Abstract
Hemoglobin-vesicles (HbVs) are artificial oxygen carriers encapsulating purified and concentrated hemoglobin solution in phospholipid vesicles (liposomes) and their safety and efficacy as a transfusion alternative have been evaluated. Because of the absence of enzymatic methemoglobin reduction system in HbV, the level of ferric methemoglobin (metHb) increases gradually after intravenous administration. Our previous studies clarified that the glycolytic electron energies, charged as NAD(P)H in red blood cells (RBC), are donated to reduce metHb compartmentalized in HbV via a water-soluble electron mediator such as methylene blue [MB; 3,7-bis(dimethylamino)phenothiazinium chloride], which freely shuttle across both RBC biomembrane and HbV lipid membrane. Herein, we tried to test repeated injections of MB after the massive HbV administration (28 mL/kg) to hemorrhagic shocked Wistar rats (n = 3). MB was injected (3.1 mg/kg) at 7, 24 and 48 h after HbV administration. Every MB injection showed rapid reduction of metHb and gradual reversal increase. As a result, the functional life span of HbV was significantly extended over 60 h. It is expected that further optimization of injection scheduling will decrease the total amount of MB and prolong the functional life span of HbV.
Introduction
Hemoglobin-based oxygen carriers (HBOCs) have been aggressively developed for clinical use aiming at resolving the problems of blood donation and transfusion systems [Citation1–3], such as (i) short shelf life of donated blood for only 3 weeks in a refrigerator; (ii) possibility of infection of known (HIV, hepatitis, etc.) or unknown pathogens; (iii) possibility of blood type mismatching by human error, and (iv) blood supply-demand unbalance in an aging society because the majority of blood donors are young people while recipients include many elder people. Hemoglobin-vesicle (HbV) is one HBOC encapsulating purified and concentrated Hb solution in phospholipid vesicles (liposomes) [Citation4,Citation5] We believe the importance of the cellular structure of red blood cells (RBCs) for retardation of any gas reactions, absence of colloid osmotic pressure, prevention of dissociation of Hb tetramer to dimers [Citation6], etc. Actually, Hb-V showed sufficient oxygen-carrying capacity in the studies of animal experimental models such as hemorrhagic shock resuscitation, hemodilution, oxygenation of ischemic tissues, etc. [Citation5].
During the Hb purification procedure from outdated and donated RBCs, carbonylhemoglobin (HbCO) is pasteurized at 60 degrees for 12 h and processed through nanofiltration to eliminate any possibility of infection [Citation7]. This Hb purification procedure also eliminates the sophisticated but thermally unstable enzymatic methemoglobin (metHb) reducing system that is originally present in RBCs. As a result, the level of metHb increases after intravenous injection. The high barrier function of liposomal lipid membrane is important to shield the escape of Hb and its degraded materials when exposed to excess amount of hydrogen peroxide [Citation8]. However, because of such high barrier function without any ionophore, metHb in HbV cannot be reduced by plasma ascorbic acid [Citation9]. Accordingly, we have struggled to establish a metHb reduction system in HbV for a long time [Citation10–13].
Recently, we have reported that intravenous injection of methylene blue [MB; 3,7-bis(dimethylamino)phenothiazinium chloride] following HbV injection demonstrated the effective reduction of metHb in HbV [Citation14–16]. We clarified the following mechanism; (i) NAD(P)H in RBCs reacts with MB to convert it to leukomethylene blue (MBH); (ii) MB and MBH shuttle freely between RBC and HbV across the hydrophobic lipid membranes; (iii) MBH is transferred into HbV and reduces metHb in HbV; (iv) the oxidized NAD(P)+ are reduced repeatedly by the glycolytic energies in RBCs with the corresponding glycolytic enzymes; and (v) other potential electron mediators are found in a series of phenothiazine compounds.
Even though one injection of MB rapidly reduced metHb in HbV in blood circulation, the level of metHb again started to increase gradually because of the rapid removal of small MB from blood circulation. In this paper, we tested multiple repeated injections of MB after massive single HbV injection as a resuscitative fluid for hemorrhagic shocked animals.
Materials and methods
Preparation of Hb-vesicles (HbVs)
HbVs were prepared using a method reported previously with only slight modifications [Citation14]. Human Hb solution was obtained through purification of outdated RBCs provided by the Japanese Red Cross Society (Tokyo, Japan). First, Hb was stabilized using carbonylation (HbCO) and pasteurized (60 °C for 12 h) for virus inactivation [Citation17]. All unstable enzymes were eliminated during this procedure. The obtained Hb solution was processed through nanofiltration (Viresolve NFP filter, Merck KGaA, Darmstadt, Germany) and concentrated by ultrafiltration to 42 g/dL. Subsequently, pyridoxal 5’-phosphate (PLP; Sigma Chemical Co., St. Louis, MO) was added to the HbCO solution as an allosteric effector at a molar ratio of PLP/Hb tetramer = 1. The HbCO solution was then mixed rigorously with lipids and encapsulated in vesicles. The lipid bilayer comprised 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, cholesterol, 1,5-O-dihexadecyl-N-succinyl-L-glutamate (Nippon Fine Chemical Co. Ltd., Osaka, Japan), and 1,2-distearoyl-sn-glycerol-3-phosphatidylethanolamine-N-PEG5000 (NOF Corp., Tokyo, Japan) at the molar composition of 5/4/0.9/0.03. The encapsulated HbCO was converted to HbO2 by exposing the liquid membranes of HbVs to visible light under an O2 atmosphere. Finally, the Hb concentration of the suspension was adjusted to 10 g/dL. The particle size distribution, 280 ± 50 nm, was measured using a light-scattering method (Nanoparticle analyzer, Model SZ-100; Horiba, Ltd., Kyoto, Japan). The original HbV had metHb level of less than 5%. The HbV suspension was mixed with 250 g/L human serum albumin (HSA) solution (Baxter AG, Vienna, Austria) to provide colloid osmotic pressure. As a result, the Hb concentration is 8.3 g/dL, and the albumin concentration in the outer phase of HbV is adjusted to about 5 g/dL.
A MB aqueous solution (Sigma-Aldrich) was prepared by dissolving it in PBS and filtration through a 0.20 µm sterilized filter. The concentration was adjusted to 0.35 mM.
Injection of HbV and MB into Wistar rats
All experimental protocols were approved by the IACUC of Biological Resource Center, A*STAR, Singapore. The protocols comply with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council – National Academy of Sciences (Washington, DC: National Academy Press, 1996). Experiments were conducted using male Wistar rats (15–17 weeks, 422–471 g b.w., n = 3). The rats were anesthetized by inhalation of 1.5%-isoflurane (Halocarbon Products Corp., North Augusta, SC, USA) mixed with air using a vaporizer (TK-7; Biomachinery, Chiba, Japan) throughout the experiment (fraction of inspired O2, FiO2 = 21%; flow rate, 1.5 L/min) while spontaneous breathing was maintained. Polyethylene catheters (SP-31 tubing, OD 0.8 mm, ID 0.5 mm; Natsume Seisakusho Co., Ltd., Tokyo) filled with saline solution containing 10 IU/ml heparin (B. Braun Medical Industries S/B, Penang, Malaysia) were introduced through the left femoral artery and vein. The arterial catheter was connected to a transducer connected to a BP Amp (MLT0670; AD Instruments) and monitored with software (PowerLab; AD Instruments) [Citation14].
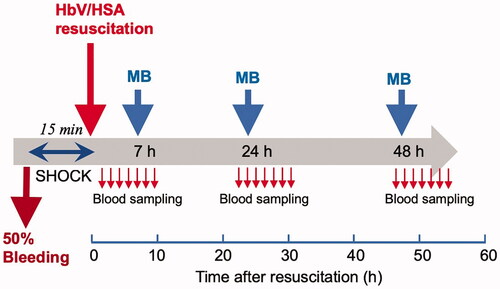
The systemic blood volume was estimated as 56 ml/kg body weight. Blood was withdrawn from the arterial line at the rate of 1 ml/min to collect 50% of whole blood (28 ml/kg). The rat was left to stabilize for 15 min. Then, HbV suspended in HSA was injected through venous line isovolemically at the rate of 1 ml/min (). Our previous study showed that this model of hemorrhagic shock and resuscitation with HbV suspended in albumin was lethal but the survival rate at 2 weeks was above 80% [Citation18]. Seven hours after injection of Hb-V when the level of metHb increased to about 40%, MB solution (0.35 mM) was injected at 28 ml/kg at the rate of 1 ml/min. Blood samples were collected through arterial line or lateral tail vein when cannulation was unavailable. After the procedure, the cannula was removed, and wound was sprayed with iodine and the rat was unanesthetized before allowing the rat to recover in cages. The rats were housed in cages in a room with a barrier against infection at the animal experimental facility. Rats were provided ad libitum access to food and water in a temperature-controlled environment with a 12-h dark/light cycle.
On the 2nd day, the rat was anesthetized again by the same procedure, and the arterial catheter was introduced to the femoral artery. Twenty-four hours after resuscitation with HbV, MB solution was injected. Just before and for a few hours after injection of MB, blood samples were collected periodically for analysis. Then the cannula was removed, the skin was sutured, disinfected, the rat was unanesthetized, and then returned to the cage. On the 3rd day, the same procedure was repeated, and after the final collection of blood sample the rat was sacrificed by anesthesia overdose.
Analysis of blood samples
Blood samples were taken hourly after injection of HbV/HSA cocktail and more frequently just before and after injection of MB. Blood samples were collected using heparinized hematocrit (Hct) capillaries (Hirschmann Laborgerate GmbH & Co. KG, Eberstadt, Germany). The samples were analyzed for Hb concentration derived from HbV, Hct, and metHb% of HbV. To measure metHb%, the Hct capillaries were centrifuged at 12,000 rpm, using Kubota 3320 Hct capillary centrifuge, for 1 min to obtain the fraction of blood plasma containing HbV. Ten µL of the fraction was diluted in 3 ml of PBS in a Thunberg cuvette fitted with a rubber cap septa. The solution was then deoxygenated using gentle N2 gas bubbling for 5 min. Consequently, HbV contained only metHb and deoxyHb. Visible light absorption spectra were measured using a spectrophotometer (V-650; Jasco Corp., Tokyo, Japan) that was equipped with an integral sphere (ISN-470) to minimize the effect of light scattering. The spectra were measured between 300–500 nm, and the level of metHb was calculated from the absorption ratios at 405 nm (metHb) and 430 nm (deoxyHb). The total Hb concentration in the plasma fraction was measured using a modified cyanomethemoglobin (metHb-CN) method (Alfresa Pharma Corp., Tokyo). The amount of ferrous Hb (HbO2 + deoxyHb) derived from HbV was calculated by subtracting the amount of ferric metHb from the amount of total Hb. The data of each plots are expressed as average ± standard deviation (n = 3).
Results
All the rats survived the lethal hemorrhagic shock of 50% circulating blood withdrawal followed by isovolemic resuscitation with HbV suspended in HSA and the succeeding MB repeated injections for over two days.
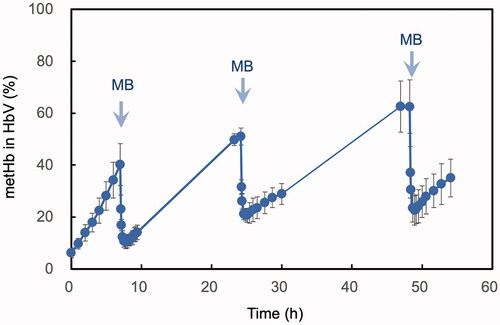
The average metHb% upon injection of HbV was 6.2 ± 1.9%. Within the next 7 h, metHb% increased to 40.2 ± 8.1% (). MB was injected at 7 h and within only 45 min metHb% decreased to 10.2 ± 2.4%. MetHb% then increased gradually to 51.0 ± 3.3% at 24 h of the experiment. Then, MB was injected again and within 55 min metHb% decreased considerably to 20.6 ± 2.7%. Again, metHb% then increased over the day to 62.5 ± 10.3%. The third MB injection led to the reduction of metHb% again to 22.6 ± 5.6%.
Figure 2. Time course of the level of ferric metHb% of HbV after intravenous administration into rats. MB was injected three times at the time points indicated with arrows, and the level of metHb% decreases spontaneously at each time. Mean ± SD (n = 3).
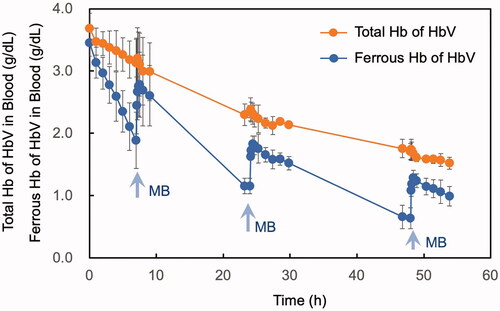
The average total Hb of HbV was 3.7 ± 0.2 g/dL just after resuscitation, and it gradually and monotonously decreased over time (, orange plots) because HbV is eliminated by reticuloendothelial system (mononuclear phagocytic system) such as spleen and liver [Citation18,Citation19]. The half-life of HbV to decrease to 1.8 g/dL concentration is estimated to be 45 h. On the other hand, the average ferrous Hb (HbO2 + deoxyHb) concentration of HbV showed different zigzag line (, blue plots). Just after injection, it was calculated to be 3.5 ± 0.2 g/dL. Within the next 7 h, it decreased to 1.9 ± 0.5 g/dL. MB was injected at 7 h and within only 45 min ferrous Hb increased to 2.8 ± 0.5g/dL. It then decreased gradually to 1.2 ± 0.1 g/dL. Within 55 min after the second MB injection ferrous Hb increased considerably to 1.8 ± 0.2 g/dL. Again, ferrous Hb decreased to 0.6 ± 0.2 g/dL at 48 h. The third MB injection again increased the ferrous Hb concentration to 1.3 ± 0.1 g/dL.
Figure 3. Time courses of the level of total Hb derived from HbV in blood (orange plots) and the level of ferrous Hb (HbO2 + deoxyHb) derived from HbV in blood (blue plots). MB was injected three times at the time points indicated with arrows, and the level of ferrous Hb derived from HbV increased spontaneously at each time. Mean ± SD (n = 3).
Discussion
Autoxidation of ferrous HbO2 to form ferric metHb and superoxide is inevitable. For all the HBOCs, it is important to consider the effect of autoxidation and how to prevent or retard it during the storage and after intravenous administration [Citation9,Citation10,Citation20–22]. In this report, we aimed to investigate the ability of MB to regulate the metHb level of HbV in rats when the rats were resuscitated with HbV suspended in HSA. Without injection of MB, it is estimated that the level of metHb in HbV would reach to 100% one day after the resuscitation by extrapolation of the initial curve of metHb formation in (0–7 h), especially because of the systemic reperfusion aftershock where reactive oxygen species and reactive nitrogen species are more excessively generated than usual in blood circulation that facilitate metHb formation. However, in the present study, we clarified that the repeated injections of MB can maintain the metHb% to a lower level thus stabilize the oxygen carrying capacity of HbV for a longer time.
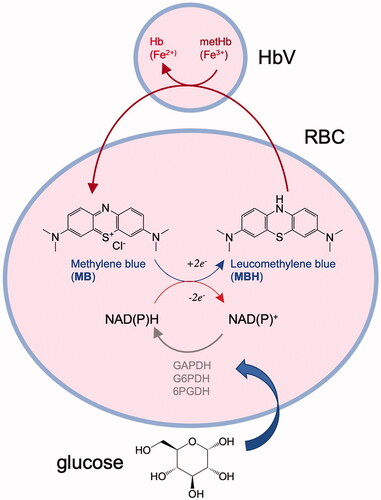
MB is a phenothiazine dye that is widely used as one of the components of the Giemsa stain solution for histopathological examination. It is also utilized widely for therapeutic purposes for neurological diseases and methemoglobinemia [Citation23–25]. In the case of methemoglobinemia, the injected MB diffuses into RBC and it is reduced by intraerythrocytic NADH and NADPH to form leukomethylene blue (MBH), which can readily reduce metHb in RBC [Citation25]. On the other hand, our previous studies clarified that MB can be utilized for the reduction of metHb of HBOCs dispersed in the extraerythrocytic plasma phase [Citation14–16]. The reduced MBH can readily permeate across the biomembrane to diffuse out to the extracellular plasma phase, where MBH permeates across the lipid membrane of HbV and reacts spontaneously with metHb compartmentalized in HbV [Citation14] (). Under the condition of sufficient plasma glucose concentration, the glycolytic energy is abundant, and the oxidized NAD+ and NADP+ are reduced repeatedly to form NADH and NADPH respectively, by the corresponding glycolytic enzymes. Actually, in a hemorrhagic shock state, the level of glucose increases considerably which is known as hyperglycemia [Citation18], which would be advantageous for glycolysis. Single injection of MB showed rapid reduction of metHb%; however, it cannot sustain the low metHb% level for a long time. The level of metHb% again started to increase because of the shorter half-life of small MB molecule in blood circulation. In the present paper, we examined the conditions of repeated MB injections in a practical model of hemorrhagic shock and resuscitation with HbV.
Figure 4. The mechanism of the indirect enzymatic reduction of ferric metHb (Fe3+), compartmentalized in HbV to form ferrous Hb (Fe2+), via an electron mediator, MB, transferring the glycolytic energies charged as NAD(P)H in RBCs. The oxidized NAD(P)+ can be reduced repeatedly by the glycolytic enzymes in RBCs. The oxidized NADP+ is energized repeatedly to NADPH using 6-phosphogluconate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) in the pentose phosphate pathway. The oxidized NAD+ is energized repeatedly to NADH by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the Embden–Meyerhof pathway.
It is reported that the time course of MB in whole blood after intravenous injection of 100 mg MB into healthy volunteers showed terminal half-life of 5.25 h [Citation26]. The body weight of the volunteers is not described in that paper; however, the dosage was approximately estimated as 1.0–2.5 mg/kg, which is quite comparable to our experimental condition of 3.1 mg/kg b.w. into rats. It is plausible that the injected MB is promptly eliminated from the blood circulation, distributed to the peripheral tissues, degraded and finally excreted through urine [Citation26]. Therefore, repeated injection of MB is required to maintain the low level of metHb in HbV.
Because the time schedule of the multiple blood collection was designed suitable for labor condition of a researcher, the interval of the injection of MB was approximately 24 h, and the graphs seem jagged in and . If the interval of the MB injections is much shorter, then the level of metHb can be maintained to less than 20% all the time for 3 days.
We utilized MB in this repeated injection study that simulates a clinical resuscitation condition because MB has been used clinically for various diseases. On the other hand, our previous in vivo study clarified that another phenothiazine dye, Azure B (AB), showed better reduction of metHb in HbV, as AB has a lower re-oxidation rate than that of MB and higher permeation through lipid membrane [Citation16]. It is expected that repeated injections of AB would show a better result for maintaining the low level of metHb after resuscitation with HbV.
Conclusion
We established a system to extract electron energies of RBCs for extracellular chemical reaction using MB that shuttles across RBC and HbV. Repeated injections of MB can maintain the high level of oxygen carrying capacity of HbV for a long time. This method is important to maximize the function of oxygen transporting capacity of HBOCs during circulation in blood.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclosure statement
Of the authors, H.S. is an inventor holding some patents related to the production and utilization of Hb-vesicles.
Additional information
Funding
References
- Chang TM. Nanobiotechnology for hemoglobin-based blood substitutes. Crit Care Clin. 2009;25:373–382.
- Alayash AI. Blood substitutes: why haven't we been more successful? Trends Biotechnol. 2014;32:177–185.
- Winslow RM. Cell-free oxygen carriers: scientific foundations, clinical development, and new directions. Biochim Biophys Acta. 2008;1784:1382–1386.
- Sakai H, Sou K, Tsuchida E. Hemoglobin-vesicles as an artificial oxygen carrier. Meth Enzymol. 2009;465:363–384.
- Sakai H. Overview of potential clinical applications of hemoglobin vesicles (HbV) as artificial red cells, evidenced by preclinical studies of the academic research consortium. JFB. 2017;8:10.
- Matsuhira T, Kure T, Yamamoto K, et al. Analysis of dimeric αβ subunit exchange between PEGylated and native hemoglobins (α2β2 tetramer) in an equilibrated state by intramolecular ββ-cross-linking. Biomacromolecules. 2018;19:3412–3420.
- Sakai H, Masada Y, Takeoka S, et al. Characteristics of bovine hemoglobin as a potential source of hemoglobin-vesicles for an artificial oxygen carrier. J Biochem. 2002;131:611–617.
- Takeoka S, Teramura Y, Atoji T, et al. Effect of Hb-encapsulation with vesicles on H2O2 reaction and lipid peroxidation. Bioconjug Chem. 2002;13:1302–1308.
- Chen G, Chang T. Dual effects include antioxidant and pro-oxidation of ascorbic acid on the redox properties of bovine hemoglobin. Artif Cells Nanomed Biotechnol. 2018;46:983–992.
- Takeoka S, Ohgushi T, Sakai H, et al. Construction of artificial methemoglobin reduction systems in Hb vesicles. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:31–41.
- Takeoka S, Sakai H, Kose T, et al. Methemoglobin formation in hemoglobin vesicles and reduction by encapsulated thiols. Bioconjugate Chem. 1997;8:539–544.
- Teramura Y, Kanazawa H, Sakai H, et al. Prolonged oxygen-carrying ability of hemoglobin vesicles by coencapsulation of catalase in vivo. Bioconjugate Chem. 2003;14:1171–1176.
- Sakai H, Masada Y, Onuma H, et al. Reduction of methemoglobin via electron transfer from photoreduced flavin: restoration of O2-binding of concentrated hemoglobin solution coencapsulated in phospholipid vesicles. Bioconjugate Chem. 2004;15:1037–1045.
- Sakai H, Li B, Lim WL, et al. Red blood cells donate electrons to methylene blue mediated chemical reduction of methemoglobin compartmentalized in liposomes in blood. Bioconjugate Chem. 2014;25:1301–1310.
- Kettisen K, Bülow L, Sakai H. Potential electron mediators to extract electron energies of RBC glycolysis for prolonged in vivo functional lifetime of hemoglobin vesicles. Bioconjugate Chem. 2015;26:746–754.
- Ghirmai S, Bülow L, Sakai H. In vivo evaluation of electron mediators for the reduction of methemoglobin encapsulated in liposomes using electron energies produced by red blood cell glycolysis. Artif Cells Nanomed Biotechnol. 2018;46:1364–1372.
- Sakai H, Takeoka S, Nishide H, et al. Convenient method to purify hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:651–656.
- Sakai H, Seishi Y, Obata Y, et al. Fluid resuscitation with artificial oxygen carriers in hemorrhaged rats: profiles of hemoglobin-vesicle degradation and hematopoiesis for 14 days. Shock. 2009;31:192–200.
- Sou K, Klipper R, Goins B, et al. Circulation kinetics and organ distribution of Hb-vesicles developed as a red blood cell substitute. J Pharmacol Exp Ther. 2005;312:702–709.
- Zhang J, Wang Y, You GX, et al. Conjugation with 20 kDa dextran decreases the autoxidation rate of bovine hemoglobin. Artif Cells Nanomed Biotechnol. 2018;46:1436–1443.
- Faivre B, Menu P, Labrude P, et al. Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream. Literature review and outline of autooxidation reaction. Artif Cells Blood Substit Immobil Biotechnol. 1998;26:17–26.
- Jeong ST, Ho NT, Hendrich MP, et al. Recombinant hemoglobin(alpha 29leucine → phenylalanine, alpha 96valine → tryptophan, beta 108asparagine → lysine) exhibits low oxygen affinity and high cooperativity combined with resistance to autoxidation. Biochemistry. 1999;38:13433–13442.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol. 2013;9:242–249.
- Ozal E, Kuralay E, Yildirim V, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79:1615–1619.
- Bradberry SM. Occupational methaemoglobinaemia. Mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev. 2003;22:13–27.
- Peter C, Hongwan D, Küpfer A, et al. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–250.