Abstract
Long non coding RNAs (lncRNAs) are important in the occurrence and development of various cancers. They have been considered to participate in many processes of diseases. In this study, we aimed at investigating expression level and clinical significance of lncRNA X91348 in hepatocellular carcinoma (HCC). The expression of X91348 in tissue and serum samples from patients with HCC and from healthy people was detected through quantitative real-time polymerase chain reaction (qRT-PCR). X91348 expression was decreased in patients with HCC compared with healthy controls no matter in tissue or serum samples. The relationship between X91348 expression and clinicopathologic characteristics was analysed. The result demonstrated that tumour size, HBsAg and Child-Pugh were vital influencing factors for X91348 expression, which revealed X91348 may be involved in the progress of HCC. Kaplan–Meier analysis was used to evaluate overall survival of patients with different expressions of X91348. Finally, prognostic value of X91348 in HCC was assessed via cox regression analysis. X91348 was proved to be closely related to the prognosis of HCC. Taken together, the down-regulation of X91348 could be an independent diagnostic and prognostic indicator for HCC.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related deaths [Citation1,Citation2]. It accounts for about 6% of all new diagnosed cancers and each year more than 500,000 new patients are diagnosed with this malignancy around the world [Citation3,Citation4]. Surgical resection is the most effective therapeutic method for HCC [Citation5]. However, despite considerable achievements in treatment techniques, high recurrence rate and early distant metastasis of the cancer still lead to poor prognosis [Citation6]. Moreover, HCC patients also face numerous unfavourable conditions, like resistance to chemo and radiation therapies, being diagnosed at advanced stages, further tumour development, recurrence of the primary lesion and metastatic spread, all of which worsen the cases’ prognosis [Citation7–9]. Therefore, it is necessary to find some effective and accurate markers for the diagnosis and prognosis of HCC.
LncRNAs are a class of non-protein-coding RNA molecules with a length more than 200 nucleotides. They have been confirmed to play important roles in many processes, such as brain development, embryonic development, histological differentiation organogenesis, cell proliferation and metastasis [Citation10–13]. LncRNAs are considered to be potentially carcinogenic and anti-carcinogenic RNAs given its role in cell transformation [Citation14]. X91348, located on chromosome 22, is found to be down-regulated in HCC [Citation15]. However, its clinical significance in HCC has never been reported.
In this study, we detected the expression of X91348 in HCC tissue and serum specimens via qRT-PCR analysis. Meanwhile, we estimated the diagnostic and prognostic values of X91348 in HCC, hoping to provide new marker for HCC in its early detection as well as in the prognosis after therapy.
Materials and methods
Patients and samples
This study was conducted in 5th Medical Centre of Chinese PLA General Hospital and permitted by the Ethnic Committee of the hospital. Totally, 107 patients with HCC and 82 healthy volunteers with matching age and gender were collected in this study. All patients with HCC had never received any physical or chemical therapies. Written informed consent was signed by each participator in advance.
One hundred and seven pairs of HCC tissues and corresponding adjacent non-cancerous tissues were collected from patients with HCC, and frozen in liquid nitrogen immediately. Besides, 3 mL peripheral venous blood was also extracted from 107 HCC patients and 82 healthy volunteers into blood collection tube with EDTA, and serum specimens were isolated through centrifugation. Both the tissue and serum samples were stored at −80 °C for RNA extraction. Clinical factors including age, gender, tumour size, tumour site, HBsAg, AFP, portal vein tumour thrombus, Child-Pugh, therapies and neoplasm metastasis were recorded in databases. A 5-years’ follow-up was performed to estimate the prognosis of HCC. Patients who died from unexpected events or other diseases were excluded.
RNA extraction and real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated for all tissue and serum samples, respectively. Reverse transcription was carried out to synthesize the first chain of cDNA using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Finally, RT-PCR reaction was implemented in the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Comparative cycle threshold (CT) was used to calculate the expression of lncRNA X91348.
Statistical analysis
All data were stated as Mean ± SD. SPSS version 13.0 software (IBM, Chicago, IL) and GraphPad Prism version 5 (GraphPad, San Diego, CA) were used to make data analysis and design relative figures. The differences in X91348 expression between malignant and non-malignant specimens were estimated through Students’ t test. The relationship between clinicopathologic characteristics and X91348 expression was estimated via chi-square test. Receiver operating characteristic curve (ROC) was plotted to estimate diagnostic significance for X91348 in HCC. Kaplan–Meier analysis was conducted to compare overall survival for patients with different X91348 expressions while prognostic value of this X91348 was evaluated through cox regression analysis. Difference was considered to be significant when p < .05.
Results
X91348 was decreased in HCC
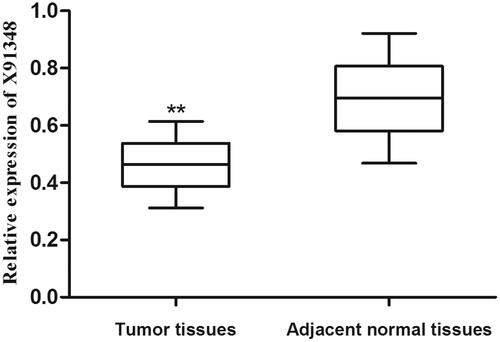
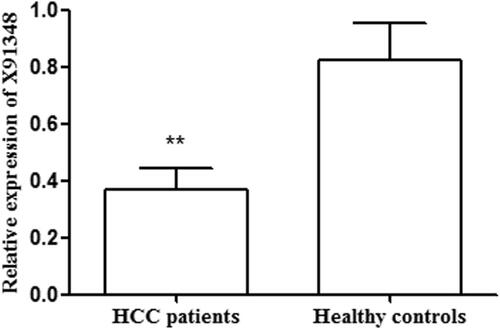
QRT-PCR analysis was taken to detect relative expression of X91348 in tissue and serum samples. As shown in , the average expression level of X91348 was lower in tumour tissues than in adjacent non-cancerous ones (0.463 vs. 0.695, p < .001). Of the patients, 54 (50.47%) showed expression level of X91348 in HCC tissues higher than the average figure (0.463) while 53 (49.53%) possessed X91348 expression lower than such figure. Meanwhile, detection results on serum sample demonstrated that X91348 expression exhibited an increasing tendency in HCC patients compared with healthy people (0.371 vs. 0.821, p < .05, ).
The relationship between clinicopathologic characteristics and X91348 expression
To explore whether X91348 participated in the development and progress of HCC, we analysed its relationship with clinicopathologic characteristics. The included HCC patients were divided into high and low expression groups according to their mean expression levels of X91348 in HCC tissues. The result demonstrated that X91348 expression was obviously influenced by tumour size (p < .001), HBsAg (p = .001), and Child-Pugh (p < .001) ().
Table 1. The relationship between clinicopathologic characteristics and X91348 expression.
Diagnostic value of X91348 in HCC
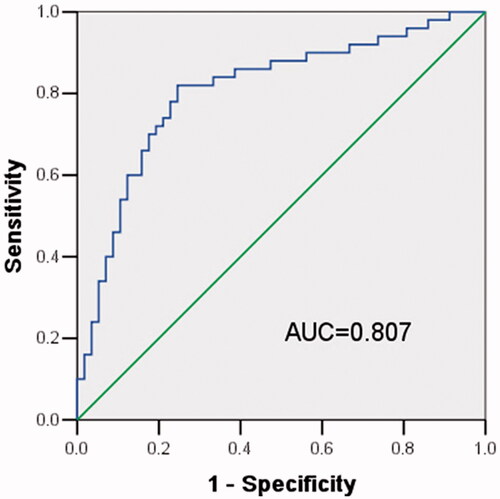
Considering aberrant expression of X91348, we inferred it might be useful in HCC diagnosis. Thus, ROC curve was established, reaching an area under curve (AUC) of 0.807 combing with a sensitivity of 82% and a specificity of 75.4% (). And the ideal cut-off value located at 0.469. These findings revealed X91348 might an independent diagnostic marker for HCC.
Association between X91348 expression and overall survival of patients with HCC
The 5-years’ follow-up was performed to explore whether X91348 influenced the prognosis of HCC. During the follow-up, 22 patients were censored with a median follow-up of 31.02 ± 15.11 months. Overall survival in patients with high X91348 expression was proved to be longer than that in patients with low expression, revealed through Kaplan–Meier analysis (). Log rank test obtained statistically significant result (p < .001). Multivariate analysis with cox regression analysis adjusting for clinicopathologic characteristics was performed to estimate prognostic value for X91348. Neoplasm metastasis (HR = 3.125, 95CI% = 1.200–8.137, p = .020) and low X91348 expression (HR = 5.527, 95CI% = 1.791–17.057, p = .003) were verified to be associated with the prognosis of HCC, and they might be independent prognostic bio-markers for the malignancy ().
Figure 4. Kaplan–Meier analysis for overall survival of patients with HCC stratified by the expression of X91348. Patients with high X91348 expression lived longer than those with low X91348 expression (Log rank test, p< .001).
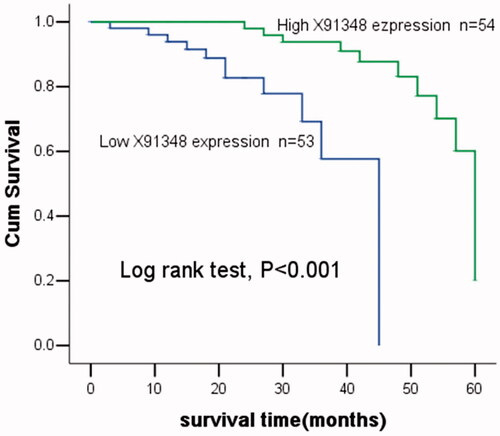
Table 2. Cox regression analysis adjusting for clinical factors to estimate prognostic value of X91348 in HCC.
Discussion
HCC accounts for approximately 90% of all primary liver cancers, representing a most malignant tumour in liver diseases [Citation16]. Generally, this disease witnesses low 5-years’ survival rate [Citation17]. Identifying new biomarkers for HCC diagnosis, prognosis and treatment are necessary to improve its actuality.
Accumulated studies have located many biomarkers for HCC diagnosis and prognosis. Among them, miRNAs, specific genes and lncRNAs play a crucial role. For instance, Yu et al. found that the combination of miR-224 and pAKT expressions can predict the prognosis in HCC cases [Citation18]. In the study of Li et al. plasma miRNA-139 can be a diagnostic and prognostic biomarker for HCC [Citation19]. Cullin1 was proved to be increased in HCC, possibly being an independent prognostic marker for the disease [Citation20]. According to Yu et al., kidney-type glutaminase was up-regulated in HCC, and its expression showed high value in the malignancy diagnosis and prognosis [Citation21]. Besides, plenty of lncRNAs have been taken as diagnostic or prognostic biomarker in HCC. H19 was the first non-coding RNA founded in HCC and considered to act as a tumour suppressor while other studies proved it as an oncogene, depending on tissue culture systems [Citation22,Citation23]. HOTAIR displayed over-expression in HCC and was associated with cell migration and invasion, but its prognostic value was insufficient [Citation24,Citation25]. In addition, MALAT1, lncRNA HEIH and lncRNA-ROR were also reported to take part in the development of HCC [Citation26–28]. X91348 was verified to be decreased in HCC [Citation15]. However, its clinical application has never been reported in any cancers.
In this study, we certified the down-regulated of X91348 in HCC using both tissue and serum samples, which was consistent with findings from previous study [Citation15]. The relationship between X91348 expression and clinicopathologic characteristics was analysed to estimate whether it was related to the development of HCC. As a result, X91348 expression was associated with tumour size, HBsAg and Child-Pugh, which manifested its participation in the progress of HCC. X91348 might act as a tumour suppressor in HCC and its down-regulation enhanced aggressive progression of the malignancy. However, the expression of X91348 was proved to be up-regulated in human renal clear cell carcinoma, revealing its oncogenic function [Citation29]. Such divergences might be attributed to different signalling pathways or targets mediated by X91348 in different types of cancers [Citation30].
Then we further explored clinical significance of X91348 in HCC. An AUC of 0.807 correspondings with high sensitivity and specificity, via establishing the ROC curve, showed its high value in the diagnosis of HCC. Kaplan–Meier analysis indicated patients possessing high X91348 expression lived much longer than those with low-X91348 expression. Meanwhile, multivariate analysis revealed X91348 had satisfactory capability to predict the prognosis of HCC, according to cox regression analysis adjusting for clinicopathologic characteristics.
In conclusion, X91348 is decreased in HCC. Its expression is influenced by some clinicopathologic characteristics, which indicates its involvement in the development of HCC. Moreover, it is clinically significant due to its high value in the diagnosis and prognosis of HCC, and it may be an independent diagnostic and prognostic indicator for cancer. However, there were many limitations in this study, such as small sample size and potential influencing factors. Further in-depth studies are essential to confirm the function of X91348 in HCC.
Disclosure statement
The authors have declared that no conflict of interest exist.
Funding
This study was supported by grants from National Key R&D Program of China [2017YFC0908401], and National Science and Technology Major Project [2018ZX10723204 and 2018ZX10302205].
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Buonaguro L. Developments in cancer vaccines for hepatocellular carcinoma. Cancer Immunol Immunother. 2015;65:93–99.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127.
- Hou J, Zhou Y, Zheng Y, et al. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25:49–63.
- Yoshiji H, Noguchi R, Ikenaka Y, et al. Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: a randomized control trial. Oncol Rep. 2011;26:1547–1553.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917.
- Chen R, Sain NM, Harlow KT, et al. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur J Cancer. 2014;50:2705–2713.
- Miao R, Luo H, Zhou H, et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol. 2014;61:840–849.
- Pollard KS, Salama SR, Lambert N, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172.
- Beniaminov A, Westhof E, Krol A. Distinctive structures between chimpanzee and human in a brain noncoding RNA. RNA. 2008;14:1270–1275.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159.
- Prensner JR, Iyer MK, Sahu A, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45:1392–1398.
- Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–61.
- Zhu J, Liu S, Ye F, et al. The long noncoding RNA expression profile of hepatocellular carcinoma identified by microarray analysis. PLoS One. 2014;9:e101707.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576.
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399.
- Yu L, Zhang J, Guo X, et al. MicroRNA-224 upregulation and AKT activation synergistically predict poor prognosis in patients with hepatocellular carcinoma. Cancer Epidemiol. 2014;38:408–413.
- Li T, Yin J, Yuan L, et al. Downregulation of microRNA-139 is associated with hepatocellular carcinoma risk and short-term survival. Oncol Rep. 2014;31:1699–1706.
- Liu W, Wang Y, Zhang C, et al. Cullin1 is up-regulated and associated with poor patients' survival in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:4001–4007.
- Yu D, Shi X, Meng G, et al. Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget. 2015;6:7619–7631.
- Yoshimizu T, Miroglio A, Ripoche MA, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci USA. 2008;105:12417–12422.
- Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329.
- Ishibashi M, Kogo R, Shibata K, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–950.
- Ding C, Cheng S, Yang Z, et al. Long non-coding RNA HOTAIR promotes cell migration and invasion via down-regulation of RNA binding motif protein 38 in hepatocellular carcinoma cells. Int J Mol Sci. 2014;15:4060–4076.
- Lai MC, Yang Z, Zhou L, et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29:1810–1816.
- Yang F, Zhang L, Huo XS, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689.
- Takahashi K, Yan IK, Kogure T, et al. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Biol. 2014;4:458–467.
- Yu G, Yao W, Wang J, et al. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377.
- Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinform. 2016;14:42–54.