Abstract
The aberrant scar is a challenging problem. Baicalein has effects in attenuating hypertrophic scar formation. Herein, the roles of baicalein in NIH-3T3 were obtained. Cells were treated by baicalein. CCK-8 method and western blot were used to detect cell viability and proliferation-related factors. Furthermore, collagen 1, collagen 3 and α-SMA expression were detected by qRT-PCR and western blot. Besides, total soluble collagen was detected by Sircol assay. In addition, the levels of NF-κB and Wnt/β-catenin signal pathways related factors were determined by western blot. The relationship between miR-9 and insulin-like growth factor (IGF)-1 were tested by luciferase reporter assay, qRT-PCR and western blot. We found baicalein cell restrained proliferation and collagen production. Besides, baicalein increased expression of miR-9 and further experiments validated that transfection with miR-9 inhibitor reversed the results led by baicalein. Moreover, baicalein decreased the phosphorylation of pathway-related proteins while transfection with miR-9 inhibitor revised this result. Otherwise, IGF-1 was authenticated as a target of miR-9 and si-IGF-1 reversed the miR-9 inhibitor-induced change in cell proliferation and collagen production. In conclusion, baicalein restrained cell proliferation and collagen production by regulating miR-9/IGF-1 axis through NF-κB and Wnt/β-catenin signal pathways.
Baicalein restrains NIH/3T3 cell proliferation and collagen production.
Baicalein restrains NF-κB and Wnt/β-catenin signal pathways.
IGF-1 is a target of miR-9.
Baicalein exerts its functions by regulating miR-9/IGF-1 axis.
Highlights:
Introduction
A scar is a general term for the appearance and histopathological changes of normal skin tissue caused by various traumas. Due to its great influence on patients’ lives, scar reduction and improvement in patient satisfaction are the motivations for different types of research to explore a better treatment for the scar [Citation1]. When scar growth exceeds a certain limit, various complications occur, such as the destruction of appearance and dysfunction, which bring great physical and mental pain to patients [Citation2,Citation3]. The excessive proliferation of fibroblasts and collagen synthesis are the pathological features of scar hyperplasia [Citation4]. Therefore, it is of great significance to search for a medicine that can inhibit the excessive proliferation of fibroblasts and collagen synthesis for the clinical treatment of scar hyperplasia.
Radix scutellariae is a traditional Chinese medicine that contains a variety of flavonoids, with baicalein being the most important [Citation5]. A number of previous studies searched the roles of baicalein in various aspects, such as antioxidant effects [Citation6], inhibiting fibrillation [Citation7], antitumor effects [Citation8] and anti-inflammatory activity [Citation9] and so on. Recent developments in the field of scar treatment have led to a renewed interest in baicalein due to its role in attenuating hypertrophic scar formation [Citation10]. This interesting finding motivated us to investigate deeper about the roles of baicalein in the regulation of fibroblasts proliferation and collagen in order to provide more basic researching data and foundations for further studies in the treatment of scar using herbal medicine.
The regulation system by herbal medicines draws attention throughout the world. The experience of former studies gave us insights about the potential possible regulative role of miRNAs, which exists in most of the biological processes. Baicalein altered the expression of several miRNAs including miR-106 [Citation11], miR-124 [Citation12], miR-21 [Citation13] and so on in different diseases. miR-9 was reported to play important roles in the fibrogenic transformation of human dermal fibroblasts [Citation14]. Combined with this crucial finding, we intended to explore whether miR-9 also participated in baicalein in fibroblast NIH/3T3 cells.
In recent years, the relationship between insulin-like growth factor (IGF)-1 and the scar has received increasing attention [Citation15]. Studies displayed that the expression of IGF-1 in the wound area was upregulated after the injury of cornea, skin and other tissues [Citation16], and exogenous IGF-1 could improve the healing of skin and wounds [Citation17]. In addition, studies confirmed a negative correlation between miR-9 and IGF-1 in glioblastomas [Citation18]. Therefore, we speculated that miR-9 may play a role by targeting IGF-1. The overall structure of the study was set to investigate how baicalein worked in NIH/3T3 cells.
Material and method
Cell culture and treatment
NIH/3T3 cells were obtained from American Type Culture Collection (ATCC, Rockville, USA) and cell cultures were prepared by strictly following ATCC culture method. Cells were cultured using Dulbecco’s Modified Eagle’s Medium, (DMEM, Catalog No. 30–2002, ATCC), in which 10% bovine calf serum (ATCC) was added. The culture was set at 37 °C and 5% CO2 environment.
Baicalein (98%) was provided by Sigma-Aldrich (St. Louis, USA). In addition, baicalein was dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich), and after that diluted with DMEM. The treatment time for baicalein was set for 12 h.
Cell counting kit-8 (CCK-8) assay
Cells were seeded in 96-well plate with 100 μl cell supernatant solution (around 5000 cells/well). After stimulation, culture mediums were removed and then a new culture medium was added with CCK-8 solution (Solarbio, Beijing, China). Then, the cells were maintained in an incubator for 1 h at 37 °C in humidified 95% air and 5% CO2. The absorbance was determined using a Microplate Reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Western blot
RIPA lysis (Beyotime, Shanghai, China) was added to the collected cells, shaken in the Vortex finder for 30 s. 40 min later, the liquid supernatant was collected after centrifuged for 12 min at 13,500 rpm at 4 °C. The BCA approach was used to determine the concentration of protein. 10 ∼ 12% SDS-PAGE was used to isolate the same amount of protein and transfer it to the nitrocellulose membrane. Primary antibodies were added after 5% skimmed milk was sealed for 1–1.5 h at room temperature. PBST was washed three times, then secondary antibodies were used and incubated for 1 h. The Image Lab™ Software (Bio-Rad, Hercules, CA, USA)was used to detect the western blot band and Odyssey 3.0 Software (LI-COR Biosciences, Lincoln, NE, USA) was used to quantify the intensity of the bands.
qRT-PCR
RNA extracting was achieved by using Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA). For miR-9 and IGF-1 expression, firstly, miRNA to cDNA transcription was performed using the Taqman MicroRNA Reverse Transcription Kit. Secondly, cDNA was generated in a reverse transcription reaction using Taqman Universal Master Mix II (Thermo Fisher Scientific, Waltham, MA,USA). Thirdly, TaqMan MicroRNA Assay of IGF-1 and β-actin, miR-9 and U6 (Thermo Fisher Scientific) were used for determining the IGF-1 and miR-9.
Measurement of collagen protein
Sircol Assay Kit (Biocolor, Belfast, UK) was used to determine the total soluble collagen. The experiment’s results were based on the mean of duplicate data from individual samples.
Transfection
MiR-9 inhibitor and the negative control (NC) (GenePharma Co., Shanghai, China) were used in our study. Cell transfections were performed using Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA).
Reporter vectors constructs and luciferase reporter assay
The fragments of IGF-1 (containing predicted binding sites) were cloned into the pmiR-report vector (Promega, Madison, WI, USA) to form the reporter vector IGF-1 wild type (IGF-1-wt). The sequence replacing the putative binding site was named IGF-1-mutant (IGF-1-mt). The vector and the miR-9 mimic were co-transfected into HEK 293 T cells to test the luciferase activity by Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
Data management and analysis were accessed using Graphpad 6.0 statistical software (GraphPad, San Diego, CA, USA). Experimental data were collected and shown as mean ± standard deviation (SD). Statistical significance was analyzed using the data of student’s t-tests and ANOVA. p < .05 indicated a significant difference.
Results
Baicalein inhibited cell proliferation
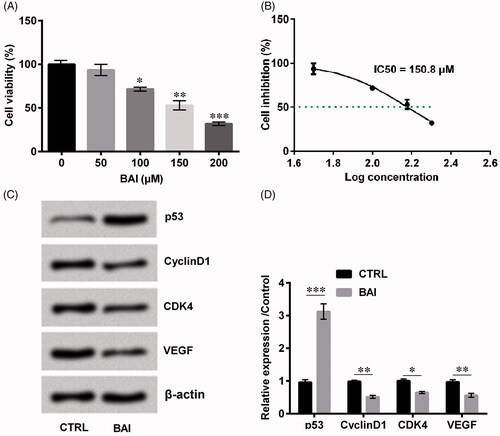
Cell viability is an important factor which can evaluate cell growth. As can be seen from , cell viability was significantly decreased in a dose-dependent manner (p < .05 or p < .01 or p < .001). We calculated that IC50 was 150.8 μM (), so the subsequent experiments used a concentration of 150 μM. In addition, we detected several proliferation-associated factors such as p53, Cyclin D1, cyclin-dependent kinase4 (CDK4) and vascular endothelial growth factor (VEGF). Interestingly, only p53 (p < .001) revealed upregulation trend while CyclinD1 (p < .01), CDK4 (p < .05) and VEGF (p < . 01), on the contrary, revealed a decreasing trend by the treatment of baicalein as relative to control (). The above results suggested that Baicalein inhibited cell proliferation.
Baicalein inhibited NIH-3T3 cell collagen synthesis
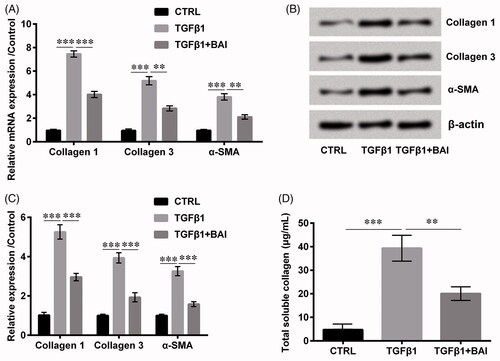
Collagen 1, collagen 3 and α-smooth muscle actin (SMA) were collagen synthesis related proteins. The results in showed that baicalein inhibited the mRNA expression levels of collagen type 1 (p < .001), type 3 (p < .01) and α-SMA (p < .01), which was induced by the treatment of transforming growth factor (TGF)-β1. Similar effects by baicalein were also shown in the expression of protein levels (p < .001, ). Furthermore, the detection of total soluble collagen revealed the same results (p < .01, ), which indicated that baicalein has the ability to inhibit collagen synthesis to some extent.
Figure 2. Baicalein alleviated collagen production. The expression of collagen 1, collagen 3 and α-SMA (A) in the mRNA level and (B, C) in protein level were determined by qRT-PCR and western blot, respectively. (D) The total soluble collagen was examined by Sircol Assay. Data is shown as mean ± SD. **p < .01; ***p < .001.
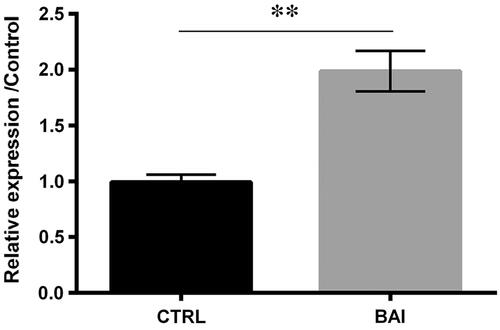
Baicalein upregulated the expression of miR-9
From the previous report, miR-9 was found to have protective effects in the fibrogenic transformation of human dermal fibroblasts [Citation14]. It can be seen from that baicalein increased the expression of miR-9 (p < .01), which indicated that miR-9 might participate in the regulative roles of baicalein in cells.
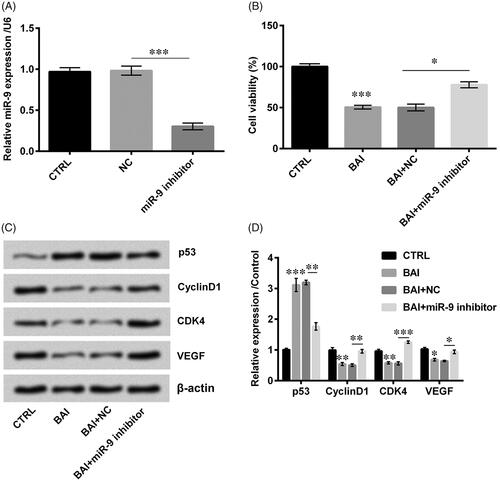
Baicalein inhibited cell proliferation by inducing miR-9 expression
To clarify the functions of miR-9 in the baicalein regulative process, the miR-9 inhibitor was transfected into cells to alter the expression of miR-9 (p < .001, ). Further results demonstrated that co-treatment with baicalein and miR-9 inhibitor statistically elevated the cell viability (p < .05, ), altered proliferation-related proteins in detailed with downregulation of p53 (p < .01) and upregulation of Cyclin D1 (p < .01), CDK4 (p < .001) and VEGF (p < .05) which revealed an opposite trend with treatment with baicalein alone (). The aforementioned results demonstrated that miR-9 downregulation influenced the cells in the opposite way, suggesting the functions of baicalein were achieved by upregulation of miR-9.
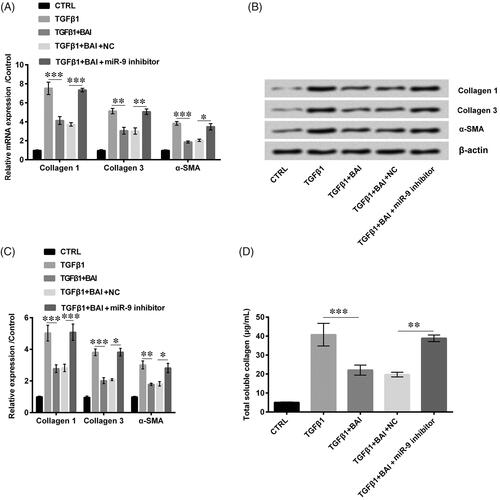
Baicalein inhibited cell collagen synthesis by inducing miR-9 expression
Similarly, we detected the expression of collagen-relative proteins collagen 1, collagen 3 and α-SMA. What stands out in the figures is the opposite trend of miR-9 silence brought out with higher expression of collagen 1 (p < .001), collagen 3 (p < .05 or p < .01) and α-SMA (p < .05) as compared to the NC in both mRNA expression levels and protein levels (). Furthermore, provided the total soluble collagen production and obviously revealed the strongly increased effects for collagen production induced by miR-9 silence as relative to the NC group (p < .01).
Figure 5. Transfection with miR-9 inhibitor promoted collagen production. The expression of collagen 1, collagen 3 and α-SMA (A) in the mRNA level and (B, C) in protein level were determined by qRT-PCR and western blot, respectively. (D) The total soluble collagen was examined by Sircol Assay. Data was shown as mean ± SD. *p < .05; **p < .01; ***p < .001.
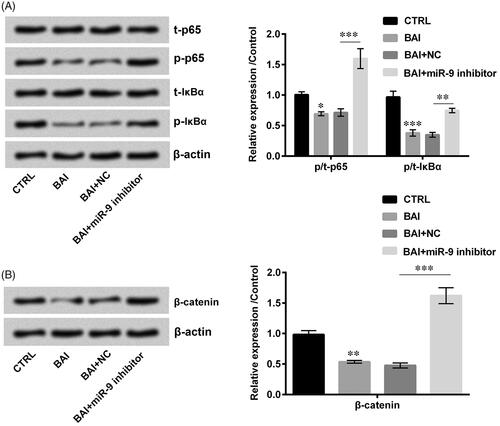
Baicalein inactivated NF-κB and wnt/β-catenin signal pathway by inducing miR-9 expression
NF-κB and Wnt/β-catenin are two important signal pathways which are reported to be involved in the biological progression of cutaneous scarring [Citation19]. Besides, Wnt/β-catenin and NF-κB signalling pathways also affect cell growth and development through a series of cascade reactions [Citation20]. The results obtained from western blot assay are shown in that exhibit Baicalein decreased the phosphorylation of p65 (p < .05), IκBα (p < .001) and β-catenin (p < .01). On the other hand, shows that co-treatment with baicalein and miR-9 inhibitor totally changed the phosphorylation of p65, IκBα and β-catenin compared with NC, which indicated that baicalein could inactivate Wnt/β-catenin and NF-κB signalling pathways through upregulation of miR-9.
IGF-1 was authenticated as a target of miR-9
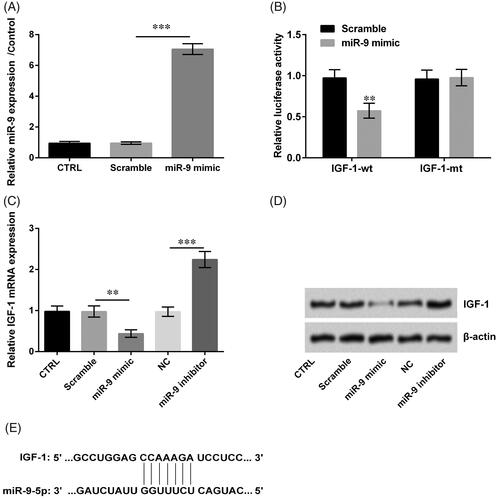
The level of miR-9 was meaningfully increased while transfected with miR-9 mimic (p < .001, ). Meanwhile, the fluorescence activity of IGF-1-wt was decreased and the fluorescence activity in IGF-1-mt was unchanged when transfected with miR-9 mimics (p < .01, ). Based on these results, we displayed miR-9 mimic decreased the IGF-1 expression while miR-9 inhibitor increased the IGF-1 expression (p < .01 or p < .001, ). Besides, IGF-1 combined miR-9 by direct interaction (). Therefore, IGF-1 was authenticated as a target of miR-9.
Figure 7. Insulin-like growth factor (IGF)-1 was authenticated as a target of miR-9. (A) miR-9 expression was detected by qRT-PCR. (B) The fluorescence activity of IGF-1 was examined by luciferase reporter assay. (C, D) The levels of IGF-1 were detected by qRT-PCR and western blot. (E) miR-9 and IGF-1 binding sites were predicted by TargetScanHuman 7.1. Result was shown as mean ± SD. **p < .01; ***p < .001.
Baicalein inhibited cell proliferation and cell collagen synthesis by regulating miR-9/IGF-1 axis
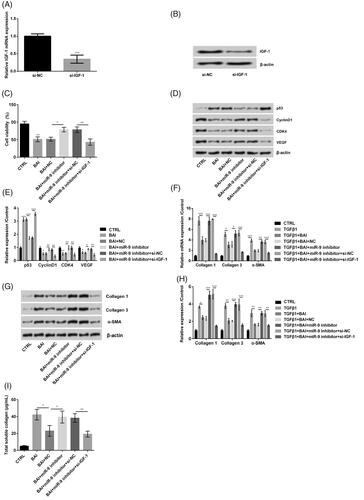
To clarify the functions of IGF-1 in the baicalein regulative process, we silenced the IGF-1 in cells (p < .001, ). When si-IGF-1 was transfected to miR-9 inhibitor-induced cells, the cell viability was statistically decreased (p < .01, ). si-IGF-1 altered proliferation-related proteins in detail with the upregulation of p53 (p < .001) and downregulation of Cyclin D1 (p < .01), CDK4 (p < .01) and VEGF (p < .01), which revealed the opposite trend on treatment with miR-9 inhibitor (). Besides, the results displayed the levels of collagen 1, collagen 3 and α-SMA decreased when si-IGF-1 was transfected into miR-9 inhibitor-induced cells (p < .001, ). Furthermore, revealed that the total soluble collagen production decreased when si-IGF-1 was transfected (p < .01). All results displayed the baicalein inhibited cell proliferation and cell collagen synthesis by regulating miR-9/IGF-1 axis.
Figure 8. Baicalein inhibited cell proliferation and cell collagen synthesis by inducing miR-9/insulin-like growth factor (IGF)-1 axis. (A, B) IGF-1 expression was detected by qRT-PCR. (C) Cell viability was examined by CCK-8. (D, E) Cell proliferation related proteins were detected by western blot. (F–H) The expression of collagen 1, collagen 3 and α-SMA in the mRNA level and in protein level were determined by qRT-PCR and western blot, respectively. (I) The total soluble collagen was examined by Sircol Assay. Data was shown as mean ± SD. *p < .05; **p < .01; ***p < .001.
Discussion
Aberrant growth skin scar is a challenging health problem because it brings great influence to the patient’s physical and spiritual life [Citation21]. The current study established an in vitro cell model using NIH/3T3 to investigate the functions of baicalein in regulating NIH/3T3 cells. Results demonstrated that baicalein mitigated cell proliferation and diminished the production of collagen production through regulation of miR-9/IGF-1 axis through NF-κB and Wnt/β-catenin signal pathways. Our experiments were based on the in vitro cell model and, firstly, explored the potential effects of baicalein in NIH/3T3 cells, in order to provide basic research for the treatment of scar.
During the biological progression of scar synthesis, fibroblasts proliferation and the aberrant collagen production exhibit crucial roles which might result in overgrowth of the scar [Citation22]. From this aspect, the inhibition of proliferation and collagen production in the growing process of fibroblast seems extremely important. That is the reason we explored the potential roles of baicalein in regulating cell proliferation and collagen production.
Baicalein is one important effective ingredient which possesses multiples effective properties in the treatment of different diseases including cancer and injuries [Citation23,Citation24]. Hence, experiments were performed to determine whether baicalein plays a role in fibroblasts proliferation and collagen production. Interestingly, data revealed that baicalein abated cell viability in a concentration-dependent manner. Furthermore, we detected the accumulated levels of proliferation-related proteins which were p53, CyclinD1, CDK4 and VEGF and found that baicalein obviously upregulated p53 while downregulating the other three factors. The upregulation of p53 normally goes along with inhibition of cell proliferation [Citation25]. While CyclinD1 [Citation26], CDK4 [Citation27] and VEGF [Citation28] are vital proliferation inducers, the downregulation of CyclinD1, CDK4 and VEGF suggested baicalein revealed proliferation suppressing roles in NIH/3T3 cells.
In another aspect, collagen production also means weighty for fibroblast growth and development. We detected the accumulated mRNA and protein levels of Collagen 1, Collagen 3 and α-SMA in NIH/3T3 cells. It can be clearly seen from results that baicalein had the ability to diminish the production of collagen 1, collagen 3 and α-SMA both in mRNA and protein levels. Previous studies pointed out that collagen 1, collagen 3 and α-SMA were found to be involved in collagen fibers [Citation29]. Furthermore, declining Collagen 1 in NIH/3T3 cells were also reported by the treatment of miR-29b [Citation22]. One powerful evidence revealed that decreasing production of Collagen 3 increased fibroblast differentiation and promoted the production of scar deposition [Citation30]. Furthermore, α-SMA suppressing also associated with scar inhibition [Citation31]. Moreover, our study demonstrated that baicalein suppressed the production of total soluble collagen. Comparing previous studies’ data with ours, we concluded that baicalein might have the potential to function as a fibroblast development inhibitor, which indicated that baicalein might have potent effects on scar reduction.
Overwhelming studies can be found about the mechanism in which baicalein functions are achieved in cells. For example, baicalein demonstrated suppressing effects in bleomycin-induced pulmonary fibrosis by regulating miR-21 [Citation32]. The current data revealed baicalein altered expression of miR-9. Further results experimentally validated the roles of miR-9 in the progression of baicalein in NIH/3T3 cells due to transfection with miR-9 inhibitor totally reversing the results both in cell proliferation and collagen production. This finding broadly supports the work of miR-9 in other studies. In cardiac fibroblast, miR-9 alleviated proliferation as well as collagen by regulating transforming growth factor β receptor type II (TGFBR2) [Citation33]. Similar results can be seen in our study that transfection with miR-9 inhibitor promoted cell proliferation and collagen production, which was consistent with the previous finding [Citation33]. However, in our study, the regulating relationship between baicalein and miR-9 was validated for the first time. Through these results, we further understand the underlying mechanism about potential roles of baicalein in the treatment of the scar.
On the other hand, we explored the NF-κB and Wnt/β-catenin signal pathways to identify whether these two signalling pathways were also involved. Baicalein inactivated these two signal pathways while transfection with miR-9 inhibitor led to the opposite result. The former study pointed out that baicalein abated kidney fibrosis with NF-κB signal pathway involved [Citation34]. Moreover, baicalein alleviated cell proliferation in acute T-lymphoblastic leukemia Jurkat cells through inactivating Wnt/β-catenin signalling [Citation35]. Through these studies, it can be seen that in the regulating network of baicalein, NF-κB and Wnt/β-catenin have great possibilities to be involved. Furthermore, our data revealing the same was consistent with previous studies [Citation34,Citation35]. On the other side, miR-9 was also reported to achieve its functions by regulating the related factors of NF-κB and Wnt/β-catenin signal pathways [Citation36,Citation37]. Our study lined up with former experiments demonstrating its great potential in regulating NF-κB and Wnt/β-catenin signal pathways in baicalein-treated fibroblast cells.
miRNA normally exerts its functions in cells by targeting IGF-1. For instance, a study displayed miR-206 targeted IGF-1 in astrocytes [Citation38]. Other study displayed miR-130b-3p worked in lung fibrosis by targeting IGF-1 [Citation39]. Furthermore, a study had confirmed miR-9 and IGF-1 were reversely related [Citation18]. In this paper, we innovatively found that IGF-1 was identified as a target gene of miR-9. Moreover, we discovered when si-IGF-1 was transfected into cells, the NIH/3T3 cell proliferation and collagen production, which were caused by miR-9 inhibitor, were statistically decreased. This suggested that si-IGF-1 also inhibited scar growth in NIH/3T3 cells.
In conclusion, our study creatively used NIH/3T3 cells to establish in vitro cell model and investigate the roles of baicalein, miR-9 and IGF-1 in regulating cell proliferation and collagen production. Furthermore, we also investigated the potential functions of baicalein at NF-κB and Wnt/β-catenin signal pathways and the relationship between miR-9 and IGF-1. Our study, firstly, induced and validated the regulating system in baicalein, miR-9 and IGF-1 and also revealed the possible regulating roles of baicalein in the treatment of scar in the future.
Disclosure statement
Authors declare that there is no conflict of interests.
References
- Li Q, Zhang C, Fu X. Will stem cells bring hope to pathological skin scar treatment? Cytotherapy. 2016;18:943–956.
- Yannas IV, Tzeranis DS, So PTC. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Rep and Reg. 2017;25:177–191.
- Gras C, Ratuszny D, Hadamitzky C, et al. miR-145 contributes to hypertrophic scarring of the skin by inducing myofibroblast activity. Mol Med. 2015;21:296–304.
- Fan C, Dong Y, Xie Y, et al. Shikonin reduces TGF-beta1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med. 2015;36:985–991.
- Yu H, Chang J-S, Kim SY, et al. Enhancement of solubility and dissolution rate of baicalein, wogonin and oroxylin A extracted from Radix scutellariae. Int J Pharm. 2017;528:602–610.
- Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865.
- Zhu M, Rajamani S, Kaylor J, et al. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–26857.
- Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000;55:951–955.
- Lee W, Ku SK, Bae JS. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation. 2015;38:110–125.
- Zhang YF, Zhou SZ, Cheng XY, et al. Baicalein attenuates hypertrophic scar formation via inhibition of the transforming growth factor-beta/Smad2/3 signalling pathway. Br J Dermatol. 2016;174:120–130.
- Jiang L, Song H, Guo H, et al. Baicalein inhibits proliferation and migration of bladder cancer cell line T24 by down-regulation of microRNA-106. Biomed Pharmacother. 2018;107:1583–1590.
- Dong N, Xu B, Shi H, et al. Baicalein inhibits amadori-glycated albumin-induced MCP-1 expression in retinal ganglion cells via a microRNA-124-dependent mechanism. Invest Ophthalmol Vis Sci. 2015;56:5844–5853.
- Cui X, Sun X, Lu F, et al. Baicalein represses TGF-beta1-induced fibroblast differentiation through the inhibition of miR-21. Toxicol Appl Pharmacol. 2018;358:35–42.
- Miguel V, Busnadiego O, Fierro-Fernández M, et al. Protective role for miR-9-5p in the fibrogenic transformation of human dermal fibroblasts. Fibrogenesis Tissue Repair. 2016;9:7.
- Unahabhokha T, Sucontphunt A, Nimmannit U, et al. Molecular signallings in keloid disease and current therapeutic approaches from natural based compounds. Pharm Biol. 2015;53:457–463.
- Trosan P, Svobodova E, Chudickova M, et al. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012;21:3341–3350.
- Nagano T, Nakamura M, Nakata K, et al. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:3810–3815.
- Ho KH, Chen PH, Hsi E, et al. Identification of IGF-1-enhanced cytokine expressions targeted by miR-181d in glioblastomas via an integrative miRNA/mRNA regulatory network analysis. Sci Rep. 2017;7:732.
- Huang C, Akaishi S, Ogawa R. Mechanosignalling pathways in cutaneous scarring. Arch Dermatol Res. 2012;304:589–597.
- Ma B, Hottiger MO. Crosstalk between Wnt/beta-catenin and NF-kappaB signalling pathway during inflammation. Front Immunol. 2016;7:378.
- Brown BC, Moss TP, McGrouther DA, et al. Skin scar preconceptions must be challenged: importance of self-perception in skin scarring. J Plast Reconstr Aesthet Surg. 2010;63:1022–1029.
- Cheng J, Wang Y, Wang D, et al. Identification of collagen 1 as a post-transcriptional target of miR-29b in skin fibroblasts: therapeutic implication for scar reduction. Am J Med Sci. 2013;346:98–103.
- Liu C, Wu J, Xu K, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–1512.
- Wang L, Ling Y, Chen Y, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48.
- Afanasyeva EA, Mestdagh P, Kumps C, et al. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011;18:974–984.
- Zhang H, Zhang X, Ji S, et al. Sohlh2 inhibits ovarian cancer cell proliferation by upregulation of p21 and downregulation of cyclin D1. Carcinogenesis. 2014;35:1863–1871.
- Du B, Wang Z, Zhang X, et al. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLOS One. 2014;9:e88022.
- Kalishwaralal K, Banumathi E, Pandian SRK, et al. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf B Biointerfaces. 2009;73:51–57.
- Mak KM, Chu E, Lau KHV, et al. Liver fibrosis in elderly cadavers: localization of collagen types I, III, and IV, alpha-smooth muscle actin, and elastic fibers. Anat Rec. 2012;295:1159–1167.
- Volk SW, Wang Y, Mauldin EA, et al. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194:25–37.
- Chen R, Zhang Z, Xue Z, et al. Focal adhesion kinase (FAK) siRNA inhibits human hypertrophic scar by suppressing integrin alpha, TGF-beta and alpha-SMA. Cell Biol Int. 2014;38:803–808.
- Gao Y, Lu J, Zhang Y, et al. Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21. Pulm Pharmacol Ther. 2013;26:649–654.
- Li J, Dai Y, Su Z, et al. MicroRNA-9 inhibits high glucose-induced proliferation, differentiation and collagen accumulation of cardiac fibroblasts by down-regulation of TGFBR2. Biosci Rep. 2016;36:e00417.
- Wang W, Zhou P-h, Xu C-g, et al. Baicalein attenuates renal fibrosis by inhibiting inflammation via down-regulating NF-kappaB and MAPK signal pathways. J Mol Hist. 2015;46:283–290.
- Liu X, Liu S, Chen J, et al. Baicalein suppresses the proliferation of acute T-lymphoblastic leukemia Jurkat cells by inhibiting the Wnt/beta-catenin signalling. Ann Hematol. 2016;95:1787–1793.
- Gu R, Liu N, Luo S, et al. MicroRNA-9 regulates the development of knee osteoarthritis through the NF-kappaB1 pathway in chondrocytes. Medicine (Baltimore). 2016;95:e4315.
- Yu T, Liu K, Wu Y, et al. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/beta-catenin signalling pathway. Oncogene 2014;33:5017–5027.
- Liu TJ, Wang B, Li QX, et al. Effects of microRNA-206 and its target gene IGF-1 on sevoflurane-induced activation of hippocampal astrocytes in aged rats through the PI3K/AKT/CREB signalling pathway. J Cell Physiol. 2018;233:4294–4306.
- Li S, Geng J, Xu X, et al. miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLOS One. 2016;11:e0150418.