410–416.
- Xiong G, Luo H, Zuo G, et al. Novel porous graphene oxide and hydroxyapatite nanosheets-reinforced sodium alginate hybrid nanocomposites for medical applications. Mater Characteriz. 2015;107:419–425.
- Khattab RM, Badr HA, Zawrah MF. Effect of processing techniques on properties of porous TiO2 and TiO2/hydroxyapatite composites. Ceram Int. 2018;44:8643–8649.
- Saber-Samandari S, Yekta H, Ahmadi S, et al. The role of titanium dioxide on the morphology, microstructure, and bioactivity of grafted cellulose/hydroxyapatite nanocomposites for a potential application in bone repair. Int J Biol Macromol. 2018;106:481–488.
- Xia X, Shen J, Cao F, et al. A facile synthesis of hydroxyapatite for effective removal strontium ion. J Hazard Mater. 2019;368:326–335.
- Yan Y, Zhang X, Mao H, et al. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl Surf Sci. 2015;329:76–82.
- Gao F, Wang Q, Gao N, et al. Hydroxyapatite/chemically reduced graphene oxide composite: environment-friendly synthesis and high-performance electrochemical sensing for hydrazine. Biosens Bioelectron.2017;97:238–245.
- Ahmadi S, Mohammadi I, Sadrnezhaad SK. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf Coatings Technol. 2016;287:67–75.
- Farzin A, Ahmadian M, Fathi MH. Comparative evaluation of biocompatibility of dense nanostructured and microstructured Hydroxyapatite/Titania composites. Mater Sci Eng C.2013;33:2251–2257.
- Hosseinzadeh H, Ramin S. Fabrication of starch-graft-poly(acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int J Biol Macromol. 2018;106:101–115.
- Zeng Y, Pei X, Yang S, et al. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf Coat Technol. 2016;286:72–79.
- Klébert S, Balázsi C, Balázsi K, et al. Spark plasma sintering of graphene reinforced hydroxyapatite composites. Ceram Int. 2015;41:3647–3652.
- Moldovan M, Prodan D, Sarosi C, et al. Synthesis, morpho-structural properties and antibacterial effect of silicate-based composites containing graphene oxide/hydroxyapatite. Mater Chem Phys. 2018;217:48–53.
- Olad A, Bakht Khosh Hagh H. Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering. Comp Part B: Eng. 2019;162:692–702.
- Depan D, Pesacreta TC, Misra R. The synergistic effect of a hybrid graphene oxide-chitosan system and biomimetic mineralization on osteoblast functions. Biomater Sci. 2014;2:264–274.
- Mohandes F, Salavati-Niasari M. Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/graphene oxide/hydroxyapatite nanocomposite. RSC Adv. 2014;4:25993–26001.
- Edwin N, Saranya S, Wilson P. Strontium incorporated hydroxyapatite/hydrothermally reduced graphene oxide nanocomposite as a cytocompatible material. Ceram Int. 2019;45:5475–5485.
- Zhang Q, Liu Y, Zhang Y, et al. Facile and controllable synthesis of hydroxyapatite/graphene hybrid materials with enhanced sensing performance towards ammonia. Analyst. 2015;140:5235–5242.
- Maddinedi SB, Mandal BK. Biofabrication of reduced graphene oxide nanosheets using terminalia bellirica fruit extract. Curr Nanosci. 2016;12:1–8.
- Justin R, Chen B. Characterisation and drug release performance of biodegradable chitosan-graphene oxide nanocomposites. Carbohydr Polym. 2014;103:70–80.