Abstract
Aim
Reports on the association of the CGG repeat length in the FMR1 gene with the severity of idiopathic POI are inconclusive. Therefore, a meta analysis was performed to investigate the relationship between the expansion of repeat CGG and idiopathic POI risk.
Methods
Up to January 2019, 18 case-control or cohort studies involving 3022 idiopathic POI patients and 8461 controls were included for meta analysis.
Results
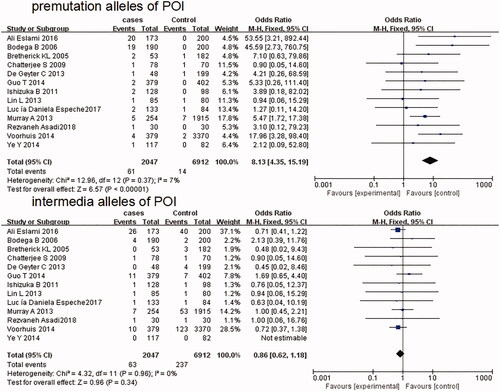
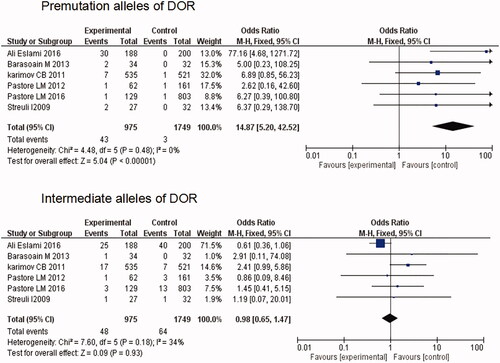
Thirteen studies, including 2047 cases and 6912 controls, met our criteria for the assessment of the premutation and intermediate repeat length in patients patients with POI. Compared with controls, FMR1 gene premutation is significantly associated with POI (OR = 8.13; 95% CI: 4.35–15.19; p < .00001), whereas there was no significant correlation between intermediate repeat length and POI (OR = 0.86; 95% CI: 0.62–1.18; p = .34). Six studies, representing 975 patients and 1749 controls, were eligible for evaluation of the premutation and intermediate repeat length in diminished ovarian reserve (DOR). The association between premutation and DOR was significant (OR=14.87; 95% CI:5.20-42.52; p < 0.00001), while no significant correlation of intermediate size to DOR was found in the case-control comparison (OR = 0.98; 95% CI: 0.65–1.47; p = 0.93).
Conclusion
There is a close association between premutation of the FMR1 gene and increased susceptibility to idiopathic POI of each stage and no correlation between intermediate repeat length of the FMR1 gene and the severity of idiopathic POI.
Introduction
Primary ovarian insufficiency (POI), a term to describe a wide range of impaired ovarian function, is a heterogeneous disorder diagnosed in women before the age of 40 years [Citation1,Citation2]. It starts with diminished ovarian reserve (DOR) (regular or irregular menstrual cycle, subfertility, normal or slightly elevated follicle-stimulating hormone(FSH) levels, low anti-mullerian hormone (AMH), antral follicle counts (AFC) < 5–7 (2–10 mm in diameter)); and finally reaches to premature ovarian failure (POF) (amenorrhea, significantly elevated FSH levels, infertility) [Citation3,Citation4]. The incidence of POF is about 1:10,000 in women by the age of 20 years, 1:1000 in women under 30 years and 1:100 in women under 40 years old [Citation5]. Ovarian dysfunction and follicle depletion are two major mechanisms in the development of POI [Citation1]. A variety of known causes may be involved in the heterogeneity of POI including enzyme deficiency, autoimmune disease, infections and iatrogenic factors. But for most of the cases, the etiology is idiopathic [Citation3,Citation6]. According to the epidemiological studies of several large cohorts of idiopathic POI, the incidence of familial cases varies from 4 to 31%, strongly a suggesting genetic component of POI [Citation7], and some genetic aberrations, such as the X chromosome-linked defects and autosomal monogenic forms, had been demonstrated involving the pathogenesis of POI [Citation8].
The prevalence of the X-linked gene FMR1 (the fragile X mental retardation 1 gene) premutation is 2 ∼ 7% in sporadic POI patients and up to 13% in women with familial forms [Citation9], and several studies have demonstrated that women with POI were associated with the expansion of repeat CGG trinucleotide segments of DNA located in the 5’ untranslated region of FMR1. Recent studies also suggested that the CGG repeat length, background modifier genes, and environmental factors are related to the severity of POI [Citation10–12]. It is conceivable that the interaction of genetic, epigenetic, and environmental factors play roles at each stage of POI. However, current individual studies showed inconsistent results regarding the role of the CGG repeat length and the severity of POI [Citation13–33]. A single study may have been underpowered in clarifying the associations of the CGG repeat length with POI susceptibility. Therefore, a meta analysis of all relevant published randomized and cohort studies was carried out in this study to further explore the relationship between the expansion of repeat CGG and the risk of idiopathic POI.
Methods
All relevant studies published until January 2019 were identified from PubMed, EMBASE, and China National Knowledge Infrastructure using a combined free text and the following MeSH search strategy: (“fragile X mental retardation I” OR “FMR1”) AND (“premature ovarian failure” OR “POF” OR “primary ovarian insufficiency” OR “POI” OR “poor ovarian response” OR “POR” OR “diminished ovarian reserve” OR “DOR” OR “early menopause”). No language restriction was applied. References from these studies were also scrutinized to identify other relevant studies by a manual search of the original publications.
Two authors (Huang J and Zhang WX) extracted the available articles independently. Titles of all articles retrieved from the database were screened. We excluded studies without results on the relationship between the CGG repeat length and POI. Of the articles with the overlapping data, we only selected the publication with the most extensive information. The abstracts of relevant articles investigating the relationship between the expansion of repeat CGG and POI risk were examined and all studies that could potentially be included in the review were retrieved. The eligible articles had to meet all the following criteria: (i) there is quantitative information on the estimated risk of the CGG repeat length in the FMR1 gene for idiopathic POI, (ii) case-control or cohort design, (iii) contained complete information about the CGG repeat length identified by PCR ABI sequencing analyzer or by PCR Southern blot analysis, and (iv) clear definition of idiopathic POI. The exclusion criteria were as follows: (i) the research was not for the CGG repeat length in the FMR1 gene and idiopathic POI, (ii) review articles, animal studies, case reports, or unpublished reports, (iii) reports containing no usable data, and (iv) duplicate publications or paper less than 20 sample size. The following data were abstracted from the eligible studies: the first author’s name; year of publication; country; ethnicity, design type of study, methodology, diagnostic criteria for POI and its severity, and sample size of case and control groups.
In the present study, idiopathic POI was defined as [Citation4]: (i) none of the patients showed an abnormal karyotype; (ii) none of the patients had a family history of the fragile X syndrome; (iii) patients caused by medical interventions, such as chemotherapy and/or radiation were excluded. DOR was defined as: women with regular or irregular cycles and/or elevated FSH levels > 10 IU/L and/or antral follicle counts (AFC) < 5–7 (2–10 mm in diameter) and/or a poor response to controlled ovarian hyperstimulation (COH) before the age of 40 years [Citation18,Citation19]. POI was defined as: (i) secondary amenorrhea more than three months; and (ii) FSH level >40 IU/L [Citation22]. The premutation of FMR1 is defined as more than 55 but less than 200 CGG repeats, the intermediate of FMR1 is defined as a CGG repeat length of 45 to 54, whereas those less than 45 are termed as normal [Citation34]. The allele with the longest repeat length of FMR1 was evaluated.
The meta analyses were performed using RevMan 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The strength of the association between the CGG repeat length in the FMR1 gene and the severity of idiopathic POI was measured by ORs with their 95% CIs. The Mantel–Haenszel method was applied to estimate pooled effect sizes. Heterogeneity of the exposure effects was evaluated graphically using forest plots. Statistical heterogeneity was assessed by the measure of the I2. An I2 measurement greater than 50% was considered to denote heterogeneity. A random-effect model was used in case of substantial heterogeneity and a fixed-effect model in the absence of heterogeneity. Two sides p-values less than .05 was considered statistically significant.
Results
With the search strategy applied, 50 published articles were identified as possible for evaluating the association of the CGG repeat length in the FMR1 gene with POI. There were 18 studies retrieved on the basis of the search criteria at last (, and ), involving 3022 POI patients and 8461 controls. Fifteen review articles, three case report papers, two animal studies, four methodologically inconsistent articles and three unextractable papers, as well as four papers not involved in the CGG repeat length, were excluded. Another paper written by Murry et al., which enrolled the same participants as their latest publication, were also excluded. Meanwhile, one study [Citation20] with a sample size of DOR more than 20 were included in the analysis. Detailed characteristics of each study are described in . The distributions of intermediate and premutation alleles of the included studies in women with POI and DOR were presented in .
Figure 1. Flow diagram of screening for relevant articles in meta-analysis of data on the CGG repeat length and idiopathic POI.
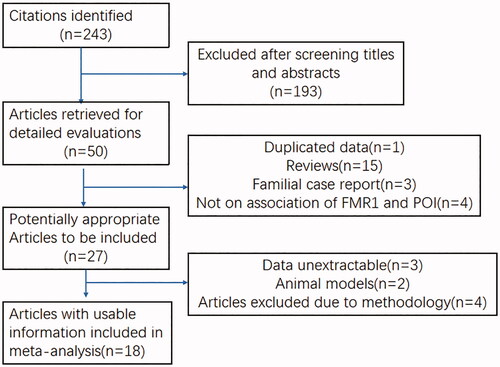
Table 1. Characteristics of studies included in the Meta-Analysis between the CGG repeat length in the FMR1 gene and idiopathic POI.
Table 2. The distributions of intermediate and premutation alleles of the included studies in women with POI and DOR.
Thirteen studies, including 2047 cases and 6912 controls, met our criteria for the assessment of the premutation and intermediate alleles in patients with POI [Citation13,Citation14,Citation17,Citation19,Citation21–23,Citation27–29,Citation31,Citation32]. In 13 POI studies, the frequency of premutation varied from 0.5 to 11.6% among cases and from 0.0 to 1.4% among controls, while the frequency of intermediate varied from 0.0 to 15.0% among 13 POI studies and from 0.0 to 20.0% among controls (). Fixed effects models were used according to the heterogeneities in the comparisons (I2 < 50%, fixed effect model). As shown in , the pooled fixed effect OR and corresponding 95% CI of premutation in POI compared with controls was 8.13 (4.35–15.19), suggesting that premutation of FMR1 gene is significantly associated with POI (p < .00001). But no significant association of intermediate alleles with POI was found (OR = 0.86; 95% CI: 0.62–1.18; p = .34).
Six studies, representing 975 patients and 1749 controls, were eligible for evaluation of the premutation and intermediate alleles in DOR [Citation15,Citation18–20,Citation30,Citation33]. The prevalence of premutation varied from 1.3 to 15.95% among cases and from 0.0 to 0.6% among controls, while the prevalence of intermediate varied from 1.6 to 13.3% among DOR, and from 0.0 to 20.0% among controls (). The association between premutation and DOR was significant (p < .00001), with a pooled fixed effect OR of 14.87 (5.20–42.52) between case and control groups. No significant correlation of intermediate alleles to DOR was found in the case control comparison (OR = 0.98; 95% CI: 0.65–1.47; p = .93) ().
Discussion
In this study, we evaluated the association between the FMR1 CGG repeat lengths and the severity of idiopathic POI using Meta-analysis. Eighteen studies were included in this study, we found that the FMR1 premutation was significantly associated with increased risks for both POI (OR = 8.13; 95% CI: 4.35–15.19; p < .00001) and DOR (OR = 14.87; 95% CI:5.20–42.52; p < .00001), whereas no correlation between the FMR1 CGG intermediate repeat length and the severity of idiopathic POI.
Several studies had demonstrated the association between some candidate genes and the risk of POI [Citation8]. For example, Aittomaki et al. [Citation35] identified that the variant in the follicle-stimulating hormone receptor gene was associated with hypergonadotropic ovarian dysgenesis, and Kovanci et al. [Citation36] found that GDF-9 might be a susceptibility gene of POF. The association between the CGG repeat length in the FMR1 gene and POI risk has been a topic of particular interest, but the results from individual studies were inconsistent and sometimes contradictory, especially for idiopathic POI. In 2005, a case-control study [Citation13] revealed that the prevalence of premutation in idiopathic POI patients increased significantly compared with control women, and there was a significant association between POI and intermediate expansions. In another prospective cohort study performed in Canada, Bretherick et al. [Citation14] found a significant increase in the frequency of intermediate expansions with the risk of POI, considering that the FMR1 gene plays a significant role in the occurrence of idiopathic POI. Currently, a similar study on Chinese population also supported the notion that the intermediate-sized FMR1 CGG repeat alleles in idiopathic POI patients were significantly more than that in the control group [Citation27]. However, in a population-based study on predominated European individuals, Murray et al. [Citation22] revealed that premutation-sized FMR1 repeats are substantial risk factors for the POI, whereas intermediate alleles were not significant risk factors for the POI. De Geyter et al. [Citation21] in their prospective cohort study found that neither of the categories of FMR1 CGG repeat length expansions (premutation, intermediate range) was more prevalent in infertile women with POI than control women, and nor CGG repeat length was correlated with the severity of primary ovarian insufficiency. Moreover, interesting results were found that there was a significant increase in the number of intermediate and premutation FMR1 alleles in DOR patients compared with controls [Citation15,Citation20,Citation33]. A study performed by Eslami A et al. [Citation19] found that the frequency of FMR1 premutation in both DOR and POI patients was significantly higher than that in control group, while no significant differences in the frequency of intermediate alleles were observed between POI patients and control subjects. Another larger case-control study carried out by Bennett et al. [Citation16] did not reveal a positive association between different intermediate-range and idiopathic POI. Recently, a review on FMR1 and the continuum of primary ovarian insufficiency conducted by Sullivan et al. supported the viewpoint that increased CGG repeat numbers may play a role in POI etiopathogenesis and could be helpful to identify, counsel, and treat the women with the FMR1 premutation [Citation4].
It is difficult to interpret the inconsistencies between the role of the CGG repeat length and the severity of POI. Additional research is needed to clarify the relationship between the expansion of repeat CGG and the risk of idiopathic POI. In this study, the most comprehensive meta analysis based on all currently available data was performed to investigate the associations of the CGG repeat length with idiopathic POI susceptibility, and the results showed that FMR1 premutation was significantly associated with increased risks for both idiopathic POI and DOR (OR = 8.13 and OR = 14.87, respectively; p < .05). In addition, it was demonstrated that intermediate size was not significantly associated with idiopathic POI and DOR (OR = 0.86 and OR = 0.98, respectively; p > .05). To our knowledge, this is the first meta analysis on the association of the CGG repeat length with idiopathic POI, and the results might be helpful to genetic counselors to give patients more reasonable interpretation between the CGG repeat length in the FMR1 gene and the severity of idiopathic POI. Currently, the mechanisms of FMR1 premutation and intermediate alleles increasing POI risk are not fully known. Some molecular mechanisms (such as increased FMR1 mRNA, possibly X inactivation and FMRP levels), might play a role in ovarian dysfunction [Citation4].
One of the important limitations of this study concerns the lack of analyses stratified by other related susceptible factors, such as race and ethnicity have not been conducted in the present study because insufficient data were available from the primary studies. Race/ethnic differences in the FMR1 CGG repeat distribution have been reported [Citation37–39]. However, the results from individual studies were inconsistent. Genereux and Laird used eight general population studies and found that the Asian and non-Asian populations had similar distribution curves, but the Asian curve was left-shifted and “almost completely non-overlapping” relative to the non-Asian distribution [Citation38]. A study performed by Pastore et al. [Citation18] found that DOR cases had fewer CGG repeats in the shorter FMR1 allele than controls among Whites, but this was not significant among Asians. White cases had fewer CGG repeats in the shorter allele than Asian cases. No significant differences were found in the high normal/intermediate range between cases and controls or by race/ethnic group within cases in the longer allele. Despite the limitation, the data of the present meta-analysis did show an association of FMR1 premutation with increased susceptibility to idiopathic POI and its severity and a non-correlation of intermediate size with increased risk to idiopathic POI.
In conclusion, our analysis supports the association of premutation with increased susceptibility to idiopathic POI (POI and DOR) and further pointed out that intermediate alleles might not be correlated with idiopathic POI and its severity. Further studies are warranted to confirm the meta analysis conclusion.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614.
- Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499–509.
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921.
- Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29:299–307.
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606.
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410.
- van Kasteren YM, Hundscheid RD, Smits AP, et al. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod. 1999;14:2455–2459.
- Qin Y, Jiao X, Simpson JL, et al. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21:787–808.
- Wittenberger MD, Hagerman RJ, Sherman SL. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465.
- Tejada MI, García-Alegría E, Bilbao A, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15:945–949.
- 1.Allen EG, Sullivan AK, Marcus M, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152.
- Spath MA, Feuth TB, Smits AP, et al. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med. 2011;13:643–650.
- Bodega B, Bione S, Dalprà L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21:952–957.
- Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382.
- Streuli I, Fraisse T, Ibecheole V, et al. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil and Steril. 2009;92:464–470.
- Bennett CE, Conway GS, Macpherson JN, et al. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25:1335–1338.
- Ishizuka B, Okamoto N, Hamada N, et al. Number of CGG repeats in the FMR1 gene of Japanese patients with primary ovarian insufficiency. Fertil Steril. 2011;96:1170–1174.
- Pastore L, Young SL, Manichaikul A, et al. Distribution of the FMR1 gene in females by race-ethnicity: women with diminished ovarian reserve versus women with normal fertility (SWAN study). Fertil Steril. 2017;107:205–211.
- Eslami A, Farahmand K, Totonchi M, et al. FMR1 premutation: not only important in premature ovarian failure but also in diminished ovarian reserve. Human Fertil. 2016;20:1–6.
- Barasoain M, Barrenetxea G, Huerta I, et al. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene 2013;521:145–149.
- De Geyter C, M’Rabet N, De Geyter J, et al. Similar prevalence of expanded CGG repeat lengths in the fragile X mental retardation I gene among infertile women and among women with proven fertility: a prospective study. Genet Med. 2014;16:374–378.
- Murray A, Schoemaker MJ, Bennett CE, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med. 2014;16:19–24.
- Chatterjee S, Maitra A, Kadam S, et al. CGG repeat sizing in the FMR1 gene in Indian women with premature ovarian failure. Reprod Biomed Online. 2009;19:281–286.
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study-preliminary data. Am J Med Genet. 1999;83:322–325.
- Uzielli ML, Guarducci S, Lapi E, et al. Premature ovarian failure (POF) and fragile X premutation females: from POF to to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am J Med Genet. 1999;84:300–303.
- Gleicher N, Weghofer A, Oktay K, et al. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19:385–390.
- Lin L, Yu D, Du X, et al. Research of FMR1 gene CGG repeat polymorphism with premature ovarian failure. J Hebei Med Univ. 2013;34:1031–1034.
- Ye Y, Lan X, Cong J, et al. Analysis of CGG repeats in FMR1 in Chinese women with idiopathic premature ovarian failure. Reprod Biomed Online. 2014;29:382–387.
- Guo T, Qin Y, Jiao X, et al. FMR1 premutation is an uncommon explanation for premature ovarian failure in Han Chinese. PLOS One. 2014;9:e103316.
- Pastore LM, Young SL, Baker VL, et al. Elevated prevalence of 35-44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci. 2012;19:1226–1231.
- Rezvaneh A, Davood OM, Hamid G, et al. Premutations of FMR1 CGG repeats are not related to idiopathic premature ovarian failure in Iranian patients: a case control study. Gene. 2018;676:189–194.
- Espeche L, Violeta C, Ianina F, et al. Distribution of FMR1 and FMR2 repeats in Argentinean patients with primary ovarian insufficiency. Genes. 2017;8:194–203.
- Karimov CB, Moragianni VA, Cronister A, et al. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum Reprod. 2011;26:2077–2083.
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–587.
- Aittomaki K, Lucena JL, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968.
- Kovanci E, Rohozinski J, Simpson JL, et al. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril. 2007;87:143–146.
- Weiss K, Orr-Urtreger A, Kaplan Ber I, et al. Ethnic effect on FMR1 carrier rate and AGG repeat interruptions among Ashkenazi women. Gen Med. 2014;16:940–944.
- Genereux DP, Laird CD. Why do fragile X carrier frequencies differ between Asian and non-Asian populations? Genes Genet Syst. 2013;88:211–224.
- Crawford DC, Meadows KL, Newman JL, et al. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002;110:226–233.