Abstract
Background/Aim
Ginsenoside Rg1 exerts a beneficial effect in many kidney diseases. But little work has been done to confirm whether ginsenoside Rg1 could also exert a protective effect on anti-glomerular basement membrane (anti-GBM) glomerular nephritis (GN). We aimed to explore the role of ginsenoside Rg1 in attenuating anti-GBM GN in vitro and in vivo and investigate the mechanism under its action.
Methods
Interleukin-1β (IL-1β) treated podocytes were used as a cell model. A total of 20 mice were used to build the Anti-GBM GN mice model. Real-time PCR analysis (RT-PCR), Western blot analysis and ELISA assay were conducted to detect related indicators in this study. The statistical analysis was performed using GraphPad Prism software 6.0.
Results
Ginsenoside Rg1 attenuates IL-1β-induced inflammation and apoptosis in podocytes. NRF2 expression can be inhibited by IL-1β, whereas reversed by ginsenoside Rg1. NRF2 inhibitor ML385 can significantly reverse the effects of ginsenoside Rg1 on inflammation and apoptosis induced by IL-1β, and block the inhibitory effect of ginsenoside Rg1 on IL-1β activated MAPK pathway. In addition, ginsenoside Rg1 could improve anti-GBM GN injury in vivo.
Conclusion
Ginsenoside Rg1 inhibits the anti-GBM GN damage through regulating NRF2 pathway in vitro and in vivo. Our findings provide new insight and mechanism of ginsenoside Rg1 to prevent anti-GBM GN.
Introduction
Glomerular nephritis (GN) is a kind of allergic inflammation characterized by glomerular damage, which is the most common cause of renal failure disease [Citation1]. The early symptoms of GN are not obvious, but the late stage of GN can cause renal failure, which seriously threatens the health and life of patients [Citation2,Citation3]. Although corticosteroids, cytotoxic drugs and immunosuppressants currently used clinically have significant effects on GN, they all have obvious side effects and are not suitable for long-term use [Citation4,Citation5]. Therefore, primary GN, especially anti-glomerular basement membrane (anti-GBM), has not yet been completely cured by drugs with little side effects.
Recent studies have shown that most primary and secondary GN are immune-mediated inflammatory diseases [Citation6]. Anti-GBM GN, which is serious and progressing rapidly, often occurs in weeks to months if treatment measures are not performed in time, so it is also called rapid progressive GN [Citation7]. The construction of an animal model of anti-GBM GN is a method of using the anti-GBM antibody to induce the pathological changes of human extra capillary proliferative GN [Citation8]. It has been widely used in the study of pathogenesis, prevention and treatment of extra capillary proliferative GN. Podocytes are terminally differentiated cells distributed in the outer layer of the glomerular basement membrane, which participate in glomerular globulin filtration and GBM synthesis, and provide structural support for glomerular capillaries. Because podocyte proliferates poorly, once it is damaged, glomerulosclerosis can occur quickly [Citation9].
Ginsenoside is a steroid compound, which is mainly extracted from the roots, stems and leaves of Ginseng [Citation10]. As a famous Chinese herbal medicine, ginseng has been widely used in kidney diseases for hundreds of years [Citation11]. Ginsenoside Rg1, the main component of ginseng, has been shown to prevent membranous nephropathy in rats by eliminating metabolites of reactive oxygen species [Citation12]. In addition, previous reports also showed that Ginsenoside Rg1 has anti-inflammatory and anti-apoptotic effects in many diseases [Citation13]. However, the role of Ginsenoside Rg1 on anti-GBM GN is still unclear.
In the present study, we will study the effects of Ginsenoside Rg1 on inflammation, apoptosis and immune response induced by GBM in vivo, and on IL-1β-induced podocyte inflammation and apoptosis. In addition, we will further explore the potential molecular mechanism under the action of Ginsenoside Rg1.
Materials and methods
Reagents
Ginsenoside Rg1 (purity > 95%) was purchased from DiDakexiang Biological Co., Ltd (Guizhou, China). It was dissolved in phosphate buffered saline (PBS) as a stock solution for further investigation.
Cell culture and treatment
MPC5 (Mount Sinai School of Medicine, USA), conditionally immortalized mouse podocyte cell line was used for our experiments. Podocytes were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS (Gibco, USA) in a humidified atmosphere with 5% CO2. Ginsenoside Rg1 (80 ng/mL) was exposed to podocytes co-cultured with recombinant human IL-1β protein (10 ng/mL) (R&D, USA) in presence or absence of NRF2 inhibitor ML385 (5 μM,) (Selleck, USA) for 48 h.
Animal experiment
A total of 20 male mice were purchased from The Beijing Vital River Laboratory Animal Technology Co., Ltd and housed in the animal facility. All animal studies were approved by the Institutional Animal Care Committee. Anti-GBM GN mice model was induced by an intraperitoneal injection of 0.5 mg/20 g BW of normal rabbit IgG (Santa Ana, USA) emulsified with complete Freund’s adjuvant (Difco, USA) at 8 weeks of age. And 5 days later, anti-GBM antibody (Santa Ana, USA) or vehicle was injected into the tail vein at a dose of 1.5 mg total protein/g body weight for three consecutive days. Mice were sacrificed 4 weeks after inducing anti-GBM nephritis. Blood and kidney samples were collected for further examination.
RT-PCR analysis
Total RNA extracted with Trizol (Invitrogen, USA) was quantified with 400 ng and carried out synthesis by reverse transcription by using oligo(dT) priming method (Takara, Japan). Then Q-PCR was performed using SYBR Green qPCR Master Mix (Bio-Rad, USA). T GAPDH was used for normalizing to detect relative mRNA expression. The 2−△△CT method was used to determine its expression.
Western blot analysis
Total protein was extracted with lysis buffer and quantified with 40 μg. The proteins were separated on 10–12% sodium dodecyl sulfate-polyacrylamide gels and then transferred to nitrocellulose membrane. Then the blot was incubated with the following primary antibodies (1:1000, CST, USA): Bax, Bcl-2, NRF2, p-ERK, ERK, p-P38, P38were used, and GAPDH was used as a loading control.
Mouse anti-rabbit IgG and rabbit anti-mouse glomerular basement membrane (GBM)
ELISA
Terminal serum of Mouse anti-rabbit IgG and IgG rabbit anti-mouse GBM antibodies were detected using the respective ELISA kit as described detailly [Citation14].
Immunohistochemical staining
The kidney tissues of normal and anti-GBM groups were collected separately and were stained with PAS. The specific detail steps were performed according to DAB substrate kit (Thermo Scientific, MA, USA)
Statistical analysis
All data are presented as “means ± SD”. The significance of differences between groups was determined by one-way analysis of variance (ANOVA, Bonferroni) using Graph pad prism 7.0.
p<.05 was considered statistically significant.
Results
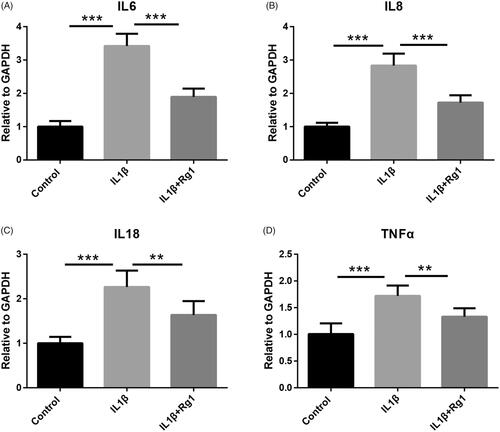
Ginsenoside Rg1 inhibits IL-1β-induced the expression of inflammatory cytokines in podocytes
To investigate the effect of Ginsenoside Rg1 on the expression of inflammatory cytokines in podocytes, podocytes were treated by IL-1β and cultured with or without Ginsenoside Rg1. The RT-PCR analysis results showed that the expression levels of inflammatory factors IL-6, IL8, IL-18 and TNF-a were significantly induced by IL-1β, while these elevated gene expressions were significantly decreased by Ginsenoside Rg1 ().
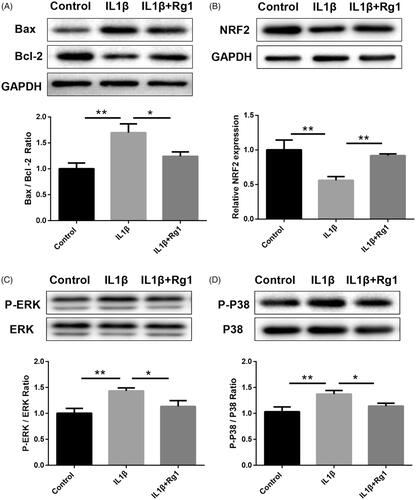
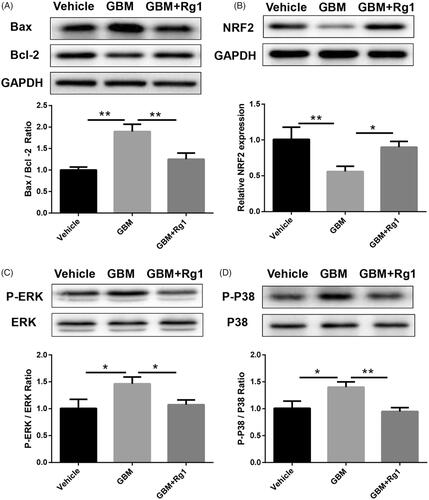
Ginsenoside Rg1 attenuates IL-1β-induced apoptosis and activated NRF2 signalling
To further study the role of Ginsenoside Rg1 on apoptosis induced by IL-1β, we used western blot analysis to detect the apoptotic protein expression of Bax and Bcl-2. We found that Ginsenoside Rg1 could significantly inhibit IL-1β-induced apoptosis (). Besides, we found that NRF2, an important gene involved in the development of GN, can be significantly inhibited by IL-1β while can be reversed after Ginsenoside Rg1 treatment (). Moreover, western blot analysis also revealed that Ginsenoside Rg1 could significantly inhibit IL-1β induced p-p38 and p-ERK1/2 expression ().
Figure 2. Ginsenoside Rg1 attenuated IL1β-induced apoptosis and activated NRF2 signalling. (A) Western blot analysis indicated Ginsenoside Rg1 protected podocytes from IL1β-induced apoptosis, (n = 3). (B–D) Ginsenoside Rg1 activated NRF2 signalling and inhibited IL1β-stimulated ERK and P38 pathway (n = 3). *p < .05; **p < .01; versus respective control.
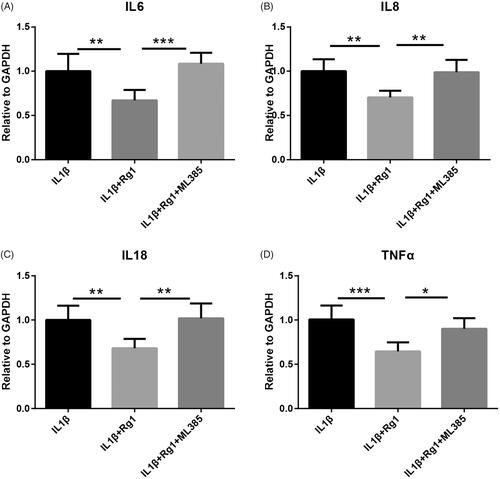
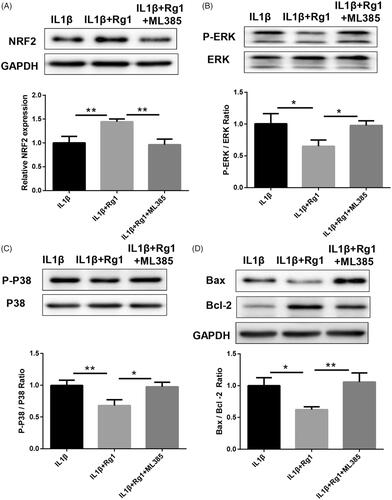
NRF2 inhibitor ML385 inhibited NRF2 signalling and promoted apoptosis attenuated by Rg1 in podocytes
NRF2 inhibitor ML385 was performed to reduce Ginsenoside Rg1-elevated NRF2 expression to verify the role of NRF2 in Ginsenoside Rg1 inhibiting IL-1β-induced apoptosis (). Western blot analysis showed that ML385 could partially block the inhibitory role of Ginsenoside Rg1 on IL-1β-induced p-p38 and p-ERK pathways (). Similarly, the inhibitory effect of Ginsenoside Rg1 on IL-1β-induced apoptosis () and inflammatory cytokines expression () was partially reversed by ML385 in vitro. These results suggest that Ginsenoside Rg1 inhibits IL-1β-induced apoptosis and inflammatory response by regulating the NRF2 pathway
Figure 3. NRF2 inhibitor ML385 inhibited NRF2 signalling and promoted IL-1β-induced apoptosis in podocytes. (A–C) ML385 inhibited NRF2 signalling and re-activated ERK and P38 signalling pathway (n = 3). (D) ML385 diminished the inhibitory effect of Ginsenoside Rg1 on attenuating apoptosis in podocytes (n = 3). *p < .05; **p<.01; versus respective control.
Ginsenoside Rg1 improved anti-GBM GN induced injury in vivo
To obtain in vivo evidence of Ginsenoside Rg1’s effect on GN, we first constructed a mouse model of anti-GBM GN, the anti-GBM GN mice showed significant cresc entformation (). PCR analysis results showed that the expressions of IL-6, IL-8, IL-18 and TNF-a in GN were significantly increased, while could be significantly attenuated by Ginsenoside Rg1 (). In addition, anti-IgG, anti-GBM elevated in anti-GBM GN, CD163 and CD206 was reduced after Ginsenoside Rg1 treatment (). Consistent with results in vitro, Rg1 attenuated anti-GBM GN induced apoptosis and reversed signalling of NRF2, P-ERK and P-P38 ().
Figure 5. Rg1 improved anti-GBM glomerulonephritis induced inflammation in vivo. (A) Immunostaining showed that anti-GBM glomerulonephritis model was successfully constructed. (B–E) Rg1 decreased expression of inflammatory cytokines in anti-GBM glomerulonephritis model via RT-PCR analysis (n = 6). (F–I) Rg1 attenuated immune response induced expression of total IgG, anti-GBM, CD163 and CD206. *p < .05; **p < .01; ***p < .001; versus respective control.
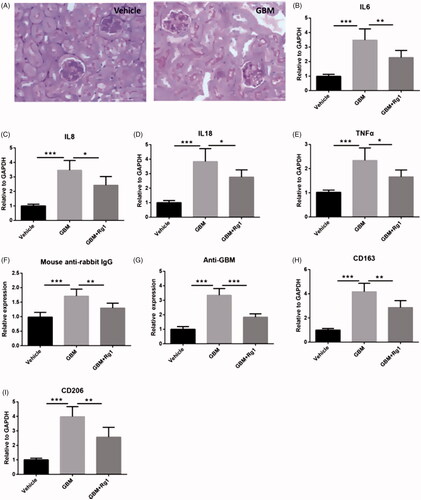
Figure 6. Ginsenoside Rg1 showed beneficial effect on inhibiting apoptosis and activated NRF2 signalling in mice. (A) Ginsenoside Rg1 protected renal function from apoptosis (n = 3). (B–D) Ginsenoside Rg1 activated NRF2 signalling and inhibited ERK and P38 pathway in vivo (n = 3). *p < .05; **p < .01; versus respective control.
Discussion
A large number of experimental and clinical studies have proved that most human GN is a disease mediated by both inflammation and immunity [Citation15]. The role of the immune response (mainly cyclic and in situ immune complexes) in the pathogenesis of nephritis has been widely recognized [Citation16]. Studies have confirmed that immune response is the initial cause of many patients with GN [Citation17]. On this basis, some inflammatory mediators (such as complement, interleukin, and TNF-a) are involved, which eventually lead to glomerular injury and clinical symptoms.
The treatment of GN includes symptomatic treatment, glucocorticoid combined with immunosuppressive drugs, plasma exchange, hemodialysis and kidney transplantation [Citation18]. Glucocorticoids and immunosuppressants are still the main therapies for anti-GBM GN [Citation19]. Inhibiting inflammation and immunity is one of the important mechanisms of these drugs. But all these drugs have obvious side effects. For example, glucocorticoids can lead to a decline in the body’s ability to fight infection [Citation20]. The clinical application of these drugs is more or less limited, so it is necessary to find new, better and more effective drugs to treat GN.
Chinese herbal medicine is one of the most promising natural medicines for the treatment of kidney diseases internationally recognized [Citation21]. Ginsenoside belongs to plant triterpenoid saponins and is the main active ingredient of ginseng [Citation10]. More than 50 ginsenoside monomers have been extracted and identified [Citation12]. Previous studies have shown that Ginsenoside Rg1 has significant protective effects on many kidney diseases [Citation22]. Fan Yanling et al. revealed that ginsenoside Rg1 could antagonize subacute renal injury in vivo and this role may due to inhibiting oxidative stress damage [Citation23]. Mao Nan et al. demonstrated that ginsenoside Rg1 attenuates podocytes of the mouse from aldosterone-activated injury in vitro [Citation24]. Our study results showed that ginsenoside Rg1 can significantly inhibit IL-1β-induced inflammation and apoptosis in podocytes. In addition, ginsenoside Rg1 can also reduce inflammation, apoptosis and immune damage in anti-GBM GN in mice.
Most GN with pathological changes similar to human nephritis can be reproduced stably in animals. In anti-GBM GN, the expression of many immune indicators will change significantly, such as CD123, CD206 [Citation25]. In this experiment, we constructed mice anti-GBM GN, and detected the CD123, CD206, anti-IgG and anti-GBM indicators to evaluate the effect of ginsenoside Rg1. The NRF2 and MAPK pathways, which have been demonstrated to be involved in the development of GN [Citation26–28], were also confirmed could be regulated by ginsenoside Rg1 in this study. We found that NRF2 inhibitor ML385 reversed the inhibitory effect of ginsenoside Rg1 on inflammation and apoptosis.
In conclusion, this study demonstrated that ginsenoside Rg1 attenuates the anti-GBM GN injury in vitro and in vivo through regulating the NRF2 pathway. Our findings provide new insight and mechanism of ginsenoside Rg1 to prevent anti-GBM GN.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Wieliczko M, Dylewska M.iga nephropathy-prognostic factors and treatment. Wiad Lek. 2016;69:707–710.
- Krebs CF, Panzer U. Plasticity and heterogeneity of th17 in immune-mediated kidney diseases. J Autoimmun. 2018;87:61–68.
- Sato M, Takei T, Moriyama T, et al. Long-term outcomes of initial therapy for idiopathic membranous nephropathy. Clin Exp Nephrol. 2017;21:842–851.
- Pfister F, Buttner-Herold M, Amann K. (immuno-)pathology of drug side effects in the kidney. Pathologe. 2018;39:576–582.
- Jefferson JA. Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol. 2018;13:1264–1275.
- Nagy J, Sagi B, Mate J, et al. considerations on the treatment of IGA nephropathy on the basis of the results of the latest studies (stop-igan, testing, nefigan). Orv Hetil. 2017;158:1946–1952.
- Arranz O, Ara J, Rodriguez R, et al. rapid-detection GBM-ANCA Elisa. An emergency tool for the early diagnosis of type I and II rapidly progressive glomerulonephritis. Nefrologia 2001;21:349–354.
- Suzuki Y, Yamada H, Ito M. antinephritic effect of md-805 [(2r, 4r)-4-methyl-[n2-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-l-arginyl]-2-piperidine-carboxylic acid monohydrate], a new antithrombin agent, on crescentic-type anti-GBM nephritis in rats. Nihon Jinzo Gakkai Shi. 1984;26:463–473.
- Takaki T, Ohno N, Saitoh S, et al. Podocyte penetration of the glomerular basement membrane to contact on the mesangial cell at the lesion of mesangial interposition in lupus nephritis: a three-dimensional analysis by serial block-face scanning electron microscopy. Clin Exp Nephrol. 2019;23:773.
- Kim YJ, Jeon JN, Jang MG, et al. Ginsenoside profiles and related gene expression during foliation in panax ginseng meyer. J Ginseng Res. 2014;38:66–72.
- Yokozawa T, Dong E. Role of ginsenoside-rd in cisplatin-induced renal injury: Special reference to DNA fragmentation. Nephron 2001;89:433–438.
- Li Y, Wang F, Luo Y. Ginsenoside rg1 protects against sepsis-associated encephalopathy through beclin 1-independent autophagy in mice. J Surg Res. 2017;207:181–189.
- Zu G, Guo J, Che N, et al. Protective effects of ginsenoside rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the wnt/beta-catenin pathway. Sci Rep. 2016;6:38480.
- El Nakeeb N, Saleh SA, Massoud YM, et al. Serum ferritin as a non-invasive marker in the prediction of hepatic fibrosis among egyptian patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2017;1:112–119.
- McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12:1162–1172.
- Westhorpe CLV, Norman MU, Hall P, et al. Effector CD4(+) t cells recognize intravascular antigen presented by patrolling monocytes. Nat Commun. 2018;9:747.
- Woollard KJ, Pusey CD. The heterogeneous mononuclear phagocyte system of the kidney. Kidney Int. 2014;85:1011–1014.
- Hou W, Huang G, Cao X, et al. Suppression of experimental autoimmune glomerulonephritis by tryptophan. J Nephrol. 2014;27:19–28.
- Wang W, Chen N. Treatment of progressive iga nephropathy: an update. Contrib Nephrol. 2013;181:75–83.
- Wofsy D, Hillson JL, Diamond B. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum. 2013;65:1586–1591.
- Wang YJ, He LQ, Sun W, et al. Optimized project of traditional chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol. 2012;139:757–764.
- Xie XS, Liu HC, Wang FP, et al. Ginsenoside rg1 modulation on thrombospondin-1 and vascular endothelial growth factor expression in early renal fibrogenesis in unilateral obstruction. Phytother Res. 2010;24:1581–1587.
- Fan Y, Xia J, Jia D, et al. Mechanism of ginsenoside rg1 renal protection in a mouse model of d-galactose-induced subacute damage. Pharm Biol. 2016;54:1815–1821.
- Mao N, Cheng Y, Shi XL, et al. Ginsenoside rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacol Sin. 2014;35:513–522.
- Han Y, Ma FY, Tesch GH, et al. C-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-gbm glomerulonephritis in the rat. Lab Invest. 2011;91:978–991.
- Ebihara S, Tajima H, Ono M. Nuclear factor erythroid 2-related factor 2 is a critical target for the treatment of glucocorticoid-resistant lupus nephritis. Arthritis Res Ther. 2016;18:139.
- Im SR, Im SW, Chung HY, et al. Cell- and nuclear-penetrating anti-dsdna autoantibodies have multiple arginines in cdr3 of vh and increase cellular level of perk and bcl-2 in mesangial cells. Mol Immunol. 2015;67:377–387.
- Wang C, Blough E, Arvapalli R, et al. Acetaminophen attenuates glomerulosclerosis in obese zucker rats via reactive oxygen species/p38mapk signalling pathways. Free Radic Biol Med. 2015;81:47–57.