Abstract
Identification of specific cell markers is crucial for recognizing functionally healthy nucleus pulposus (NP) cells. The objective of this study was to investigate the role of CD24 expression in adult human NP cells. Cells were retrieved from NP tissues of 20 patients (aged 17–44) operated on for lumbar disc herniation. Based on CD24 expression, NP cells were separated by sorting and then used to examine phenotypic behavior, the effects of culture conditions and cellular senescence pathway related proteins. CD24 expression was positive in 35.5 ± 3.7% (range 9.1–65.2%) of NP cells. Consistently, normoxic expansion and serial passages in monolayers decreased percentage positivity for CD24 in NP cells. CD24– NP cells showed a markedly decreased GSK-3β activity and increased mitogen-activated protein kinase phosphorylation accompanying by an increased β-catenin expression. Higher levels of matrix metalloproteinases, as well as lower levels of ACAN and COL2 in CD24– cells, indicated the breakdown and reduced the formation of key extracellular matrix components. CD24+ NP cells presented a more favorable phenotype while CD24– cells showed a more prominent cellular senescence fate. CD24 in NP cells may be a surrogate marker of healthy cells, in the cell-based therapeutic treatment of degenerative disc disorders.
Introduction
Degeneration of intervertebral disc (IVD) is the main cause of low back pain that leads to morbidity and the health burden of patients. Pathophysiologic evidence indicates that IVD degeneration begins in the nucleus pulposus (NP) [Citation1,Citation2]. When NP degenerates and its properties compromised, the IVD may fail to transmit intervertebral forces properly and then various degenerative processes develop [Citation3].
Transplantation of autologous NP cells demonstrates the potential for treating IVD degeneration [Citation4]. NP tissues obtained during lumbar disc surgeries have been used as sources of NP cells for cell transplantation [Citation5–7]. NP cells with young and healthy phenotype are said to enhance therapeutic success.
A collection of markers has been identified for defining the phenotype of NP cells [Citation8]. These markers were discovered in studies of various animals and humans of different ages. Manifestations and role of each of these markers are not completely understood in the NP cells of human adults, who comprise the main target population of cell-based therapeutics for treating IVD related degeneration. The current study first identified the expression profiles of brachyury T [Citation9–11], CD155 [Citation12] and CD24 [Citation13–15], considered as markers of young and healthy NP phenotypes, in NP cells retrieved from adult patients. As the percentage positivity of CD24 was different from the other tested markers, and then additional studies were conducted to investigate the role of CD24 expression in adult human NP cells.
Methods and methods
Harvest and cultivation of NP cells from subjects
Retrieval and use of human NP tissues were reviewed and approved by the Research Ethical Committee (Approval #201412137RIND) of the National Taiwan University Hospital. Human NP tissues were obtained aseptically from a sample of 20 patients, consisting of 13 males and 7 females, with an average age of 30.6 years (range: 17–44), who underwent microdiscectomy surgery for lumbar disc herniation (). Informed consent was obtained from each patient for collection of samples. Degeneration of studied IVDs was graded according to Pfirrmann’s classification using T2-weighted magnetic resonance imaging [Citation15]. Immediately after retrieval, human NP tissues were minced as small fragments and incubated in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Sigma-Aldrich, St. Louis, MO, USA) containing 0.2% collagenase (Sigma-Aldrich) at 37 °C overnight for cell isolation. Isolated NP cells were then cultivated in standard growth medium, DMEM/F-12 containing 10% fetal bovine serum (FBS) (Gibco, Life Technologies, NY, USA), 1% Antibiotic-Antimycotic (15240062, Gibco) and 50 mg/mL L-ascorbic acid (Sigma-Aldrich). Cell isolation, expansion and subsequent experiments for human NP tissues from case No. 1-8 and 18-20 were conducted exclusively in a hypoxic environment (US Autoflow NU-4950; NuAire, Plymouth, MN, USA) with a mixture of 5% O2, 5% CO2 and 90% N2. Human NP tissues from case No. 9-17 were divided into two portions, for the purpose of testing isolation and expansion of cells under hypoxic (5%) or normoxic (21%) conditions. Cells isolated from retrieved NP tissues and adhered to tissue culture polystyrene plates were used for experimental purposes.
Table 1. Demographic data of the studied cases and percentage of CD24+ NP cells in each set of experiments.
Flow cytometry for detection of selected markers of young healthy NP cells
Upon 80% confluence at first passage (P1) under normoxic or hypoxic expansion, NP cells were collected and then suspended in ice-cold PBS supplemented with 2% FBS, treated with monoclonal antibodies for detection of brachyury T (Millipore, Billerica, MA, USA), CD155 and CD24 (Becton Dickinson, Franklin Lakes, NJ, USA). Cells were then examined by flow cytometry to detect percentage positivity of each marker. Correlations among these three markers and the age of patients were identified.
Separation of CD24+ cells and CD24– cells by fluorescence-activated cell sorting
Due to its unique pattern of expression, CD24 was selected for further experiments. Upon 80% confluence at P1 under hypoxic expansion, cells from human NP tissues of case No. 5-11 were harvested by AccutaseTM cell detachment solution and divided into two groups of cells based on the expression of CD24 (CD24+ and CD24–) in flow cytometry. Each group of cells was collected by using a protocol for sorting (Cell-Quest software, Becton Dickinson, Franklin Lakes, NJ, USA) and tested. Cells were sorted using FACSAriaIII (BD Bioscience, San Jose, CA, USA).
Sorted and non-sorted NP cells were seeded into the 24-well culture plates and cultured in the standard growth medium. The medium was changed every 3 days. After reaching 80% confluence, the NP cells were fixed and incubated with primary anti-CD24 antibody (1:80, 311101, BioLegend) at 4 °C overnight and then conjugated with a secondary antibody containing a far-red fluorescent dye (1:400, A-21203, donkey anti-mouse IgG (H + L) highly cross-adsorbed secondary antibody, Alexa Fluor 594, Invitrogen™, Thermo Fisher Scientific, Wilmington, DE, USA) for an additional 30 min. Finally, the NP cells were stained with 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI, 300 μM, D1306, Invitrogen™, Thermo Fisher Scientific) for 10 min and imaged using the confocal microscopy.
Growth kinetic of CD24+ and CD24– NP cells
Proliferation of sorted and non-sorted NP cells was analyzed. NP cells (P3) were seeded onto the 24-well culture plates at a cell density of 5000 and 10,000 cells/cm2, respectively. At predetermined intervals, the cells were detached and counted to determine the cell number. In addition, cell proliferation was further verified by using BrdU immunostaining (ab125306, BrdU Immunohistochemistry Kit, Abcam, Cambridge, UK).
Factors affecting percentage positivity of CD24 in adult human NP cells
Sorted NP cells from case No. 1-6 were expanded in monolayer under hypoxia to induce serial expansion. The percentage of positive CD24 expression was then measured at P1, P3 and P5 cells.
The impact of oxygen concentrations during isolation and expansion of CD24 expression in NP cells were also examined. Immediately after retrieval, NP tissues from case No. 9-17 were divided into two portions for tests on isolation and expansion of cells under hypoxic (5%) or normoxic (21%) conditions. Percentage positivity of CD24 expression was measured upon 80% confluence at P1.
mRNA expressions for CD24+ and CD24– cells
Total RNA was extracted from CD24+ and CD24– NP cells (case No. 6-11) immediately after sorting using a kit (PureLink micro-to-midi total RNA purification system; Invitrogen, Carlsbad, CA). RNA was treated with RNase-free DNase (Qiagen, Chatsworth, CA) to eliminate any residual DNA during the extraction process. An aliquot of RNA extract was used to quantify the RNA yield by recording the absorbance at 260 nm using the NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Fisher Scientific). The RNA was reverse transcribed into cDNA by using High-Capacity cDNA Reverse Transcription kits (Applied Biosystems, Foster City, CA). Complement DNA was subjected to each polymerase chain reaction amplification using TaqMan Gene Expression Master Mix and Assays-on-Demand Gene Expression probes on an ABI PRISM 7900HT Fast Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific). The expression profiles of aggrecan (ACAN), decorin (DCN), type I collagen (COL1), type II collagen (COL2), type X collagen (COL10), SOX9, Fas-associated death domain protein (FADD), transforming growth factor beta 1 (TGF-β1), bone morphogenetic protein-2 (BMP-2), matrix metalloproteinase (MMP)-2, -13 and hypoxia inducible factor (HIF)-1α, -1β and -2α were examined with the endogenous control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The expression of mRNA level for each target gene was normalized to the housekeeping gene GAPDH.
Western blot analysis of senescence associated pathway
For whole cell extractions, the NP cells (case No. 5, 6, 7, 10, 11) with or without expression of CD24 were lysed using the CellLytic NuCLEAR extraction kit (Sigma-Aldrich). Total cellular proteins were separated with SDS-PAGE and blotted onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Non-specific binding was blocked with 5% milk powder and 0.2% Tween 20 in Tris-buffered saline. The membrane was incubated with primary antibodies against mouse p44/42 mitogen-activated protein kinase (MAPK) (total MAPK, Erk1/2, 4696, 1:5000; Cell Signaling, Danvers, MA, USA), rabbit phospho-p44/42 (p-p44/42 MAPK, Erk1/2 Thr202/Tyr204, 4370, 1:2000; Cell Signaling), rabbit phospho-p38 MAPK (p-p38 MAPK, Thr180/Tyr182, 4631, 1:2000; Cell Signaling), rabbit Akt (1:2000, Cell Signaling), rabbit phospho-Akt (p-Akt, Ser473, 4691, 1:2000; Cell Signaling), rabbit glycogen synthase kinase (GSK)-3α/β (1:2000, 4060, Cell Signaling), rabbit phospho-serine 21/9-GSK-3β (pSer9-GSK-3β, 9331, 1:2000; Cell Signaling), rabbit β-catenin (1:1000, Cell Signaling), mouse GAPDH (1:5000, ab8245, Abcam) and mouse α-tubulin (1:5000, T5168, Sigma) overnight at 4 °C. To detect p-Akt, pSer9-GSK-3β, p-p38 MAPK and p-p44/42 MARK, protein extracts were pre-treated with phosphatase inhibitors before Western blot analysis. Primary antibodies were recognized using either a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:10,000; GeneTex, Irvine, CA, USA) or an HRP-conjugated rabbit anti-mouse secondary antibody (1:10,000, GeneTex, Irvine, CA, USA). Secondary antibodies were detected with enhanced chemiluminescence and imaged using a western blot imaging/gel documentation instrument. The sizes of resolved protein bands were verified with the precision protein standard (Bio-Rad, Hercules, CA, USA). The bands of each targeted protein were quantified and expressed as relative densitometry.
Statistical analysis
Quantitative data from multiple experiments were expressed as the mean ± SE (standard error of the mean). Wilcoxon matched-pairs signed ranks test was applied to verify the significance of differential expressions between CD24+ and CD24– cells as well as the percentage positivity of CD24 expressions under different culture conditions.
Results
Expression of brachyury T, CD155 and CD24 in subjects
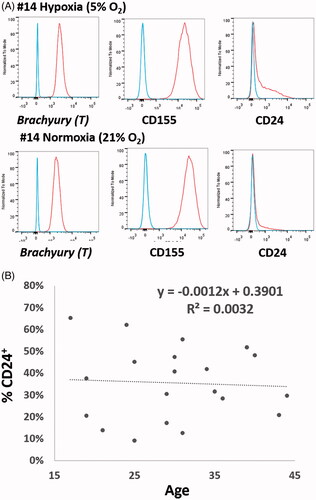
NP cells from all 20 cases showed high positive expressions of brachyury T (99.79 ± 0.14%; range 99.3–100.0%) and CD155 (98.73 ± 2.20%; range 92.6–99.9%) under both hypoxic or normoxic expansions (). No significant differences were noticed in the brachyury T and CD155 expressions between normoxic and hypoxic cultivations. There was also no correlation between the expressions of these two makers and the ages of patients. On the contrary, the percentage positivity for CD24 in NP cells varied among patients. Demographic data of included cases and percentage of CD24+ cells in each set of experiments are shown (). However, the percentage of CD24+ NP cells was not significantly correlated with age in this patient group (aged 17–44) ().
Figure 1. (A) NP cells show high positive expressions of brachyury T and CD155 either under hypoxic or normoxic expansion. No significant differences were noticed in brachyury T and CD155 expression between normoxic and hypoxic cultivations. On the contrary, the percentage positivity for CD24 in NP cells varied among patients. (B) There was also no correlation between the rates of CD 24 expression and the age of patients.
Isolation, passage and oxygen concentrations to the CD24 expressions in human NP cells
After isolation and expansion for passage under hypoxia (), CD24 expression was positive in 35.47 ± 3.74% (range 9.1–65.2%) of NP cells from these 20 cases. Cells sorted according to a positive expression of CD24 were stained pronouncedly to CD24 than that of non-sorted cells (). However, there was no significant difference in the growth kinetic between CD24+ and CD24– NP cells (). This finding was also confirmed by BrdU staining ().
Figure 2. (A) CD24+ and CD24– NP cells were sorted according to CD24 expression. (B) Sorted CD24+ NP cells were pronouncedly stained with CD24 than that of non-sorted cells. (C) There was no significant difference in the growth kinetic between CD24+ and CD24– NP cells. (D) BrdU staining further verified CD24 expression did not influence cell proliferation. (E) NP cells isolated and expanded under hypoxia showed significantly higher rate of CD24 expression than those under normoxia. (F) Serial passages in monolayers caused decreased percentage positivity for CD24 in human NP cells. The percentage positivity for CD24 in P1 NP cells was 43.57 ± 4.81%, 34.17 ± 8.67% in P3 cells and 18.33 ± 5.77% in P5 cells.
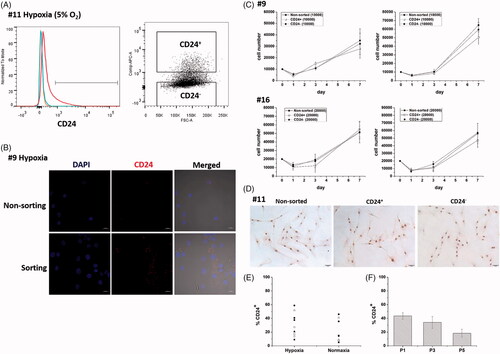
NP cells (case Nos. 9–17) isolated and expanded under hypoxia showed significantly higher rate of CD24 expression (31.80 ± 6.25%; range 9.1–65.2%) than those under normoxia (20.31 ± 5.33%; range 5.1–46.0%; p < .05) (). In NP cells from case No. 1–6, percentage positivity for CD24 was 43.57 ± 4.81% (range 30.4–62.0%) in P1 cells, 34.17 ± 8.67% (range 9.2–60.9%) in P3 cells and 18.33 ± 5.77% (range 1.9–34.3%) in P5 cells. Serial passages in monolayers caused a decrease in percentage positivity for CD24 in NP cells. Significant differences were found between P1 and P5 cells and between P3 and P5 cells (p < .05) ().
mRNA expression pattern in CD24+ and CD24– NP cells
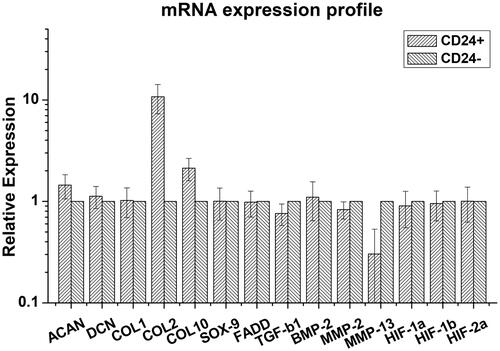
Relative expressions of all target genes in CD24+ and CD24– NP cells are shown (). CD24+ NP cells expressed significantly higher levels of ACAN (1.44 ± 0.40 fold, range 1.17–2.26-fold; p < .05), COL2 (10.76 ± 3.46 fold; range 4.84–15.56-fold; p < .001) and COL10 (2.12 ± 0.57 fold; range 1.37–2.69-fold; p < .05), but significantly lower level of MMP-2 (0.83 ± 0.10 fold; range 0.70–0.92-fold; p < .05), MMP-13 (0.30 ± 0.27 fold; range 0.03–0.65-fold; p < .05) and TGF-β1 (0.73 ± 0.18 fold; range 0.48–0.96-fold; p < .05) relative to those of CD24– NP cells. Expression levels of DCN, COL1, SOX9, FADD, HIF-1α, -1β and -2α were similar in the two cell groups.
Figure 3. CD24+ NP cells expressed significantly higher levels of ACAN (p < .05), COL2 (p < .001) and COL10 (p < .05), but significantly lower level of MMP-2 (p < .05), MMP-13 (p < .05) and TGF-β1 (p < .05) relative to those of CD24– NP cells. Otherwise, expression levels of DCN, COL1, SOX9, FADD, HIF-1α, –1β and –2α were similar in two groups of cells.
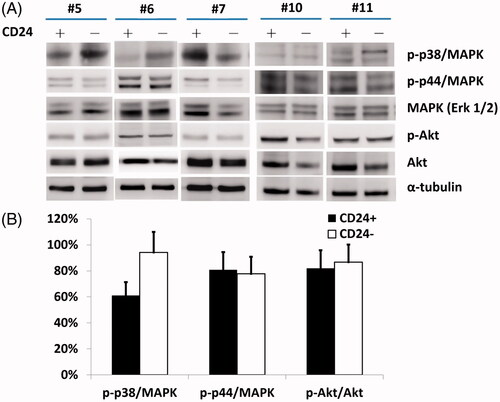
Western blot analysis of senescence associated pathway
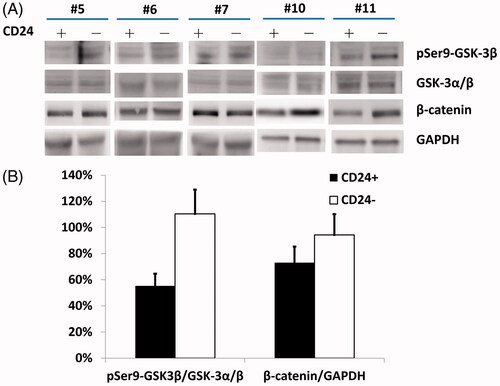
There was a significant increase in pSer9-GSK-3β levels without substantial changes in the total GSK-3α/β levels (, lanes 1 and 2; statistics in ; p < .01), in parallel with the increase in β-catenin expression (, lane 3; statistics in ; p < .05) in CD24– cells compared to CD24+ cells.
Figure 4. (A) CD24+ NP cells displayed increase in pSer9-GSK-3β levels without substantial changes in the total GSK-3α/β levels (lanes 1 and 2) in parallel with the increase in β-catenin expression (lane 3) compared to CD24– cells. (B) Relative densitometry by Western blotting showed that CD24+ NP cells had significantly higher pSer9-GSK-3β and β-catenin expressions relative to those of CD24– cells.
Western blotting further showed that there was a significantly higher level of p-p38 MAPK in CD24– cells compared to CD24+ cells (, lane 1; statistics in ; p < .05). However, there were no substantial changes in the levels of either p-p44/42 MAPK or total MAPK (Erk1/2) proteins (, lanes 2 and 3; statistics in ).
Discussion
Several cell surface markers have been used to characterize the phenotype of healthy NP cells, which may be served as a cell source for IVD regeneration [Citation8]. However, their individual presentation in NP cells, isolated from the herniated NP fragments, is not fully understood. Although brachyury T, CD155 and CD24 have all been suggested as markers for young healthy NP cells, the current study revealed that the positive expression rates of these three markers were different in NP cells of human adults. Since brachyury T and CD155 are highly expressed (> 95%), these 2 markers are less likely to serve as indicators for healthy cells in adult human NP tissues. By contrast, the differential rates of CD24 expression in cells retrieved from human NP tissues suggest its potential role as an indicator of healthy cells that may be used for cell-based therapies treating IVD degeneration.
CD24, a glycosylphosphatidylinositol anchored cell surface protein, is expressed in various cells such as neurons, preB cells, T cells and some cancer cells [Citation16–19]. CD24 is also highly expressed in rat and human NP cells and human chordoma [Citation13]. CD24 has been defined as a healthy NP marker and identified as a human notochord-specific marker [Citation8,Citation20]. In comparison with non- and moderately-degenerated NP, a significant decrease in CD24 expression has been demonstrated in severely degenerated NP [Citation21]. However, studies performed on NP progenitor cells or immortal NP cell lines indicate that CD24+ cells are at a more advanced maturation stage while CD24– cells constitute a more immature stage phenotype [Citation14,Citation22]. Although CD24 has been recognized as a characteristic NP marker, the function of CD24 in NP physiology is still unknown [Citation8]. The current study demonstrated that cellular proliferation was not related to CD24 expression in human NP cells.
The percentage positivity for CD24 in human NP cells is yet to be discovered. The current study of 20 cases, aged 17–44 years, showed that the percentage positivity for CD24 in cells derived from adult human NP was 35.19 ± 3.91% (range 9.1–65.2%). This percentage was compatible with the study done by Richardson et al. (30.0 ± 26.4%; range 0.6–65.0%; n = 5; age unknown) and another study on juvenile NP by Tang et al. (32.5 ± 3.3%; n = 4; age 6–16) [Citation12,Citation21]. Although the averages of percentages were similar in these three studies, the ranges were wide indicating that the expression rate of CD24 was highly variable in a case dependent manner. Although Tang et al. demonstrated that CD24 expression in aged NP (4.4 ± 1.9%; n = 7; age 39–69) was low [Citation12], the current study (age 17–44) and the findings of Richardson et al. (age 22–80) showed that the percentage positivity for CD24 in human NP cells was not correlated with age [Citation21].
For the evaluations of biological therapies for IVD degeneration, the productions of collagens and proteoglycans, major components of extracellular matrix (ECM) in the NP, are widely used markers [Citation23]. The current study revealed that CD24+ NP cells expressed a relatively higher level of the long chain proteoglycan, aggrecan, while the expression levels of the short chain proteoglycan, decorin, were similar between CD24+ and CD24– NP cells. It is known that large aggregating proteoglycans provide more strength than those of non-aggregating proteoglycans, an increase in the synthesis of aggrecan may represent a better tissue property in the matrix regenerated by CD24+ NP cells [Citation24]. Furthermore, CD24+ NP cells showed significantly higher expression of type II collagen, another important ECM component, compared to that of CD24– cells [Citation25,Citation26]. CD24– NP cells had relatively higher mRNA levels of MMPs and TGF-β1, which represents a catabolic process indicative of an increase in the breakdown in ECM and reduced formation of type II collagen. Therefore, CD24+ cells could be considered possessing a more favorable phenotype of NP cells. However, there was no substantial difference in the expression levels of SOX9 and HIFs between CD24+ and CD24– NP cells, which revealed that the overexpression of aggrecan and type II collagen in CD24+ NP cells is not mediated by the mechanisms associated with these factors. On the other hand, the expression of type X collagen in CD24+ NP cells was shown to be twofold higher than in CD24– cells. Although immature, type X collagen appears more frequently as the NP degenerates [Citation27,Citation28], and expression of type X collagen could also be an early event of chondrogenic differentiation in mesenchymal stem cells [Citation29].
One important feature shared by senescent cells in the IVD is cell cycle arrest in G1-S transition [Citation30]. In response to the loss of environmental growth factors, oxidative stress and inhibition of GSK-3β have been shown to halt G1-S transition by up-regulating β-catenin signals [Citation31,Citation32]. GSK-3β modulated β-catenin phosphorylation and caused proteasome-mediated degradation [Citation33]. When the phosphorylation of β-catenin by GSK-3β is decreased, β-catenin stabilizes, accumulates and moves into the nucleus to trigger cell cycle arrest. Since the inhibition of GSK-3β results in the accumulations of β-catenin and subsequently the activation of Wnt/β-catenin cascade to promote cell senescence, the current study examined the relationship between CD24 expression and the GSK-3β-β-catenin cascade. GSK-3β is constitutively active in resting cells, requiring phosphorylation by upstream kinases such as Akt to inactivate it, the expression levels of pSer9-GSK-3β were checked to serve as a surrogate for its activity [Citation34]. Phosphorylation of serine 9 triggers inhibition of GSK-3β activity through activation of the PI3K/Akt signaling pathway [Citation35,Citation36]. Consistent with results of previous studies, the current study demonstrated a decreased activity of GSK-3β and an increased expression of β-catenin in the CD24– NP cells, reinforcing the contention that loss of the CD24 surface marker in NP cells may indicate the senescence fate of human NP cells [Citation31,Citation32]. The observation of decreased GSK-3β activity with increased β-catenin expression may indicate that cellular senescence fate may be more prominent in NP cells retrieved from human adults.
In addition to the β-catenin signaling pathway, the p38-MAPK which is activated by various external stimuli could upregulate the downstream p53-p21-Rb and p16-Rb pathways to trigger cellular senescence [Citation37,Citation38]. In addition to β-catenin, activation of MAPK, especially p38 MAPK, is also senescence-inducing [Citation39]. p38 MAPK is involved in numerous cellular processes related to IVD degeneration including apoptosis and provoked expressions of catabolic enzymes [Citation40,Citation41]. A previous study has reported that CD24 may promote cell growth and induced activation of extracellular signal-regulated kinases, including p38 MAPK [Citation42]. This evidence further supports our findings that loss of CD24 leads to senescence of NP cells and suggests that the MAPK pathway may also play a role in the underlying mechanism. Considered together, the findings of the current study suggest that cellular senescence in adult human NP cells without CD24 expression would be the consequential inactivation of GSK3β activity with resulting accumulations of β-catenin and up-regulation of p-p38-MAPK expression. Senescent cells have an abnormal gene expression pattern with a proinflammatory phenotype, which characterized by the overexpression of MMPs and deregulations in growth factors and cytokines [Citation43]. Activation of β-catenin signaling is shown to promote MMP expression and induce IVD cell senescence [Citation44]. Higher expressions of MMP-2, MMP-13 and TGF-β1 in CD24– NP cells could, therefore, be a part of the manifestations related to cellular senescence.
IVD cells typically experience a dedifferentiation process when grown in monolayer culture [Citation45]. Human NP cells cultivated under normoxia displayed poorer expressions of aggrecan and type II collagen while promoting expressions of MMPs [Citation46]. Not only compromising the overall phenotypic behaviors, serial passages in monolayer and cultivation under normoxic environment also diminish the percentage positivity of CD24 in human NP cells. Further investigations on treatments or stimuli that may aid in increasing the population of CD24+ cells would also be potential targets for cell-based therapies for NP regeneration.
Conclusion
A substantial proportion of cells from adult human NP tissues expressed the CD24 surface marker. CD24+ NP cells expressed significantly higher levels of aggrecan and type II collagen representing a healthier phenotype. The actual mechanism is unlikely to be related to SOX9- or HIFs-medicated pathways. With the decreased activity of GSK-3β, increased expression of β-catenin and activation of p38 MAPK, NP cells without expression of CD24 surface marker possess a more prominent fate of cellular senescence. Percentage positivity for CD24 in human NP cells is negatively affected by serial passages in monolayer and oxygen concentration during the process of isolation/expansion. Further investigations are warranted to discover factors or conditions that may propagate the proportion of CD24+ NP cells which may promote the chance of developing effective cell-based therapies for IVD degeneration.
Acknowledgements
The authors are grateful for the technical support provided by the image core at the First Core Lab of National Taiwan University College of Medicine and the Microscopy Core Facility, Department of Medical Research, National Taiwan University Hospital. The authors thank Department of Medical Research in the National Taiwan University Hospital for technical assistance.
Disclosure statement
The authors confirm that there are no conflicts of interest.
Additional information
Funding
References
- Adler JH, Schoenbaum M, Silberberg R. Early onset of disk degeneration and spondylosis in sand rats (Psammomys obesus). Vet Pathol. 1983;20:13–22.
- Phélip X. Why the back of the child? Eur Spine J. 1999;8:426–428.
- Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003.
- Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11:S206–S214.
- Meisel HJ, Ganey T, Hutton WC, et al. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15:397–405.
- Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21.
- Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater. 2015;29:202–212.
- Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the spine research interest group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293.
- Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22.
- Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41.
- Smolders LA, Meij BP, Riemers FM, et al. Canonical Wnt signaling in the notochordal cell is upregulated in early intervertebral disk degeneration. J Orthop Res. 2012;30:950–957.
- Tang X, Jing L, Richardson WJ, et al. Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration. J Orthop Res. 2016;34:1316–1326.
- Fujita N, Miyamoto T, Imai J, et al. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–1896.
- Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264.
- Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878.
- Bruce J, Symington FW, McKearn TJ, et al. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981;127:2496–2501.
- Symington FW, Hakomori S. Hematopoietic subpopulations express cross-reactive, lineage-specific molecules detected by monoclonal antibody. Mol Immunol. Immunol. 1984;21:507–514.
- Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J Immunol. 1992;149:2533–2540.
- Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262.
- Rodrigues-Pinto R, Berry A, Piper-Hanley K, et al. Spatiotemporal analysis of putative notochordal cell markers reveals CD24 and keratins 8, 18, and 19 as notochord-specific markers during early human intervertebral disc development. J Orthop Res. 2016;34:1327–1340.
- Richardson SM, Ludwinski FE, Gnanalingham KK, et al. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep. 2017;7:1501.
- van den Akker GG, Surtel DA, Cremers A, et al. Novel immortal human cell lines reveal subpopulations in the nucleus pulposus. Arthritis Res Ther. 2014;16:R135.
- Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am. 2011;42:585–601.
- Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interaction. Instr Course Lect. 1998;47:477–486.
- Roberts S, Menage J, Duance V, et al. 1991 Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16:1030–1038.
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699.
- Roberts S, Bains MA, Kwan A, et al. Type X collagen in the human invertebral disc: an indication of repair or remodelling? Histochem J. 1998;30:89–95.
- Freemont AJ, Watkins A, Le Maitre C, et al. Current understanding of cellular and molecular events in intervertebral disc degeneration: implication for therapy. J Pathol. 2002;196:374–379.
- Mwale F, Stachura D, Roughley P, et al. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–1798.
- Kletsas D. Senescent cells in the intervertebral disc: numbers and mechanisms. Spine J. 2009;9:677–678.
- Hiyama A, Sakai D, Tanaka M, et al. The relationship between the Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc cellsignal in the intervertebral disc cell. J Cell Physiol. Physiol 2011;226:1139–1148.
- Wang F, Cai F, Shi R, et al. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr Cartil. 2016;24:398–408.
- Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor)). Oncogene. 1999;18:6615–6620.
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186.
- Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665.
- Noël A, Barrier L, Rinaldi F, et al. Lithium chloride and staurosporine potentiate the accumulation of phosphorylated glycogen synthase kinase 3β/Tyr216, resulting in glycogen synthase kinase 3β activation in SH-SY5Y human neuroblastoma cell lines. J Neurosci Res. 2011;89:755–763.
- Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403.
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signaling pathway. Genes Cells. 2003;8:131–144.
- Zhang Y, Herbert BS, Rajashekhar G, et al. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:1358–1365.
- Ariga K, Yonenobu K, Nakase T, et al. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine. 2003;28:1528–1533.
- Studer RK, Aboka AM, Gilbertson LG, et al. P38MAPK inhibition in nucleus pulposus cells: a potential target for treating intervertebral disc degeneration. Spine. 2007;32:2827–2833.
- Wang W, Wang X, Peng L, et al. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010;101:112–119.
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740.
- Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047.
- Wang JY, Baer AE, Kraus VB, et al. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001;26:1747–1751.
- Yang SH, Hu MH, Sun YH, et al. Differential phenotypic behaviors of human degenerative nucleus pulposus cells under normoxic and hypoxic conditions: influence of oxygen concentration during isolation, expansion, and cultivation. Spine J. 2013;13:1590–1596.