Abstract
Alkannin (ALK) has anti-inflammatory and anti-tumour activities. We tried to probe the underlying functions of ALK in oral squamous carcinoma (OSCC) cells growth, migration and invasion. OSCC cells viability was investigated after treatment with ALK. Then, BrdU, flow cytometry, Western blot and Transwell assays were executed to appraise proliferation, apoptosis, migration and invasion in OSCC cells with ALK stimulation. The biological functions of miR-9 were explored after miR-9 mimic/inhibitor transfection. The relevance of RECK and miR-9 was predicated by dual luciferase activity assay. JAK1/STAT3 and PI3K/AKT pathways were estimated by Western blot. Tumour formation in vivo was executed by xenograft tumours assay. We found that ALK restrained cell proliferation, facilitated apoptosis, repressed migration and invasion also interdicted JAK1/STAT3 and PI3K/AKT pathways in CAL-27 and SCC-9 cells. miR-9 expression was upgraded in OSCC tissues but decreased in OSCC cells along with ALK administration; meanwhile, overexpressed miR-9 inverted the influences of ALK in OSCC cells growth, migration and invasion. RECK was predicated as a novel target gene of miR-9, and overexpressed RECK hindered JAK1/STAT3 and PI3K/AKT pathways in OSCC cells. ALK prohibited tumour formation in vivo. In conclusion, ALK restrained OSCC cells growth, migration and invasion via adjusting miR-9/RECK axis.
ALK restrains cell growth, migration and invasion in OSCC cells;
miR-9 is enhanced in OSCC tissues but is repressed by ALK in OSCC cells;
miR-9 inverts the repressive effect of ALK on CAL-27 and SCC-9 cells;
RECK is a novel target of miR-9;
ALK or RECK hinders JAK1/STAT3 and PI3K/AKT pathways in CAL-27 and SCC-9 cells;
ALK prohibits tumour formation in vivo.
Highlights
Introduction
Oral cancers are a general term for malignant tumours that occur in the oral cavity, most of which belong to oral squamous cell carcinoma (OSCC), characterized by mucosal variation [Citation1,Citation2]. OSCC, similar with various epithelial malignancies, is prone to local metastasis, mainly in head and neck area, especially lymphatic metastasis, which can lead to the loss of oral functions, including linguistic and swallowing function [Citation3,Citation4]. With further development, OSCC can also cause local ulceration, which seriously affects the quality of life, and ultimately may endanger the life of patients [Citation5]. By now, the conventional surgery, radiotherapy and chemotherapy remain the dominating approaches for the treatment of OSCC [Citation6,Citation7]. Despite the achievements in OSCC diagnosis and remedy, the mortality and morbidity of OSCC remain exceedingly high. Hence, looking for novel and better means for OSCC treatment has practical significance.
Alkannin (ALK, chemical formula: C16H16O5; molecular weight: 288.31) also known as shikonin, isolates from the root of Boraginaceae species, such as Lithospermum erythrorhizon, Arnebia euchroma and A. guttata [Citation8]. The anti-inflammatory and anti-tumour activities of ALK have been verified in diverse illnesses. It has been utilized for remedying acute jaundiced or non-jaundiced hepatitis, chronic hepatitis and verruca plana [Citation9,Citation10]. What is more, an animal experiment found that ALK could restrain hepatic inflammation in diabetic mice [Citation11]. In the researches of cancers, ALK has been discovered to repress glioma cells growth and invasion via mediation of IQGAP/mTOR pathway [Citation12]; the anti-proliferative effect of ALK has been discovered in colorectal cancer cells [Citation13]; moreover, ALK could restrain cell metastasis by repression of matrix metalloproteinase-2/-9 (MMP-2/-9) expression in prostate cancer cells [Citation14]. However, the issues about the role of ALK in OSCC cell growth, migration and invasion have not received fully investigated.
MicroRNAs (miRNAs) are central mediators in multitudinous cellular biological processes in cancers [Citation15,Citation16]. miR-9, an MYC/MYCN-activated miRNA, which participates in regulating E-cadherin and cancer metastasis [Citation17]. Additionally, the research of Sun et al. [Citation18] certified that repression of serum miR-9 was linked to poor prognosis of the patients with OSCC. The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) gene, is an identified tumour suppressor gene, and the RECK hypermethylation is linked to the unfavourable prognosis of OSCC [Citation19]. Above studies hints that miR-9 and RECK possibly take part in regulating the nosogenesis of OSCC. Herein, we attempted to divulge the impacts of ALK on OSCC cells growth, migration and invasion, meanwhile explored the involvements of miR-9/RECK axis in the processes. Besides, JAK1/STAT3 and PI3K/AKT pathways were also probed to disclose the latent mechanisms.
Materials and methods
Collection of OSCC tumour samples
OSCC tumour tissues and the circumambient non-cancerous tissues attained from 20 patients with OSCC (10 females and 10 males, aged from 39 to 67) from 2014 to 2016 in the Binzhou Medical University Hospital were used in the present study. These tissues were detached before the commencing of radiotherapy or chemotherapy. The segregated samples were immediately frozen and stored at −80 °C. All these participants provided an informed consent. The study was supported by Binzhou Medical University Hospital.
Cell culture and treatment
CAL-27 and SCC-9 cells were procured from American Type Culture Collection, (ATCC, Rockville, MD, USA), meanwhile cultivated in ATCC-formulated DMEM (Hyclone, Logan, UT, USA), embodying 10% FBS (Invitrogen, Carlsbad, CA, USA). Above cells were placed in a 5% CO2 incubator under the conventional culture condition. ALK procured from Sigma (St. Louis, MO, USA) was diluted in methanol and allocated in different concentrations (0–20 µM). These above-cultured cells were administrated by ALK for 12 h in the related experiments.
Cell transfection
The short-hairpin RNA (shRNA) directed against RECK and the full length of RECK sequences were constructed in U6/GFP/Neo plasmids or pcDNA3.1 plasmids (GenePharma, Shanghai, China), which were named as sh-RECK and pc-RECK. Plasmids of sh-NC and pcDNA3.1 were regarded as the severally controls of sh-RECK and pc-RECK. Additionally, miR-9 mimic, inhibitor and the NC mimic/inhibitor were synthesized (Life Technologies, Frederick, MD, USA) and transfected into OSCC cells. Lipofectamine 3000 reagent (Life Technologies) was implemented for cell transfections on the basis of the reagent direction. Above-transfected OSCC cells were gathered and employed in the next experiments after 48 h post-transfection.
Cell viability assay
CAL-27 and SCC-9 cells were stimulated with ALK and then were co-cultivated with 10 µL Cell Counting Kit-8 solution (CCK-8, Dojindo, Gaithersburg, MD, USA) for another 2 h in 5% CO2 incubator under the conventional culture environment. A Microplate Reader (Multiskan EX, Thermo Labsystems, Helsinki, Finland) was ultimately for the evaluation of the absorbance at 450 nm.
Bromodeoxyuridine (BrdU) assay
Cell proliferation of disposed and transfected CAL-27 and SCC-9 cells was estimated by utilizing BrdU procured from Sigma on the basis of its specifications. The concentration of 1 mg/mL BrdU solution was supplemented to above-mentioned cells for co-cultivating 3 h before calculating to mark proliferating cells. At least, 1000 cells were counted in the diverse treatment group. Each condition contained at least five replicates.
Cell apoptosis assay
Annexin V-Phycoerythrin (PE) Kit (Beyotime Biotechnology, Shanghai, China) was implemented to assess cell apoptosis in CAL-27 and SCC-9 cells after ALK stimulation or miR-9 mimic transfection. These cells were gathered and washed twice with PBS (Beyotime Biotechnology), simultaneously centrifuged at 1000 rpm for 5 min. Afterwards, 5 µL Annexin V-PE solution was co-incubated with cells for 15 min in the absence of light. Flow cytometry (Beckman Coulter, Fullerton, CA, USA) was done to measure the percentage of apoptotic cells.
Migration and invasion assay
After administration with ALK or transfection with miR-9 mimic/inhibitor, Transwell chamber (Corning, Cambridge, MA, USA) was conducted for the assessment of cell migration and invasion. Transwell assessment for cell invasion was necessary matrigel (BD Biosciences, San Diego, CA, USA) for blocking the upper chamber. Above-involved cells were assembled, re-suspended and configured the concentration for 3 × 105 cells/well. The 600 µL complete medium was added into the lower chamber, synchronously, 200 µL cell suspension was supplemented into the upper chamber. After incubation, these cells were fastened by methyl alcohol, in the meantime dyed with 0.5% crystal violet (Sigma) for 20 min. Cells on the membrane surface at the bottom of the upper chamber were cleared via employing a cotton swab. The migrating and invading cells were observed and measured by a microscope (Olympus, Tokyo, Japan).
Dual luciferase activity assay
The PCR assay was executed for the amplification of the fragment from RECK 3′-UTR with the putative miR-9-binding site. The PCR products were subsequently cloned into pMiR-report vector (Promega, Madison, WI, USA). The plasmids of miR-9 mimic or NC mimic along with RECK 3′-UTR were co-transfected into CAL-27 cells by utilizing Lipofectamine 3000 (Life Technologies). For the determination of luciferase activity, the dual-luciferase assay system (Promega) was ultimately appraised.
Real-time quantitative PCR (RT-qPCR)
The total RNA isolated from OSCC tissues, non-cancerous tissues or two different cell lines (CAL-27 and SCC-9) via exploiting TRIzol reagent (Invitrogen). The 25 µg RNA served as template was added to allocate the reverse transcriptions system utilizing miScript II RT Kit (Qiagen, Valencia, CA, USA). miR-9 and RECK expression levels were assessed through utilizing SYBR Green PCR Master Mix (ABI, Foster City, CA, USA). U6 and β-actin were regarded as endogenous control, and data were gauged through the classical 2−ΔΔCt method [Citation20].
Western blot assay
After administration or transfection, the proteins from the treated CAL-27 and SCC-9 cells were extracted by RIPA lysis buffer (Beyotime Biotechnology) supplemented with a protease inhibitor cocktail (Calbiochem, Billerica, MA, USA). The consistency of protein samples was quantified by utilizing BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). Subsequently, the protein samples were electrophoresed by SDS-PAGE and transferred onto a PVDF membrane. The PVDF membrane was sealed whit 5% BSA, and subsequently co-incubated with the primary antibodies of CyclinD1 (ab226977), p53 (ab31333), Pro-Caspase-3 (ab32499), Cleaved-Caspase-3 (ab49822), total (t)-JAK1 (ab125051), phosphor (p)-JAK1 (ab138005), t-STAT3 (ab68153), p-STAT3 (ab76315), t-PI3K (ab191606), p-PI3K (ab182651), t-AKT (ab18785), p-AKT (ab38449), RECK (ab115844) and β-actin (ab8227, Abcam, Cambridge, MA, USA) overnight at 4 °C. After washing with PBS, the PVDF membranes were co-cultured with a secondary antibody (ab205718, Abcam) for 1 h at the room temperature. The Western blots were visualized by exploiting Western Blotting Luminol Reagent (Santa Cruz, CA, USA), as well as the internal parameters of these bands were quantified through the Image Lab™ Software (Bio-Rad, Hercules, CA, USA).
In vivo assay
The 40 athymic nude mice (age, 8–9 weeks; weight, 20–25 g) were procured from Shanghai Laboratory Animal Center of Chinese Academy of Sciences (Shanghai, China). The mice were fed in cages with free watering under a 12 h light/dark cycle environment. The in vivo experiment was supported by the Ethics Committee of Binzhou Medical University Hospital. In this experiment, the above mice were assigned into four groups of 10 mice each, the groups were respectively named as Control, ALK, ALK + NC mimic and ALK + miR-9 mimic. For creating an OSCC model, SCC-9 cells transfected with or without NC mimic and miR-9 mimic vectors were administered extraorally into the floor of the mouth in the above mice. After the creation of the model, the mice in Control group were injected with 2 mg/kg PBS once daily. The mice in the ALK group were injected with 2 mg/kg ALK once daily after creating a model for six weeks. The mice in ALK + NC mimic group were injected with 2 mg/kg ALK once daily for as long as four weeks after creating a model for eight weeks. The mice in ALK + miR-9 mimic group were injected with 2 mg/kg ALK once daily for as long as four weeks after creating a model for eight weeks. The above-involved mice were executed after 12 weeks and the tumour weight and tumour volume were detected. Outside of this, RT-qPCR and immunohistochemistry were then performed the miR-9 expression and cell proliferation activity.
Ki67 immunohistochemistry
As described previously [Citation21], the paraffin-embedded tissue sections (5 mm) were immunostained with an anti-Ki67 antibody (ab16667, Abcam). The number of Ki67-positive cells was subsequently counted in randomly selected 10 microscopic fields at 400× magnification.
Statistical assay
Data in the current study were described with mean ± SD followed by three independent experiments. GraphPad statistical software (San Diego, CA, USA) with ANOVA following Tukey post-test was implemented for statistical analysis. p < .05 was passed for a statistically significant result.
Results
ALK repressed OSCC cells proliferation and facilitated apoptosis
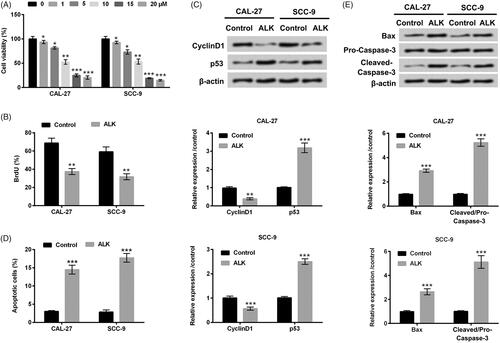
ALK with the concentrations ranged from 1–20 µM was exploited to stimulate CAL-27 and SCC-9 cells. Cell viability was repressed by ALK administration at the concentrations of 1, 5 (p < .05), 10 (p < .01), 15 and 20 µM (p < .001, ). Consideration of the 50% repressive effect of ALK on cell viability was approximately presented at 10 µM, thus, we utilized 10 µM ALK for stimulation of CAL-27 and SCC-9 cells in the following experiments. Afterwards, ALK stimulation evidently restrained the percentage of BrdU-positive cells (p < .01, ), additionally inhibited CyclinD1 and accelerated p53 expression (p < .01 or p < .001, . Further, cell apoptosis, as well as Bax and Cleaved-Caspase-3, were all accelerated by ALK stimulation (p < .001, ). The above-mentioned observations exhibited that ALK repressed cells proliferation and facilitated apoptosis in OSCC cells.
Figure 1. ALK restrained cell proliferation and accelerated apoptosis in OSCC cells (A) The viabilities of CAL-27 and SCC-9 cells tested by CCK-8 were investigated after administration with ALK (1, 5, 10, 15 and 20 µM). (B) The percentage of BrdU-positive cells examined by BrdU assay was explored in 10 µM ALK-stimulated cells. (C) Expression of CyclinD1 and p53 assessed by Western blot, (D) cell apoptosis analyzed flow cytometry and (E) protein levels of Bax, Pro-Caspase-3 and Cleaved-Caspase-3 measured by Western blot were all probed in 10 µM ALK-stimulated cells. *p<.05, **p<.01, ***p<.001.
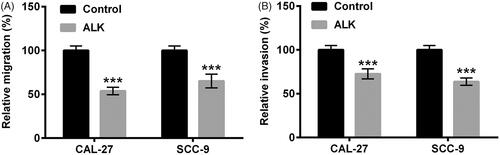
ALK restrained OSCC cells migration and invasion
Cell migration and invasion were then tested in CAL-27 and SCC-9 cells after ALK administration (10 µM). In , we discovered that ALK administration prominently abated cell migration in OSCC cells (p < .001). Similarly, cell invasion was also repressed by ALK administration in OSCC cells (p < .001, ). Collective above data indicated the suppressive effect of ALK on OSCC cell migration and invasion.
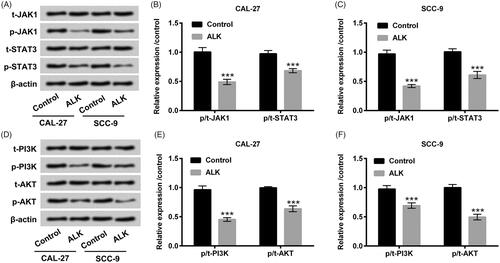
ALK impeded JAK1/STAT3 and PI3K/AKT pathways in OSCC cells
We next probed the impacts of ALK on JAK1/STAT3 and PI3K/AKT pathways. Western blot analytical results revealed that ALK stimulation notably repressed the phosphorylation levels of JAK1 and STAT3 in both CAL-27 and SCC-9 cells (p < .001, ). In addition, the phosphorylation levels of PI3K and AKT were restrained by ALK administration (p < .001, ). Nevertheless, there was no distinct variation of JAK1, STAT3, PI3K and AKT protein levels after stimulation with ALK (). The discovery testified that ALK could impede JAK1/STAT3 and PI3K/AKT pathways in OSCC cells.
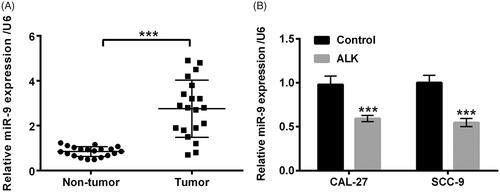
miR-9 expression was presented in OSCC tissues and cells
miR-9 expression in 20 OSCC tissues and the para-carcinoma tissues was investigated through RT-qPCR assessment. displayed that miR-9 expression was enhanced in OSCC tissues as a comparison with that in the para-carcinoma tissues (p < .001). We next observed that miR-9 expression was memorably declined in CAL-27 and SCC-9 cells after administration with ALK (p < .001, ). Above findings testified the abnormal expression of miR-9 in OSCC tissues and cells, indicating that miR-9 likely played a central role in the pathogenesis of OSCC.
Figure 4. miR-9 expression was enhanced in OSCC tissues but repressed by ALK in OSCC cells. (A) Expression level of miR-9 determined by RT-qPCR was probed in 20 OSCC tissues and the para-carcinoma tissues. (B) Expression level of miR-9 assessed by RT-qPCR was explored in OSCC cell lines after ALK stimulation. ***p<.001.
ALK repressed OSCC cells growth, migration and invasion via decreasing miR-9 expression
After transfection with miR-9 mimic and the relative control, cell proliferation, apoptosis, migration and invasion were reassessed in CAL-27 and SCC-9 cells accompanied by ALK stimulation. Cell transfection efficiency was presented in , revealing that miR-9 expression was dramatically raised in CAL-27 and SCC-9 cells (p < .001) after miR-9 mimic transfection. Afterwards, we discovered that the percentage of BrdU-positive cells was overtly enhanced by miR-9 overexpression in ALK-stimulated cells (p < .01 or p < .001, ). Acceleration of CyclinD1 and repression of p53 were observed in cells accompanying miR-9 mimic transfection and ALK stimulation (p < .01 or p < .001, ). miR-9 overexpression repressed cell apoptosis and the relative factor expression of Bax and Cleaved-Caspase-3 in ALK-disposed cells (p < .001, ). Besides, cell migration and invasion were all augmented by miR-9 overexpression in ALK-stimulated cells (p < .01 or p < .001, ). The above-mentioned data corroborated that miR-9 inversed the impacts of ALK on cell growth, migration and invasion in OSCC cells.
Figure 5. ALK inhibited OSCC cells growth, migration and invasion through repression of miR-9. (A) Expression level of miR-9 determined by RT-qPCR was investigated in OSCC cells after miR-9 mimic transfection. (B) The percentage of BrdU-positive cells tested by BrdU assay, (C and D) expression of CyclinD1 and p53 determined by Western blot, (E) cell apoptosis analyzed by flow cytometry, (F and G) protein levels of Bax, Pro-Caspase-3 and Cleaved-Caspase-3 detected by Western blot, (H) cell migration and (I) cell invasion analyzed by Transwell assay were all probed in OSCC cells after transfection with miR-9 mimic accompanied by ALK stimulation. **p<.01, ***p<.001, ns: no significance.
To further certify the adjusted impacts of miR-9 on ALK-affected cell growth, migration and invasion, the miR-9 inhibitor and inhibitor NC vectors were utilized to transfect into SCC-9 cells again. In Supplementary Figure 1(A–C), we discovered that miR-9 repression obviously restrained the percentage of BrdU-positive cells (p < .01), meanwhile declined CyclinD1 expression (p < .01) and upgraded p53 expression (p < .001) in ALK-stimulated SCC-9 cells. In addition, cell apoptosis, as well as Bax and Cleaved-Caspase-3 expression, were all expedited by miR-9 inhibition in ALK-stimulated SCC-9 cells (p < .01, Supplementary Figure 1(D–F)). Besides, miR-9 repression was also further prohibited cell migration and invasion (p < .01, Supplementary Figure 2(A,B)) in ALK-stimulated SCC-9 cells. The above-associated discoveries further confirmed the involvement of miR-9 in ALK-affected cell growth, migration and invasion in OSCC cells.
RECK was a novel target of miR-9
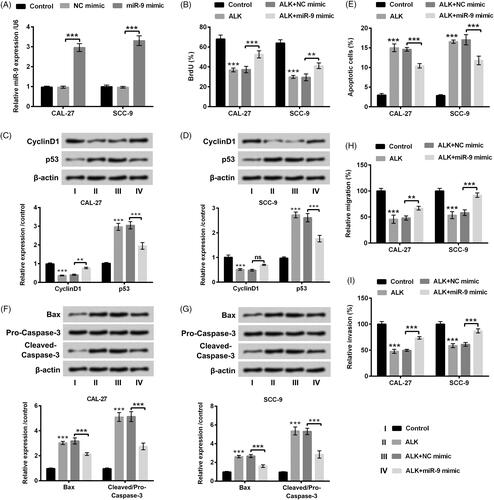
In the subsequent experiments, we investigated the correlation between RECK and miR-9. The interesting results revealed that ALK administration signally accelerated RECK expression in CAL-27 and SCC-9 cells (p < .001). Whereas, the promoting effect of ALK on RECK expression was inversed by overexpressed miR-9 (p < .001, ). More importantly, the relative luciferase activity of RECK-wt with miR-9 mimic transfection was memorably repressed as a comparison with the corresponding control (p < .001, ). There was no obvious variation tendency of the luciferase activity in RECK-mut with miR-9 mimic or NC mimic transfection (). All the above observations certified that RECK possibly was a novel target gene of miR-9.
Figure 6. RECK was a novel target gene of miR-9. (A) Expression level of RECK determined by RT-qPCR was explored in OSCC cells after transfection with miR-9 mimic accompanied by ALK stimulation. (B) Protein level of RECK examined by Western blot was investigated in OSCC cells after transfection with miR-9 mimic accompanied by ALK administration. (C) The correlation between RECK and miR-9 tested by dual luciferase activity assay was explored in OSCC cells after RECK-mut and RECK-wt, miR-9 mimic and NC mimic co-transfection. ***p<.001.
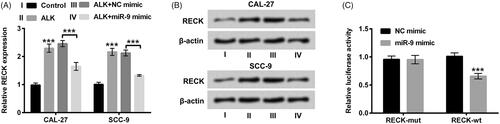
RECK hindered JAK1/STAT3 and PI3K/AKT pathways in OSCC cells
We ultimately probed the impacts of RECK on JAK1/STAT3and PI3K/AKT pathways. The vectors of pc-RECK, sh-RECK and the severally controls were respectively transfected into CAL-27 and SCC-9 cells. The mRNA and protein levels of RECK were all increased after pc-RECK transfection but decreased after sh-RECK transfection in CAL-27 and SCC-9 cells (p < .001, ). Western blot assay results disclosed that the phosphorylation levels of JAK1 and STAT3 were all depressed by RECK overexpression (p < .001, ). Likewise, the phosphorylation levels of PI3K and AKT were also restrained by RECK overexpression (p < .001, ). Nevertheless, the elevation of phosphorylated JAK1, STAT3, PI3K and AKT were presented in CAL-27 and SCC-9 cells after sh-RECK transfection (p < .001, ). There was no apparent variation of total JAK1, STAT3, PI3K and AKT in CAL-27 and SCC-9 cells (). The discoveries indicated that RECK could hinder JAK1/STAT3 and PI3K/AKT pathways in OSCC cells.
Figure 7. RECK hindered JAK1/STAT3 and PI3K/AKT pathways in OSCC cells. (A) Protein and mRNA expression levels of RECK analyzed by Western blot and RT-qPCR were explored in OSCC cells after transfection with pc-RECK, sh-RECK and the several controls. Protein levels of (B and C) total (t)-JAK1, phosphor (p)-JAK1, t-STAT3 and p-STAT3, likewise (D and E) total (t)-PI3K, phosphor (p)-PI3K, t-AKT and p-AKT determined by Western blot was explored in OSCC cells after transfection with pc-RECK, sh-RECK and the several controls. ***p<.001.
ALK prohibited tumour formation in vivo
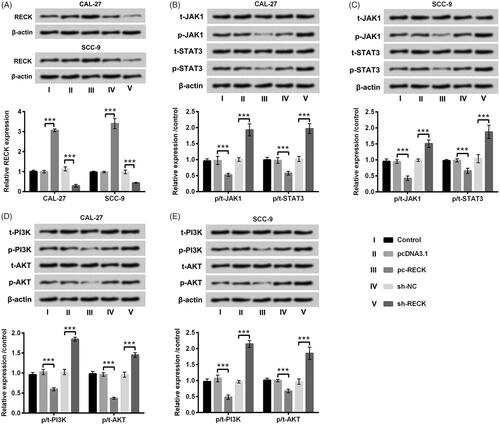
The 40 athymic nude mice were assigned into four groups, including Control, ALK, ALK + NC mimic and ALK + miR-9 mimic, and then tumour weight and volume were determined. In , we discovered that the tumour weight and volume were clearly restrained by ALK administration (p < .001). But, the repressive functions were overturned by overexpressed miR-9 (p < .01, ). Further, in in vivo model, miR-9 expression was obviously declined in ALK group, as a comparison with that in Control group (p < .001), while was upgraded in ALK + miR-9 mimic, as contrasted with that in ALK + NC mimic (p < .001, ). Likewise, the number of Ki67-positive cells was also repressed in ALK group (p < .001), but was expedited in ALK + miR-9 mimic (p < .01, ). All these above results explained that ALK could repress tumour formation via adjusting miR-9 expression in vivo.
Figure 8. ALK prohibited tumour formation in vivo. The 40 athymic nude mice were assigned into four groups, including Control, ALK, ALK + NC mimic and ALK + miR-9 mimic. Tumor (A) weight and (B) volume were evaluated via xenograft tumours assay. (C) miR-9 expression in above-mentioned experimental group was determined via RT-qPCR. (D) The number of Ki67-positive cells was estimated via immunohistochemistry. **p<.01, ***p<.001.
Discussion
OSCC is the most usual oral tumour, which is prone to metastasis to lymph nodes [Citation22]. The classical treatments have been unable to achieve a satisfactory therapeutic effect. Thus, it is imperative to search for a neoteric and valid treatment for OSCC. The present study intended to research the functions of ALK in OSCC cells growth, migration and invasion. The observations disclosed that ALK restrained OSCC cell growth, migration and invasion, meanwhile impeded JAK1/STAT3 and PI3K/AKT pathways. What’s more, abnormally expressed miR-9 was discovered in OSCC tissues and cells, and miR-9 overexpression inversed the alterations of ALK in OSCC cells. Further, RECK was considered to be a newfangled target of miR-9 and overexpressed RECK hindered JAK1/STAT3 and PI3K/AKT pathways in OSCC cells. Besides of these, in vivo experiment revealed that ALK repressed tumour formation via adjusting miR-9 expression.
Various traditional Chinese medicines (TCMs) have been corroborated to possess anti-tumour feature [Citation23]. Evidence from Yang et al. [Citation24] discovered that Icaritin could repress the progression of OSCC through hindering STAT3 signalling. In addition, Tubeimoside-1 (TBMS1) has been certified to refrain OSCC cell proliferation, migration and to accelerate apoptosis in vitro [Citation25]. These findings seem to hint that TCMs are an effective agent for OSCC treatment. ALK, an active constituent procured from Boraginaceae species, has been utilized as a natural red dye for a long time, and the anti-inflammatory and anti-tumour peculiarities of ALK have been testified in disparate illnesses [Citation11]. While, whether ALK exerts the anti-tumour effect on OSCC is still vague. Our experiment results disclosed that ALK repressed cell proliferation, facilitated apoptosis as well as suppressed migration and invasion in OSCC cells. The meaning observations demonstrated that ALK exhibited the anti-tumour impact on OSCC cells.
miRNAs, such as miR-340, miR-188 and miR-17/20a have been found to mediate OSCC cells metabolic shift, proliferation, invasion and migration [Citation26–28]. miR-9 is a fatal miRNA, which has been discovered in leukaemia function as an essential oncogenic gene [Citation29]. Additionally, methylation of miR-9 is regarded as a specific and sensitive biomarker for OSCC [Citation30]. Xiao et al. [Citation31] found that Curcumin could suppress OSCC cell proliferation by adjusting miR-9 expression. Nevertheless, whether miR-9 as a key regulator participates in mediating the anti-tumour activity of ALK in OSCC remain ambiguous. The present study disclosed that miR-9 was up-regulated in OSCC tissues, but down-regulated by ALK in CAL-27 and SCC-9 cells. More interestingly, overexpression of miR-9 inverted the impacts of ALK on OSCC cell growth, migration and invasion, suggesting that miR-9 acted as an important role in the nosogenesis of OSCC.
As a novel suppressor of malignancy, RECK has been regarded as a vinculum to link with oncogenic signalling and extracellular matrix remodelling [Citation32]. RECK has also been shown to be a neoteric MMPs inhibitor, which is capable of repressing tumour invasion, angiogenesis and metastasis [Citation33]. An amusing research demonstrated that silencing of RECK was a crucial event in oral cancer invasion through regulating GSK3β pathway [Citation34]. However, the impacts of RECK on OSCC are still indistinct. In our study, we observed that RECK expression was enhanced by ALK administration, but the alterations were declined after miR-9 mimic transfection. More importantly, we predicted that RECK might be a novel target gene of miR-9 in OSCC cells. The functions of RECK in OSCC cells growth, migration and invasion merit further research.
Activation of STAT3 is conducive to malignant progression in diverse cancers, which can be phosphorylated by JAK family [Citation35]. A study from Sen et al. [Citation36] reported that suppression of JAK could abrogate the activation of STAT3, as well as repressed tumour growth of head and neck squamous cell carcinoma. In addition, STAT3 expression is discovered to be associated with clinicopathological parameters and survival of OSCC, which is regarded as a molecular target in RNA interference-based treatment of OSCC [Citation37,Citation38]. Likewise, the PI3K/AKT pathway also plays a significant role in OSCC. Zhu et al. [Citation39] revealed that Paraoxonase 3 could facilitate OSCC cell proliferation and metastasis through PI3K/AKT. Li et al. [Citation40] affirmed that Muc-1 could aggrandize OSCC cell migration and invasion through the mediation of PI3K/AKT signalling. In our study, the observations showed that JAK1/STAT3 and PI3K/AKT pathways were hindered by ALK administration, also blocked by overexpression of ROCK. The outcomes indicated the involvements of JAK1/STAT3 and PI3K/AKT pathways in OSCC pathogenesis.
For the further certification of the anti-tumour action of ALK in OSCC, we executed an in vivo experiment to investigate the functions of ALK in tumour formation. We selected 40 athymic nude mice in this experiment and assigned these mice into four groups, as Control, ALK, ALK + NC mimic and ALK + miR-9 mimic. The tumour weight and volume were subsequently determined. The experiment disclosed that ALK stimulation clearly prohibited tumour weight and volume. But, interestingly, the repressive impacts were overturned by overexpression of miR-9. Further, we also discovered that miR-9 expression and the number of Ki67-positive cells were both impeded in ALK group, but were both ascended in ALK + miR-9 mimic group. These findings hinted that ALK restrained tumour formation via mediating miR-9 expression in vivo.
Collectively, the research uncovered that ALK restrained OSCC cell growth, migration and invasion through mediating miR-9/RECK axis. Inactivation of JAK1/STAT3 and PI3K/AKT pathways evoked by ALK or RECK might be involved in regulating the anti-tumour effect of ALK on OSCC cells. Further, ALK restrained tumour formation might via adjusting miR-9 expression in vivo. More thorough experiments are still required to corroborate this hypothesis.
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure statement
The authors declare that there are no conflict of interests.
References
- Iyer S, Thankappan K, Balasubramanian D. Early detection of oral cancers. Current status and future prospects. Curr Opin Otolaryngol Head Neck Surg. 2016;24:110–114.
- Ng JH, Iyer NG, Tan MH, et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39:297–304.
- Abdul-Aziz MA, Amin AK, El-Rouby DH, et al. Lymphangiogenesis in oral squamous cell carcinoma: correlation with VEGF-C expression and lymph node metastasis. Int J Dentistry. 2017;2017:7285656.
- Takes RP, Rinaldo A, Silver CE, et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012;48:775–779.
- Aldridge T, Paraneetharan BP, et al. Epstein-Barr-virus-related mucocutaneous ulceration that mimics oral squamous cell carcinoma: the importance of recognising this new condition. Br J Oral Maxillofac Surg. 2017;55:418–419.
- Fan K-H, Chen Y-C, Lin C-Y, et al. Postoperative radiotherapy with or without concurrent chemotherapy for oral squamous cell carcinoma in patients with three or more minor risk factors: a propensity score matching analysis. Radiat Oncol. 2017;12:184–184.
- Hosni A, Huang SH, Xu W, et al. Distant metastases following postoperative intensity-modulated radiotherapy for oral cavity squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2017;143:368–375.
- Wu FY, Tang CY, Guo YM, et al. Transcriptome analysis explores genes related to shikonin biosynthesis in Lithospermeae plants and provides insights into Boraginales’ evolutionary history. Sci Rep. 2017;7:4477.
- Kourounakis AP, Assimopoulou AN, Papageorgiou VP, et al. Alkannin and shikonin: effect on free radical processes and on inflammation – a preliminary pharmacochemical investigation. Arch Pharm Pharm Med Chem. 2002;335:262–266.
- Liu T, Xia Y, Li J, et al. Shikonin attenuates concanavalin A-induced acute liver injury in mice via inhibition of the JNK pathway. Mediat Inflamm. 2016;2016:2748367.
- Xue W, Fan Z, Li Y, et al. Alkannin inhibited hepatic inflammation in diabetic Db/Db mice. Cell Physiol Biochem. 2018;45:2461–2470.
- Gao C, Liang C, Nie Z, et al. Alkannin inhibits growth and invasion of glioma cells C6 through IQGAP/mTOR signal pathway. Int J Clin Exp Med. 2015;8:5287–5294.
- Huu Tung N, Du GJ, Wang CZ, et al. Naphthoquinone components from Alkanna tinctoria (L.) Tausch show significant antiproliferative effects on human colorectal cancer cells. Phytother Res. 2013;27:66–70.
- Chen Y, Zheng L, Liu J, et al. Shikonin inhibits prostate cancer cells metastasis by reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2 pathways. Int Immunopharmacol. 2014;21:447–455.
- Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732.
- Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292.
- Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256.
- Sun L, Liu L, Fu H, et al. Association of decreased expression of serum miR-9 with poor prognosis of oral squamous cell carcinoma patients. Med Sci Monit. 2016;22:289–294.
- Long NK, Kato K, Yamashita T, et al. Hypermethylation of the RECK gene predicts poor prognosis in oral squamous cell carcinomas. Oral Oncology. 2008;44:1052–1058.
- Adnan M, Morton G, Hadi S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2−ΔΔCt method. Mol Cell Biochem. 2011;357:275–282.
- Wang YW, Wang SJ, Zhou YN, et al. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785–797.
- Ren ZH, Zhang CP, Ji T. Expression of SOX2 in oral squamous cell carcinoma and the association with lymph node metastasis. Oncol Lett. 2016;11:1973–1979.
- Tseng CY, Lin CH, Wu LY, et al. Potential combinational anti-cancer therapy in non-small cell lung cancer with traditional Chinese medicine Sun-Bai-Pi extract and cisplatin. PLOS One. 2016;11:e0155469.
- Yang JG, Lu R, Ye XJ, et al. Icaritin reduces oral squamous cell carcinoma progression via the inhibition of STAT3 signaling. Int J Mol Sci. 2017;18:132 (1-15).
- Wu T, Cui H, Xu Y, et al. The effect of tubeimoside-1 on the proliferation, metastasis and apoptosis of oral squamous cell carcinoma in vitro. Onco Targets Ther. 2018;11:3989–4000.
- Xu P, Li Y, Zhang H, et al. MicroRNA-340 mediates metabolic shift in oral squamous cell carcinoma by targeting glucose transporter-1. J Oral Maxillofac Surg. 2016;74:844–850.
- Wang L, Liu H. microRNA-188 is downregulated in oral squamous cell carcinoma and inhibits proliferation and invasion by targeting SIX1. Tumor Biol. 2016;37:4105–4113.
- Rather MI, Nagashri MN, Swamy SS, et al. Oncogenic microRNA-155 down-regulates tumor suppressor CDC73 and promotes oral squamous cell carcinoma cell proliferation: implications for cancer therapeutics. J Biol Chem. 2013;288:608–618.
- Chen P, Price C, Li Z, et al. miR-9 is an essential oncogenic microRNA specifically overexpressed in mixed lineage leukemia-rearranged leukemia. Proc Natl Acad Sci USA. 2013;110:11511–11516.
- Minor J, Wang X, Zhang F, et al. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2012;48:73–78.
- Xiao C, Wang L, Zhu L, et al. Curcumin inhibits oral squamous cell carcinoma SCC-9 cells proliferation by regulating miR-9 expression. Biochem Biophys Res Commun. 2014;454:576–580.
- Kowshik J, Mishra R, Sophia J, et al. Nimbolide upregulates RECK by targeting miR-21 and HIF-1α in cell lines and in a hamster oral carcinogenesis model. Sci Rep. 2017;7:2045–2045.
- Kato K, Long NK, Makita H, et al. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Br J Cancer. 2008;99:647–654.
- Pramanik KK, Singh AK, Alam M, et al. Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer. Tumor Biol. 2016;37:1–12.
- Liao J, Xu T, Zheng JX, et al. Nitidine chloride inhibits hepatocellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway. Int J Mol Med. 2013;32:79–84.
- Sen M, Pollock NI, Black J, et al. JAK kinase inhibition abrogates STAT3 activation and head and neck squamous cell carcinoma tumor growth. Neoplasia. 2015;17:256–264.
- Shah NG, Trivedi TI, Tankshali RA, et al. Stat3 expression in oral squamous cell carcinoma: association with clinicopathological parameters and survival. Int J Biol Mark. 2006;21:175–183.
- Klosek SK, Nakashiro K, Hara S, et al. Stat3 as a molecular target in RNA interference-based treatment of oral squamous cell carcinoma. Oncol Rep. 2008;20:873–878.
- Zhu L, Shen Y, Sun W. Paraoxonase 3 promotes cell proliferation and metastasis by PI3K/Akt in oral squamous cell carcinoma. Biomed Pharmacother. 2017;85:712–717.
- Li P, Xiao LY, Tan H. Muc-1 promotes migration and invasion of oral squamous cell carcinoma cells via PI3K-Akt signaling. Int J Clin Exp Pathol. 2015;8:10365–10374.