Abstract
Allergen-specific immunotherapy is widely used for allergic rhinitis and asthma treatment worldwide. This study explored the efficacy and safety of sublingual immunotherapy (SLIT) with the extracts of Dermatophagoides Farinae (D. farinae Drops) on house dust mites (HDM)-induced atopic dermatitis (AD). 239 patients with HDM-induced AD were recruited and exposure to a multi-centre, randomized, double-blind, and placebo-controlled clinical trials for 36 weeks, which were randomly divided into placebo and sublingual D. farinae Drops groups (high-dose, medium-dose and low-dose), respectively. Statistical analysis was performed in three groups: Full Analysis Set, Per Protocol Set and Safety Set. 48 cases have withdrawn from the study before the end of study. As primary outcomes, significant decreases in scoring atopic dermatitis and total medication score were showed in medium-dose and high-dose D. farinae Drops groups. In the sixth visit, the skin lesion area showed a statistically significant difference between high-dose/medium-dose D. farinae Drops group and placebo group (p < .05). Most adverse events are slight, and no life-threatening adverse drug reaction happened. Our research demonstrates the beneficial effect of SLIT with high or medium dose D. farinae Drops on AD, and the treatment was well tolerated.
Introduction
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease with highly intractable pruritus [Citation1]. Almost 10–20% of children and 1–3% of adults suffer from AD worldwide [Citation2]. The main clinical symptoms of AD are pruritic, eczematous, erythematous and skin lesions with serous exudate or crusts [Citation3]. More seriously, about half of the patients with severe AD will get asthma and about two-thirds of them will develop allergic rhinitis [Citation4]. AD can be divided into two categories: intrinsic AD and extrinsic AD [Citation5]. Extrinsic AD accounts for ∼80% of all cases, which shows sensitivity to the environmental allergens, such as house dust mite (HDM), pollen and so on [Citation6]. Allergens, like HDM and pollen, can through skin barrier or respiratory tract, promote the polarization of T helper type 2 cells, elevate the sensitization of specific IgE in serum and aggravate the symptoms of AD [Citation7].
Allergen-specific immunotherapy (ASIT) is recognized as a highly effective practice in the treatment of AD, including in the therapy of natural allergens-caused allergic diseases [Citation8,Citation9]. The rationale of ASIT is lifting the tolerance of patients to the specific allergen by gradually increasing the dose administration of allergen [Citation10]. Its beneficial clinical effects have been demonstrated in the treatment of allergic asthma, atopic rhinitis and hymenoptera allergy [Citation11–13]. In generally, ASIT includes subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) [Citation14]. Due to its higher safety and lower compliance, SLIT has been recommended by the World Allergy Organization (WAO) as an alternative to SCIT for patients with allergic rhinitis and asthma [Citation15].
As a major allergen, HDM can be found worldwide where human beings lives and contains a number of mites, such as Dermatophagoides farinae, Dermatophagoides microceras, Dermatophagoides pteronyssinus and Euroglyphus maynei [Citation16]. SLIT with standardized extracts of D. farinae (D. farinae Drops) has been applied currently in Chinese clinical II phase practice [Citation17]. Qin et al. proved that SLIT with D. farinae Drops could reduce the need of patients with AD for medicine [Citation17]. In this study, we want to further explore the efficacy and safety of SLIT with D. farinae Drops on patients with HDM-induced AD. Moreover, this study will provide a clinical basis for design and drug administration of the clinical phase III trial.
Materials and methods
Study design
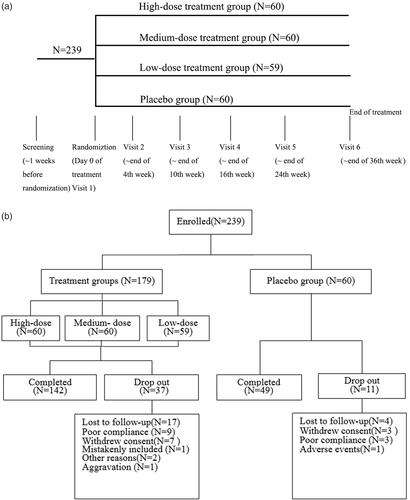
The study was a clinical phase II, multi-centre, randomized, double-blind, and placebo-controlled trial with 4 parallel groups. Eligible patients were randomly divided into four groups, placebo control group, high-dose D. farinae Drops group, medium-dose D. farinae Drops group and low-dose D. farinae Drops group averagely using a computer-based randomization program (). After randomization, patients were assigned to receive relevant treatment for 36 weeks, and regularly received follow-ups, which consist of visit 1–6 in 0, 4, 10, 16, 24 and 36 weeks, respectively. In each visit, diary cards (containing whether take medicine as required) were retrieved, scoring atopic dermatitis (SCORAD) [Citation18], medicine use score (whether use Levocetirizine Hydrochloride Tablets, Mometasone Furoate Cream, Mupirocin Ointment or Clarithromycin Tablets to relieve clinical symptoms), self-evaluation of patients and adverse events (AE) were recorded. In visit 1 and 6, patients were given a completed medical examination, including physical examination and laboratory test.
This study was approved by the Ethics Committee of The Second Affiliated Hospital, Zhejiang University School of Medicine and registered at clinicaltrials.gov (NCT01471119). This study has got IRB approval from all the clinical centres. Written informed consents were obtained from all participants before any protocol-specific procedures were undertaken.
Patients
A total of 239 patients were recruited from October 2011 to February 2014 in The Second Affiliated Hospital, Zhejiang University School of Medicine (48 cases), Huashan Hospital Affiliated to Fudan University (40 cases), Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (40 cases), Peking University Third Hospital (44 cases), Tongji Hospital, Tongji Medicial College of Huazhong University of Science & Technology (12 cases), The First Affiliated Hospital of Soochow University (20 cases), The First Affiliated Hospital, Zhejiang University School of Medicine (23 cases) and The Second Affiliated Hospital of Kunming Medical University (12 cases) for clinical trial. Main inclusion criteria include: (1) 18 to 60 years old; (2) 10 ≤ SOCRAD ≤ 40; (3) positive skin prick test results (histamine equivalent or above ++) to D. farinae stimulation. Main exclusion criteria include: (1) Patients showing higher skin prick test reaction to other allergens rather than D. farinae, and skin prick test results to these allergens showed above +++; (2) had received immunotherapy for dust mite in the preceding 3 years; (3) had clinically relevant history of immunosuppressive diseases (such as: history of HIV affection, history of malignancy), autoimmune disease or phthisis; (4) had received UV therapy or systemic treatment with glucocorticoids, leukotriene antagonists or immune-suppressor in the preceding 4 weeks; (5) asthma patients who needed a regular use of glucocorticoids and/or β2-adrenoceptor.
Procedures
Eligible patients received sublingual D. farinae Drops or placebo (only containing glycerin and saline). D. farinae Drops (Nos. 1–5 containing 1, 10, 100, 333 and 1000 μg/ml extracts of D. Farinae, respectively, in glycerinated saline) were kindly provided by Zhejiang Wolwo Bio-pharmaceutical Co., Ltd. (Zhejiang, China). Treatment was conducted by two phases: up-dosing phase (1st–10th weeks) and maintenance phase (11th–36th weeks). In up-doing phase, patients received low to high dose of sublingual D. farinae Drops or placebo treatment. The specific information on drug dosage in up-doing phase is shown in . From 11th to 36th weeks, patients took a high dose of sublingual D. farinae Drops or placebo daily as a maintenance dose.
Table 1. SLIT Dosing schedule.
In addition, patients can use some concomitant drugs, including Mometasone Furoate Cream, Levocetirizine Hydrochloride Tablets, Mupirocin Ointment or Clarithromycin Tablets to relieve clinical symptoms, such as erythema, pimples or infiltration during the trial. The use of any concomitant drugs during the trial was recorded, and the total amount of concomitant drugs use of each patient was counted for evaluation. The use of medicine count 1 point for each dose of Levocetirizine Hydrochloride Tablets, Mometasone Furoate Cream, Mupirocin Ointment or Clarithromycin Tablets. All patients were instructed to use a daily card to record every consumption of D. farinae Drops, medicine for relieve clinical symptoms or AE during the trial.
Assessment of efficacy
The therapeutic efficacy of SLIT with D. farinae Drops was assessed using SCORAD index, the use of concomitant drugs to relieve clinical symptoms in maintenance phase, the dermatology life quality index (DLQI) and the skin lesion area. The SCORAD index was recorded in each follow-up. The reduction ratio of SCORAD index was calculated by the score decrease from baselines to each follow-up.
Assessment of safety
Safety of SLIT with D. farinae Drops was evaluated by AE and general clinical laboratory evaluations. AE included death, life-threatening, hospitalization, disability, low work efficacy and deformity. General clinical laboratory evaluations included blood routine, urine routine, liver function, renal function and a urine pregnancy test.
Statistical analysis
Statistical analysis was performed in three groups: Full Analysis Set (FAS), Per Protocol Set (PPS) and Safety Set (SS). FAS included all the patients who had received at least one-time SLIT with D. farinae Drops, and could provide at least one day’s diary record. PPS included all the patients with good compliance (received more than 70% SLIT with D. farinae Drops of total SLIT with D. farinae Drops) and completed all the diary record. FAS and PPS were used for efficacy assessment. SS included all the patients who had received at least one-time SLIT with D. farinae Drops, which was used for safety assessment.
Two-tailed tests were used for statistical analysis with a significant level of 0.05. Qualitative data were evaluated by χ2 test. Ordinal categorical variables were evaluated by CMH χ2 test. Quantitative differences among multi-groups were evaluated by one-way analysis of variance or non-parametric test. Clinical efficacy index were evaluated by covariance analysis. Intro-group comparisons were evaluated by t-test or sign-rank test.
Results
As shown in and , patients in four groups were well balanced in demographics and baseline disease characteristics, as well as the results of skin prick test with various allergens.
Table 2. Baseline characteristics of the patients.
Table 3. Baseline skin prick test results.
A total of 48 cases have withdrawn from the study before the end of study (). There are 11 cases in placebo group, 10 cases in high-dose D. farinae Drops group, 14 cases in medium-dose D. farinae Drops group and 13 cases in low-dose D. farinae Drops group. There were no significant differences in withdraw rates between the placebo group and D. farinae Drops groups.
Primary efficacy endpoints
Scorad
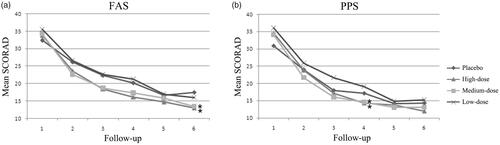
226 cases were included in FAS, and 193 cases were included in PPS. The SCORAD index of the placebo group and D. farinae Drops groups were recorded in every visit during the trail. Results in displayed that the SCORAD indexes were both decreased in the placebo group and D. farinae Drops groups. Due to the 4th visit was the first follow-up patients entered into the maintenance phase, and the 6th visit was the last one, we paid attention to the data of these two groups. The results showed that D. farinae Drops treatment groups had lower SCORAD indexes in FAS, compared to the placebo group, especially in high-dose and medium-dose D. farinae Drops groups (p < .05). In the 4th visit, the SCORAD index in PPS was also significantly decreased in high-dose and medium-dose D. farinae Drops groups, relative to the placebo group (p < .05).
Figure 2. Mean SCORAD over 36-weeks’ treatment period. (a) Mean SCORAD in FAS; (b) Mean SCORAD in PPS. *p < .05.
Clinical efficacy rate was evaluated by the percentage of cure and the change of marked effect during the whole treatment phase. The cure, marked effect, improvement, and ineffectiveness were defined by the reduction ratio of SCORAD index after 36th week sublingual D. farinae Drops treatment compared to baseline. Compared to a 38.00% (in FAS and PPS) clinical efficacy rate in placebo group, D. farinae Drops treatment groups showed higher rates (). In high-dose D. farinae Drops treatment group and medium-dose D. farinae Drops treatment group, the efficacy rate reached to 56.00 and 58.70%, respectively.
Table 4. Clinical efficacy rate.
Pharmacotherapy medication score
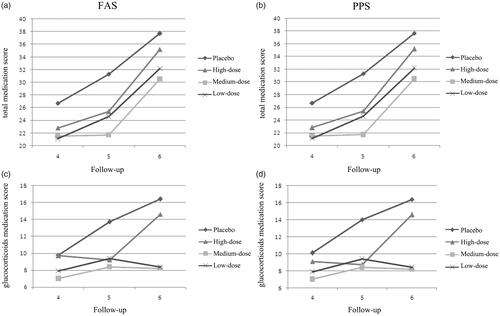
Use of concomitant drugs to relieve clinical symptoms in the maintenance phase (11th to 36th weeks) was evaluated by medication score. shows that both D. farinae Drops treatment groups and the placebo group showed an increase in total medication score from fourth to sixth follow-up. As a major therapeutic medicine for AD, the reduction of glucocorticoids dosage could directly reflect the efficacy of immunotherapy. Thus, the single topical glucocorticoids medication score (at pre-defined targeted area) was compared. From fourth to sixth follow-up, the use of glucocorticoids in the placebo and the low-dose group were increased, while the use of glucocorticoids in the high-dose and medium-dose group still remained at relatively low levels ().
Figure 3. Mean medication score from fourth to sixth follow-up. Medication score was calculated in every follow-up by days of concomitant drugs use. Every single day use of one kind of concomitant drug represented one point. (a) Mean total medication score in FAS. (b) Mean total medication score in PPS. (c) Mean topical glucocorticoids medication score in FAS. (d) Mean topical glucocorticoids medication score in PPS.
Secondary efficacy endpoints
Skin lesion area
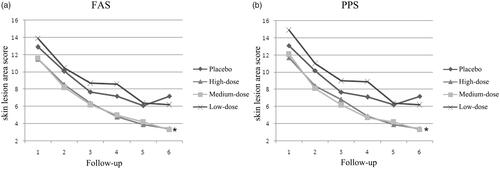
Skin lesion area was scored over the 36-weeks’ treatment. The skin lesion areas were both decreased in D. farinae Drops treatment groups and the placebo group. High-dose and medium-dose group displayed a higher decrease in skin lesion areas than the placebo group (). In the sixth follow-up, the differences among high-dose D. farinae Drops and placebo group, as well as medium-dose D. farinae Drops and placebo group were statistically significant (p < .05).
Dermatology life quality index (DLQI)
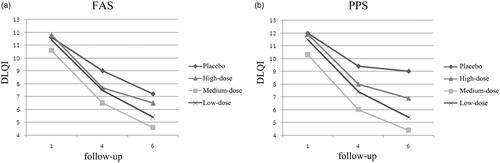
DLQI was used to evaluate whether the AD symptoms could affect the daily life of patients. DLQI was recorded during the whole 36-weeks treatment. Compared to the baseline, both the placebo group and D. farinae Drops groups had a decrease in DLQI (). The decrease of DLQI is highest in medium-dose D. farinae Drops group, followed by high-dose D. farinae Drops group, then low-dose D. farinae Drops group and placebo group.
Safety assessment
231 patients were included in SS for safety assessment. There were 41 patients who reported adverse drug reaction (ADR), 85 cases in total. Among them, 19 patients (33 cases) belonged to high-dose D. farinae Drops group, 9 patients (24 cases) belonged to medium-dose D. farinae Drops group, 6 patients (13 cases) belonged to low-dose D. farinae Drops group and 7 patients (15 cases) belonged to the placebo group (). The difference in number is statistically significant (p < .05).
Table 5. Incidence of adverse drug reaction.
Wherein, 74 cases were mild ADR, 6 cases were moderate ADR, and 5 cases were serious ADR (). Serious ADR included swelling in tough and lips (three cases, in the medium-dose group), throat pain (one case, in high-dose group), and diarrhoea (one case, in high-dose group). None of these five cases dropped out from the study. The mostly occurred ADR were swelling, itching, or numb in mouth, tough, or lips (N = 24). Most of this kind of ADR was mild and did not need treatment or dose adjustment. No differences in clinical laboratory evaluations and vital signs were observed.
Table 6. Number and severity of adverse drug reaction.
Discussion
Since Scadding G.K. firstly reported the randomized, double-blind, placebo-controlled parallel group clinical trial about SLIT in 1985 [Citation19], SLIT has been widely used in adults and children for the treatment of AD. An increasing number of reports provide evidence that SLIT is effective in allergic rhinitis and asthma treatment [Citation11,Citation12]. Until now, a few clinical trials concerning SLIT to AD have been reported [Citation20,Citation21]. In 2016, H H. Tam et al [Citation22] reported a Cochrane systematic review about SLIT for the treatment of atopic eczema, which analyzed 12 eligible trials with 733 subjects. High safety is proved by these trials. In terms of efficacy, two trials showed a significant difference in primary outcomes, while three trials showed no significance. Unfortunately, due to a high statistical heterogeneity, this review is not able to conclude a firm report whether ASIT is efficient in the treatment of AD. More clinical trials are still needed to further assess whether ASIT has a beneficial effect on AD.
Herein, we further tested the efficacy and safety of SLIT with D. farinae Drops on patients with HDM-induced AD. As a dosage exploratory clinical trial, we reported a certain decrease in SCORAD, concomitant drugs use, skin lesion area, and improvement in DLQI in high and medium-dose D. farinae Drops groups, especially in the medium-dose group, which suggests medium-dose D. farinae Drops might be the proper dose for treatment. These results suggest a positive response of AD patients to SLIT treatment. However, most of the primary outcomes didn’t show statistically significant differences. Further long term clinical trials are needed to further validate the efficacy of SLIT in the treatment of AD.
Pajno et al. conducted an 18-months’ randomized, double-blind, placebo-controlled parallel group clinical trial about SLIT to AD with 56 subjects, which indicated that SCORAD started to decrease after 9 months treatment, and showed a significant decline after 18 months treatment [Citation21]. Glover and Atherton conducted a randomized, double-blind clinical trial on immunotherapy to AD among 24 children [Citation23]. They found that there was no statistical significance after 8 months treatment. When the trial continued for another 6 months, the treatment group showed a significant lower score than the placebo group. These findings suggest that a long term for immunotherapy is necessary. In our study, we observed an increase in SCORAD from fifth to sixth follow-up in the placebo group. We regarded that it was a decline of the placebo effect. With the extension of the treatment period, the placebo effect could be diminished, and the effect of immunotherapy could be more significant. Thus, a significant improvement in a longer term’s immunotherapy is expected.
Topical glucocorticoids are the most effective therapeutic drugs to AD [Citation24]. Then, we suppose that the use of topical glucocorticoids can reduce SCORAD during the study, which explains why all the placebo and treatment groups showed a reduction in SCORAD. It also indicates that the reduction use of topical glucocorticoids could directly reflect the efficacy of SLIT. We reported a lower topical glucocorticoids medication score in all three D. farinae Drops treatment groups, especially in medium-dose D. farinae Drops group, which indicated that SLIT may reduce the use of therapeutic drugs to AD.
During the immunotherapy, patients may experience a lower risk of arising AD symptoms again [Citation25]. On the other aspect, patients also can use medicine to control the clinical symptoms during SLIT. By this way, patients were expected to have a lower possibility in AD relapse. According to a subgroup analysis evaluating the period for not using topical glucocorticoids after IGA ≤ 2, In this clinical trial, we set control over the use of glucocorticoids. When IGA ≥ 3, mometasone furoate cream could be used consistently until when IGA ≤ 1. However, most patients failed to conduct this medication rule because of relative low compliance. This may help to explain why the difference of topical glucocorticoids medication score was not statistically significant.
In this clinical trial, no serious systemic AE was happened, which proved that SLIT with D. farinae Drops is well tolerated to adult patients. Moreover, we found that a higher incidence rate of ADR in the high-dose group, and investigators speculate that a relative high-dose allergen may promote the allergic reaction in patients, which result in the AE. Therefore, it hints that an appropriate dose in the maintenance phase is important for safety consideration.
Though this clinical trial didn’t include children as clinical trial subjects, the well-tolerated of SLIT to children has been firmly testified in many previous clinical trials and found that no life-threatening AE happened in a clinical trial of SLIT to children [Citation26].
To conclude, this research investigated the efficacy and safety of SLIT with D. farinae Drops on patients with HDM-induced AD. We found that SLIT with an appropriate dosage of D. farinae Drops could reduce the SCORAD, lower the use of concomitant drugs and improve the DLQI. A low dosage of D. farinae Drops cannot have good efficacy, while the excessive dosage of D. farinae Drops may cause adverse reactions and influence the safety of the treatment. The findings our research provide experiment and clinical basis for clinical phase III trial of SLIT with D. farinae Drops on patients with HDM-induced AD. In the clinical phase III trial, we should prolong the time of study and choose a more suitable D. farinae Drops dosage.
Disclosure statement
We declare that we have no personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- Kakinuma T, Nakamura K, Wakugawa M, et al. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin Exp Immunol. 2002;127:270–273.
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258 e23.
- Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine 2015;73:311–318.
- Bülow AV, Backer V, Bodtger U, et al. S8 A nationwide real-life study: exploring the difficulties of confirming the asthma diagnosis in patients with severe asthma. Thorax 2015;70:A8.
- Park JH, Choi YL, Namkung JH, et al. Characteristics of extrinsic vs. intrinsic atopic dermatitis in infancy: correlations with laboratory variables. Br J Dermatol. 2006;155:778–783.
- Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7.
- Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy 2012;67:1475–1482.
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562.
- Bae JM, Choi YY, Park CO, et al. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–117.
- Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27.
- Zuberbier T, Bachert C, Bousquet PJ, et al. GA 2 LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–1530.
- Pipet A, Botturi K, Pinot D, et al. Allergen-specific immunotherapy in allergic rhinitis and asthma. Mechanisms and proof of efficacy. Res Med. 2009;103:800–812.
- Bonadonna P, Zanotti R, Caruso B, et al. Allergen specific immunotherapy is safe and effective in patients with systemic mastocytosis and Hymenoptera allergy. J Allergy Clin Immunol. 2008;121:256–257.
- Lawrence MG, Steinke JW, Borish L. Basic science for the clinician: mechanisms of sublingual and subcutaneous immunotherapy. Ann Allergy Asthma Immunol. 2016;117:138–142.
- Passalacqua G, Compalati E, Canonica GW. Sublingual immunotherapy: clinical indications in the WAO-SLIT position paper. World Allergy Organ J. 2010;3:216–219.
- Saleh AM, Ali HA, Ahmed SA, et al. House dust mites: a risk factor to be considered for occupational safety or source of work-related allergens. JESP. 2013;43:669–678.
- Qin YE, Mao JR, Sang YC, et al. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. 2014;53:650–655.
- Schäfer T, Dockery D, Krämer U, et al. Severity scoring of atopic dermatitis (SCORAD). Allergo J. 1997;6:410–410.
- Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16:483–491.
- Cadario G, Galluccio AG, Pezza M, et al. Sublingual immunotherapy efficacy in patients with atopic dermatitis and house dust mites sensitivity: a prospective pilot study. Curr Med Res Opin. 2007;23:2503–2506.
- Pajno GB, Caminiti L, Vita D, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007;120:164–170.
- Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;122:CD008774.
- Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to Dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy. 1992;22:440–446.
- Prakash A, Benfield P. Topical mometasone. A review of its pharmacological properties and therapeutic use in the treatment of dermatological disorders. Drugs 1998;55:145–163.
- Justicia JL, Barasona MJ, Serrano P, et al. Predicting patients at high-risk of systemic reactions to cluster allergen immunotherapy: a pilot prospective observational study. J Investig Allergol Clin Immunol. 2007;17:386–392.
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S27.