Abstract
Objective
The randomized controlled trail was carried out to investigate the influence of statin pre-treatment on clinical efficacy of carotid artery stenting (CAS).
Methods
160 eligible patients were randomly divided into statin group (n = 82) and control group (n = 78). The patients in statin group received 40 mg atorvastatin daily 7 days before operation. Major endpoints included transient ischemic attack (TIA), stroke, death, myocardial infarction (MI), and other cardiac adverse events within 30 days after CAS.
Results
Preoperative baseline information was similar between the statin and control groups (p > 0.05 for all). Within 48 h after operation, the occurrence rate of CIN (3.66% vs 8.97%, p = .019) and new infarction (4.88% vs. 14.10%, p = .045) were significantly lower in statin group than in control group. 30 days after CAS, the incidences of TIA (12.20% vs. 26.92%, p = .018), ischemic stroke (6.10% vs. 16.67%, p = .034), and other cardiac complications (7.32% vs. 19.23%, p = .026) were also significantly lower in statin group, than in the control group. Multiple analysis demonstrated that statin use exerted protective effect against ischemic stroke (OR = 0.038, 95% CI = 0.003–0.543, p = .016) and other cardiac complications (OR = 0.208, 95%CI = 0.063–0.694, p = .011).
Conclusion
Pre-treatment with statin is an effective and safe strategy to prevent from perioperative complications and to improve postoperative outcomes in patients undergoing CAS.
Introduction
Stroke represents a leading reason of deaths and disability, posing a great threat to human health around the world [Citation1]. Approximate 7% of stroke cases could be attributed to carotid artery stenosis [Citation2]. The prevalence of carotid artery stenosis is up to 1% in general population, and its morbidity sharply increases with population ageing, obesity, diabetes mellitus, smoking, hypertension, family history, etc. [Citation3,Citation4]. Timely diagnosis and treatment of carotid artery stenosis could significantly prevent the occurrence of stroke [Citation5]. Carotid artery endarterectomy (CEA) and carotid artery stenting (CAS) are two common treatments for carotid artery stenosis. Compared to CEA, CAS is minimally invasive, so the latter is accepted as an alternative to CEA, especially among those with high surgical risk [Citation6,Citation7]. However, growing evidence have demonstrated that CAS treatment is related to high risk of stroke, restenosis, mortality, as well as perioperative complications, like contrast-induced nephropathy (CIN) [Citation8–11].
CIN is frequently observed in patients experiencing CAS, due to the application of contrast reagents. A recent study reported that in chronic kidney disease patients undergoing internal CAS, the occurrence rate of CIN was up to 21% [Citation12]. CIN is closely correlated with prolonged hospital stage, high incidence of adverse events, low survival rate and poor prognosis [Citation13]. Therefore, drug treatment is in urgent need to reduce complications and to improve clinical outcomes among cases receiving CAS.
Statin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, is widely used in preventing and treating cholesterolemia and cardiovascular events [Citation14]. The drug is well tolerated with low occurrence of adverse events [Citation15]. A number of trails have estimated the efficacy of statin pre-treatment in CAS [Citation16–19]. Relevant evidence suggested that pre-treatment with statin could significantly reduce stroke occurrence, major cardiovascular events, and death among patients receiving CAS. However, the effects of statin treatment on perioperative complications, kidney function, and inflammation status in cases taking CAS are rarely reported. Additionally, the safety of statin treatment in CAS remains unclear.
In the current randomized controlled trail, we proactively investigated the influence of statin pretreatment on perioperative complications, kidney function, and postoperative clinical outcomes among patients undergoing CAS. Clinical efficacy and safety of pre-treatment with statin in CAS were estimated in our study.
Materials and methods
Study subjects
The prospective randomized controlled study was constructed in Tianjin Huanhu Hospital. Patients admitted to the Neurosurgery and Neurology departments of our hospital for CAS surgery were included in the current study. All included patients met CAS surgery indications recommended in the 2011 guidance of endovascular interventional therapy of ischemic cerebrovascular disease in China, and none of them had any contraindication of CAS. In addition, the patients presenting the following conditions would be excluded from our study: (1) severe cardiac and/or hepatorenal insufficiency; (2) statins allergy or intolerance; (3) contraindication of head magnetic resonance imaging (MRI); (4) malignancies; (5) statins usage history within 30 days before admission; and (6) against patients’ or their family’s will. Clinical information of the eligible patients, including age, gender, complications, disease history, symptoms, etc., was collected from their medical records.
The study was approved by the Ethic Committee of the hospital. Written informed consents were obtained from all patients or their guardians.
Grouping and treatments
The included patients were randomly divided into statin treatment group and control group using a computer-generated table. Staff performing CAS and statisticians were blinded to grouping.
Seven days before surgery, all patients took aspirin 100 mg/day combined with clopidogrel 75 mg/day for antiplatele therapy. Oral atorvastatin 40 mg/day was given to patients in statin group. Moreover, atorvastatin was still administered after surgery. Other treatments were same in statin and control groups. Medical care of the two groups was performed by the same team.
Clinical examinations
All of the patients received head MRI, computed tomography angiography (CTA), or cervical vascular ultrasound, magnetic resonance angiography (MRA) examinations prior to CAS treatment. 5 ml fasting blood was collected from every subject 24 h before operation. Examination indicators included neuron-specific enolase (NSE), low density lipoprotein (LDL), high density lipoprotein (HDL), cholesterol, triglyceride, apoliporotin a, apolipoprotein b, hypersenstivie C-reactive protein (hs-CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (BUN), creatinine (Cre), blood urea acid, creatine kinase (CK), and creatine kinase isoenzyme. At postoperative 48 h, head MRI and blood routine examinations were performed again.
Follow-up investigation and endpoints
Outpatient follow-up investigations were performed 30 days after operation. Major endpoints included transient ischemic attack (TIA), stroke, death, myocardial infarction (MI), and other cardiac adverse events within 30 days after CAS. In addition, changes in blood parameters, and the occurrence of contrast-induced nephropathy (CIN) and new infarction within postoperative 48 h were employed as secondary endpoints to estimate the efficacy of statin treatment in CAS.
Patients would be removed if they presented severe adverse events, such as jaundice, dysregulation of liver (ALT or AST exhibited continuous elevation, more than three times of the normal values), muscle soreness, elevated CK values, etc.
Diagnostic criteria
CIN was defined as serum Cre level increase by absolute 0.5 mg/dL or by relative 25% in comparison with baseline data within 48-72 h after intravascular injection of iodinated contrast media, without other acute renal diseases [Citation20]. In our study, the calculation of Cre clearance (Ccr) followed Cockcroft-Gault formula: Ccr = [(140-age) × weight (kg)]/[0.818 × Scr (μmol/L)] (×0.85 if female) [Citation21].
Preoperative and postoperative head MRI was performed by the same machine in our hospital. Images were analyzed by two experienced doctors who were blinded to grouping. New infarction referred to different lesions in postoperative head MRI diffusion-weighted images, which were discontinuous with preoperative ones. The enlargement of preoperative lesions was not regarded as new infarction in the current study.
TIA referred to transient neurological dysfunction caused by cerebral, spinal and retinal ischemia without acute infarction. Cerebral infarction was defined as regional blood supply disorders induced by various reasons, which resulted in ischemic and hypoxic necrosis in brain tissues, thus impairing neurological function in clinic.
If cases presented two or more of the following conditions, they were diagnosed with MI: (1) chest pain; (2) abnormalities in electrocardiograph (ECG); and (3) elevated myocardial enzyme level.
Statistical analysis
All data calculations were achieved by SPSS 18.0 software (SPSS Inc., Chicago, IL, USA), and GraphPad Prism version 5.0 (GraphPad, San Diego, CA, USA) was used for figure plotting. Continuous data were expressed as mean ± standard deviation (SD), and their comparisons between two groups were performed through student’s t-test. Classified variables were recorded as case number with percentages, and compared between groups using chi-square test. Multivariate analyses were performed using logistic regression model. p values less than 0.05 indicated statistical significance of results.
Results
General characteristics of included patients
Based on the selection criteria, a total of 160 eligible patients were enrolled in our study. There were 105 (65.63%) males and 55 (34.38%) females, with an average age of 64.87 ± 9.90 years. 59 (36.88%) patients had smoking history, while drinking history was observed in 62 (38.75%) patients. Concurrent diseases among the cases included hypertension (83, 51.88%), diabetes (76, 47.50%), hyperlipidemia (71, 44.38%) and CHD (69, 43.13%). Common indicators for CAS treatment referred to asymptomatic (41, 25.63%), transient monocular blindness (20, 12.50%), TIA (30, 18.75%), minor stroke (50, 31.25%) and major stroke (19, 11.88%). Left stenosis appeared in 100 (62.50%) patients, while 60 (37.50%) patients exhibited right stenosis.
According to computer-generated table, the patients were randomly divided into statin group (n = 82) and control group (n = 78). As displayed in , the two groups did not show significant differences in basic characteristics, revealing that they were matched. Basic information of the two groups are described in detail in .
Table 1. Baseline information of the included patients.
Laboratory examination results for the included patients before CAS treatment
Peripheral blood samples were collected from patients for laboratory examinations. The average level was 14.22 ± 5.04 ng/mL for NES, 102.26 ± 16.99 mg/dL for LDL, and 41.61 ± 5.43 mg/dL for HDL. The levels of cholesterol, triglyceride, apoliporotin a, apoloprotein b were 185.42 ± 30.52 mg/dL, 136.06 ± 39.18 mg/dL, 137.47 ± 23.51 mg/dL, 95.76 ± 19.14 mg/dL, respectively. The mean hs-CRP level among the patients was 8.74 ± 5.58 mg/L, while their ALT and AST levels were 20.71 ± 4.05 U/L and 19.96 ± 4.54U/L, respectively. The levels for BUN, serum cre and urea acid were 8.43 ± 2.38 mmol/L, 0.89 ± 0.14 μmol/L, and 4.83 ± 1.46 μmol/L, respective. Student’s t-test demonstrated that there was no obvious difference between the statin and control groups. Detailed results on laboratory examinations are summarized in .
Table 2. Blood examination results for the included patients before CAS treatment.
Blood examination results for patients 48 h after CAS treatment
At postoperative 48 h, blood samples were collected again from study subjects for laboratory examinations. Analysis results demonstrated that compared to control group, statin groups exhibited significant low levels of LDL (94.50 ± 14.40 vs. 101.37 ± 17.19, p = .007), cholesterol (147.85 ± 332.84 vs. 161.14 ± 41.29, p = .025), hs-CRP (7.92 ± 5.53 vs. 10.49 ± 7.87, p = .018), and serum Cre (1.02 ± 0.23 vs. 1.24 ± 0.42, p < .001). After CAS treatment, 10 patients (6.25%) had CIN. Furthermore, the occurrence rate of CIN was significantly lower in statin group than in control group (3.66% vs 8.97%, p = .019). Additionally, 15 (9.38%) patients showed new infarction within 48 h after CAS treatment. Chi-square test revealed that statin treatment could significantly inhibit the formation of new infarction after CAS (4.88% vs. 14.10%, p = .045). Detailed comparison results on blood examinations between statin and control groups at postoperative 48 h are listed in .
Table 3. Blood examination results for the included patients at postoperative 48 h.
Clinical outcomes of included patients within 30 days after CAS treatment
Primary end points included the occurrences of TIA, ischemic stroke, MI, other cardiac complications, and death within 30 days after CAS treatment. TIA was observed in 31 patients, accounting for 19.38%, while ischemic stroke in 18 patients (11.25%). 2 patients were diagnosed with MI, taking up 1.25%, and 21 with other cardiac complications, taking up 13.13%. Unfortunately, 5 (3.13%) patients died within 30 days after CAS surgery. Comparison on end points between statin and control groups demonstrated that statin treatment significantly decreased occurrence rate of TIA (12.20% vs. 26.92%, p = .018), ischemic stroke (6.10% vs. 16.67%, p = .034), and other cardiac complications (7.32% vs. 19.23%, p = .026) ( and ).
Figure 1. Clinical outcomes in statin and control groups within postoperative 30 days. The occurrences of transient ischemic attack (TIA), ischemic stroke and other cardiac complications were significantly different between Statin and control groups (p < .05).
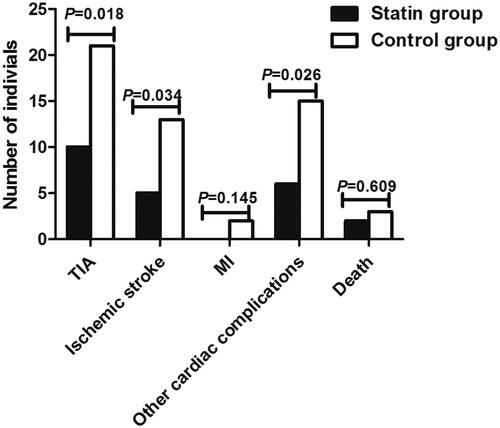
Table 4. Clinical outcomes of the patients within postoperative 30 days.
In addition, logistic regression model was adopted to estimate clinical values of statin treatment in clinical outcomes of patients undergoing CAS within 30 days. As shown in , after adjusting confusing factors, statin treatment was independently correlated with lower incidence of ischemic stroke (OR = 0.038, 95%CI = 0.003–0.543, p = .016) and other cardiac complications (OR = 0.208, 95%CI = 0.063–0.694, p = .011). Unfortunately, there were no independent relationships for statin treatment with TIA (p = .052), MI (p = .996), or death (p = .625).
Table 5. Multiple analysis estimating statin treatment values in clinical outcomes within 30 days among cases undergoing CAS.
Adverse events
In statin group, 7 patients (8.54%) faced slightly increased ALT, 5 patients (6.10%) underwent headache, while 2 patients (2.35%) exhibited elevated CK. In control group, increased ALT level was observed in 5 patients (6.41%), and headache was found in 3 patients (3.84%). Adverse events were slight, and timely treatment could improve those symptoms. None of included patients dropped out the study due to these adverse events. The two groups did not show obvious differences in adverse events, revealing the safety of statin treatment.
Discussion
Prospective randomized controlled trial was carried out to investigate clinical efficacy and safety of statin therapy in patients undergoing CAS. The eligible patients were divided into statin and control groups. Patients in statin group received oral atorvastatin 40 mg/day 7 days before surgery, and the drug was still administered after CAS treatment. 48 h after CAS, the occurrence rates of CIN and new infarction were significantly lower in statin group than in control group. Furthermore, patients exhibited significantly high levels of LDL and low levels of cholesterol, CRP, and Cre after statin treatment. The data revealed that statin treatment could reduce the risk of new infarction, improve inflammation status, and protect kidney function. Within postoperative 30 days, the occurrence rates of TIA, stroke, and cardiac complications were significantly lower in statin group, revealing the benefits of statin treatment in CAS surgery. In addition, the incidence of adverse events was similar between statin and control groups, revealing the safety of statin treatment. Taken together, statin pre-treatment was a safe approach to reduce complications and to improve clinical outcomes among patients undergoing CAS.
CAS is generally accepted as a substitutive treatment for CEA in patients with carotid artery stenosis, but this method may cause high risk of perioperative complications, stroke and mortality [Citation22]. Statin pre-treatment is a commonly used strategy to reduce perioperative and postoperative complications among patients undergoing CAS. In our study, we found that statin treatment could significantly enhance serum levels of LDL and reduce cholesterol level. Published articles have demonstrated that statin might play protective roles in cardiovascular system through suppressing hepatic cholesterol synthesis, enhancing hepatic LDL receptors, and clearing LDL-cholesterol [Citation23]. The results might explain low occurrence rate of new infarction in statin group. In addition, we also found that patients in statin group exhibited obviously lower levels of CRP than in control group. Statin usage might improve inflammation status in patients undergoing CAS. Inhibitory effect of statin on CRP level was also reported in published articles [Citation24]. Statin might exert protective effects on cardiovascular system through anti-inflammation function [Citation25].
With the introduction of endovascular techniques into the management of cardiovascular diseases, increasing demand of contrast media may damage kidney function, thus leading to high occurrence of CIN. The pathogenesis of CIN may be linked to excessive activation of inflammasome and necroptosis induced by crystal media [Citation26]. CIN represents a leading reason of hospital-acquired acute kidney failure, resulting in high hospital costs, prolonged hospital stay and increases in short- and long- term mortality [Citation27]. Therefore, CIN is a worrisome complication of CAS treatment. In our study, we found that serum Cre level was significantly decreased in statin group than in control group. Moreover, the incidence of CIN exhibited obviously downward tendency in statin group, in comparison with control group. Total morbidity of CIN was 9.38% in study population. While 14.10% cases in control group developed CIN, and only 4.88% patients suffered CIN after statin pretreatment. The data suggested that pre-treatment with 40 mg statin could inhibit the occurrence of CIN in patients undergoing CAS. Fu et al. reported that atorvastatin could prevent CIN in a dose-dependent manner after CAS. Daily 40 mg atorvastatin has been proposed as an effective dose for preventing CIN [Citation28]. A single-blind randomized study including 495 patients undergoing primary percutaneous coronary intervention demonstrated that treatment with statins could significantly reduce the occurrence rate of CIN within postoperative 48 h [Citation29]. Potential mechanisms of statin protecting renal function might lie in intrinsic cholesterol-lowering effect, anti-inflammation, antioxidant, etc. [Citation30]. Related mechanisms require further investigations.
The influences of statin pre-treatment on 30-day clinical outcomes of patients undergoing CAS were also estimated in our study. We found that statin treatment could significantly reduce the occurrence rates of TIA, ischemic stroke and other cardiac complications. Moreover, multivariate analysis demonstrated that statin treatment might significantly influence the incidence of ischemic stroke and other cardiac complications. Similar results were also obtained in published articles. For example, Colussi et al. reported that statin use could increase long-term survival and lower the occurrence of major adverse cardiovascular event after CAS [Citation17]. A recent meta-analysis suggested that statin pre-treatment could lead to decreased risk of perioperative stroke and death after CAS, but had no association with TIA or MI [Citation18]. In addition, the safety of statin treatment after CAS was also estimated. The occurrence of adverse events was similar between statin and control groups. Furthermore, no cases dropped out the investigation due to any adverse events. All of the data revealed that pre-treatment with statin was a safe strategy to improve clinical efficacy of CAS.
In conclusion, statin pre-treatment in patients experiencing CAS could significantly reduce the occurrence of CIN and new infarction, and prevent postoperative ischemic stroke and other cardiac complications, with acceptable adverse events. Statin pretreatment is an effective and safe approach to reduce perioperative complications and to improve postoperative outcomes of patients undergoing CAS. Due to the relatively small sample size, the results obtained in our study need to be verified in further studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Caprio FZ, Sorond FA. Cerebrovascular disease: primary and secondary stroke prevention. Med Clin North Am. 2019;103:295–308.
- Dharmakidari S, Bhattacharya P, Chaturvedi S. Carotid artery stenosis: medical therapy, surgery, and stenting. Curr Neurol Neurosci Rep. 2017;17:77.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
- Nguyen BM, Lin KW, Mishori R. Public health implications of overscreening for carotid artery stenosis, prediabetes, and thyroid cancer. Public Health Rev. 2018;39:18.
- Sardar P, Chatterjee S, Aronow HD, et al. Carotid artery stenting versus endarterectomy for stroke prevention: a meta-analysis of clinical trials. J Am Coll Cardiol. 2017;69:2266–2275.
- Lamanna A, Maingard J, Barras CD, et al. Carotid artery stenting: current state of evidence and future directions. Acta Neurol Scand. 2019;139:318–333.
- Serra R, Barbetta A, Fugetto F, et al. Emergent treatment of carotid stenosis: an evidence-based systematic review. Minerva Chir. 2018;73:505–511.
- Zhang L, Zhao Z, Ouyang Y, et al. Systematic review and meta-analysis of carotid artery stenting versus endarterectomy for carotid stenosis: a chronological and worldwide study. Medicine (Baltimore). 2015;94:e1060
- Salem MM, Alturki AY, Fusco MR, et al. Carotid artery stenting vs. carotid endarterectomy in the management of carotid artery stenosis: lessons learned from randomized controlled trials. Surg Neurol Int. 2018;9:85.
- Featherstone RL, Dobson J, Ederle J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): a randomised controlled trial with cost-effectiveness analysis. Health Technol Assess. 2016;20:1–94.
- McCullough PA, Young A, Shutze WP. Acute kidney injury after carotid artery stenting. JACC Cardiovasc Interv. 2015;8:1515–1517.
- Donahue M, Visconti G, Focaccio A, et al. Acute kidney injury in patients with chronic kidney disease undergoing internal carotid artery stent implantation. JACC Cardiovasc Interv. 2015;8:1506–1514.
- Paraskevas KI, Mikhailidis DP. Contrast-induced acute kidney injury in patients undergoing carotid artery stenting: an underestimated issue. Angiology. 2017;68:752–756.
- Bellosta S, Corsini A. Statin drug interactions and related adverse reactions: an update. Expert Opin Drug Saf. 2018;17:25–37.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561.
- Hussain MA, Saposnik G, Raju S, et al. Association between statin use and cardiovascular events after carotid artery revascularization. J Am Heart Assoc. 2018;7:e009745.
- Colussi G, Zuttion F, Bais B, et al. Pre-procedural statin use is associated with improved long-term survival and reduced major cardiovascular events in patients undergoing carotid artery stenting: a retrospective study. J Clin Med 2018;7:286.
- Texakalidis P, Giannopoulos S, Jonnalagadda AK, et al. Preoperative Use of Statins in Carotid Artery Stenting: A Systematic Review and Meta-analysis. J Endovasc Ther. 2018;25:624–631.
- Rizwan M, Faateh M, Dakour-Aridi H, et al. Statins reduce mortality and failure to rescue after carotid artery stenting. J Vasc Surg. 2019;69:112–119.
- Azzalini L, Spagnoli V, Ly HQ. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can J Cardiol. 2016;32:247–255.
- Millar JA. The Cockroft and Gault formula for estimation of creatinine clearance: a friendly deconstruction. N Z Med J. 2012;125:119–122.
- Oliveira PP, Vieira J, Guimaraes RB, et al. Risk-benefit assessment of carotid revascularization. Arq Bras Cardiol. 2018;111:618–625.
- Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243.
- Matsubara T, Naruse K, Arakawa T, et al. Impact of pitavastatin on high-sensitivity C-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: the PREMIUM Study. J Cardiol. 2012;60:389–394.
- Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16:435
- Mulay SR, Shi C, Ma X, et al. Novel insights into crystal-induced kidney injury. Kidney Dis (Basel). 2018;4:49–57.
- Au TH, Bruckner A, Mohiuddin SM, et al. The prevention of contrast-induced nephropathy. Ann Pharmacother. 2014;48:1332–1342.
- Fu M, Dai W, Ye Y, et al. High dose of atorvastatin for the treatment of contrast-induced nephropathy after carotid artery stenting. Am J Ther. 2017;24:e718–e722.
- Firouzi A, Kazem Moussavi A, Mohebbi A, et al. Comparison between rosuvastatin and atorvastatin for the prevention of contrast-induced nephropathy in patients with STEMI undergoing primary percutaneous coronary intervention. J Cardiovasc Thorac Res. 2018;10:149–152.
- Honore PM, Jacobs R, Hendrickx I, et al. Statins and the kidney: friend or foe? Blood Purif. 2017;43:91–96.
