Abstract
The rhizome of A. officinarum possesses immense pharmaceutical properties like antioxidant, anti-inflammatory, antiapoptotic, anticancer activities. The foremost downside of herbal-based drugs is their poor bioavailability, to trounce this we synthesized a herbal based silver nanodrug with Alpinia officinarum rhizome extract and assessed its effect against the cisplatin-induced nephrotoxicity in in vivo model. The A. officinarum biosynthesized silver nanoparticles (AG-AO) were characterized using UV-Spec, FTIR, XRD, TEM and SAED analysis. The antioxidant and the nephroprotective property of biosynthesized AG-AO nanoparticles were assessed by estimating the levels of kidney biomarkers, cytokine, inflammatory markers, free radicals and antioxidants induced in control and experimental. Antiapoptotic effect of AG-AO nanoparticles were evaluated by measuring the levels of apoptotic proteins in control and experimental rats. Further, it is confirmed with histopathological analysis of kidney tissue with haematoxylin and eosin staining. Our physical analysis confirms the biosynthesized silver nanoparticles by A. officinarum and it satisfies the qualities of potent nanoparticles to be used for medication. Our biochemical, molecular and histopathological results confirm the antioxidant, antiapoptotic, anti-inflammatory properties of AG-AO. Overall our results authentically confirm AG-AO is a potent nephroprotective drug, which can be a supplementary drug to prevent cisplatin-induced nephrotoxicity.
Introduction
Cisplatin (cis-diamminedichloroplatinum) is a globally prescribed drug for a wide variety of cancers like ovarian, testicular, bladder, lung, cervical head and neck cancer [Citation1]. It has been approved by FDA long back in the year 1968 and until now it is used as an effective drug of choice to treat both adult and pediatric cancers. It is prescribed as an antineoplastic drug alone or in combination with other drugs [Citation2–4]. More than 35% of patients treated with a high dose of cisplatin end up with renal damage, which hinders the medical usage of cisplatin as an anticancer drug [Citation5]. Even though it causes various side effects like neurotoxicity, ototoxicity, nausea, vomiting, etc., nephrotoxicity was observed in most of the patients [Citation1,Citation6]. Cisplatin targets the cancerous cells by the generation of free radicals and creating oxidative stress which leads to the apoptosis of cancerous cells. During long term usage of cisplatin, excessive free radicals generated encounters the non-proliferating cells causing apoptosis [Citation7]. Numerous studies have reported that nephrons are targeted by the cisplatin [Citation5,Citation8,Citation9]. Nephrotoxicity rendered by cisplatin limits the long term usage of cisplatin in cancer patients. Hence, it is the need of the day to develop a natural supplementary drug which protects from the cisplatin-induced side effects.
The exact mechanism of cisplatin is not yet elucidated, but the key mechanism is the oxidative stress induced by cisplatin causes DNA damage, lipid peroxidation in non-proliferative kidney cells [Citation10]. Increased levels of urea creatinine, lipid peroxidation and decreased levels of antioxidants were observed in most of the cisplatin-treated cancer patients [Citation11,Citation12]. Human and animal trials have proved the administration of antioxidants like Vitamin C, carotenoids, selenium prevented the patients from cisplatin-induced nephrotoxicity [Citation13,Citation14].
Plants are the major natural reservoir of antioxidants and they are used to treat various diseases like cancer, inflammation, hepatic disorders, kidney disorders etc., Alpinia officinarum, lesser galangal is a traditional plant of India and China [Citation15]. It is a perennial herb belonging to the ginger family Zingiberaceae possessing ornamental leaves and white flowers [Citation16]. Rhizome of A. officinarum possesses immense pharmacological traits like antitumor, antiulcer, antibacterial, and antifungal properties [Citation17–19]. Poor bioavailability was the foremost drawback of herbal-based drugs. Nanoparticles are a promising agent to overcome the poor bioavailability of herbal-based drugs. Compared to physical and chemical synthesized nanoparticles green synthesized nanoparticles are more potent, eco-friendly, economic and without any side effects [Citation20,Citation21]. Phytochemicals present in plants reduces the metals and stabilizes the ions to form nanoparticles [Citation22]. Various studies are reported the medicinal potency of silver nanoparticles synthesized from plant extracts against cancer, bacterial infection, inflammation. etc [Citation23–25].
Synthesizing nanoparticles with traditional medicinal plant A. officinarum used in Chinese and Ayurvedic medicine may be an effective supplementary drug to cancer patients to overcome the nephrotoxicity induced by cisplatin medication. Therefore, in the present study, we biosynthesized silver nanoparticles with A. officinarum rhizome extract and assessed its efficacy against cisplatin-induced nephrotoxicity in rats through modulating the apoptotic pathway.
Materials and methods
Chemicals
Silver nitrate, cisplatin and all other chemicals used in the present study were of analytical grade purchased from Sigma Aldrich, USA. The ELISA kits to assess tumor necrosis factor, interleukin 1β, NF-kappaB p65, BCL2 Associated X Protein, Bcl2, P53 were purchased from MyBiosource, USA. Colorimetric kits to assess urea, creatinine, serum albumin and nitric oxide were purchased from BioVision, USA.
Alpinia officinarum rhizome extract
Fresh healthy plants of A. officinarum were procured from the local farmers and the rhizome were separated. The rhizome was cleaned with distilled water and dried in shade, the dried rhizome was ground in a blender to a fine powder. The powder is sieved to obtain uniform finely grounded particles, then to 100 mg of rhizome powder, 25 ml of double distilled water was added and placed in the stirrer for 5 h. The rhizome extract was then used for the synthesis of silver nanoparticles.
Green synthesis of Alpinia officinarum silver nanoparticles (AG-AO)
20 ml of rhizome extract was added to the 180 ml of 1 mM silver nitrate solution and incubated at dark for 48 h, room temperature. The colour change of the solution from light brown to dark brown indicates the reduction of silver ions to form silver nanoparticles [Citation26]. The synthesized silver nanoparticles were characterized using further analysis.
Characterization of AG-AO
AG-AO UV-Vis spectrophotometric analysis
The bioreduction property of synthesized AG-AO nanoparticles were assessed by spectrophotometer (UV-3000 PC spectrometer) with the wavelength range from 300–700 nm for a period of 30 days. The absorbance was measured on 24 h, 48 h, 5th day, 15th day and 30th days to detect the stability of synthesized nanoparticles. The surface Plasmon resonance peaks were measured by plotting the wavelength on the X-axis and the absorbance on the Y-axis.
AG-AO FTIR spectroscopic analysis
The functional groups of biosynthesized AG-AO nanoparticles were evaluated using Fourier transform infra red (FTIR) spectroscopic analysis. The AG-AO nanoparticles were mixed with potassium bromide pellets and placed on the scanning disc of Nicolet 6700 spectrophotometer. The mixture was scanned at room temperature between the wavelength of 600–4000 cm−1. The process is continued until 50 scans were recorded and the data obtained were assessed using the software WINFIRST, Mattson, USA.
AG-AO XRD analysis
The X-ray diffraction pattern of biosynthesized AG-AO nanoparticles was assessed using Bruker-AXS D5005 (Siemens). The samples were measured with diffractometer equipped with copper sealed scintillation counter operated at 45 mA current, 40 kV voltage. The scanning times were fixed as 0.02 and 0.5 s respectively.
AG-AO TEM analysis
The size and shape of the biosynthesized AG-AO nanoparticles were evaluated using the transmission electron microscope (JEAO JEM 1400). The AG-AO sample was placed over the carbon-coated 400 mesh copper grid and allowed to dry at room temperature. The dried sample was assessed at an acceleration voltage of 120 kV and the images were analyzed using Image J software
AG-AO SAED analysis
The structure and nature of biosynthesized AG-AO nanoparticles were analyzed using Malvern zetasizer selected area diffraction pattern analyzer. The distance between the planes was calculated based on the patterns obtained after the SAED analysis using the formula λL × Rd = (1) where λL is a constant of the microscope, R is the ring radius, and d is the interplanar distance.
In-Vivo experimental procedure
Animals
Healthy young male Wistar rats weighing about 200–250 g were used for the current study. The animals were procured from the institutional animal house after getting ethical clearance from the institutional ethical committee. The rats were housed in a hygienic environment with a constant room temperature of 22 ± 2 °C, 65 ± 5 °C humidity with 12 h light/dark cycle. Before initiation of the experiment, the rats were allowed to acclimatize for a week in a new environment and fed with standard laboratory rat feed, reverse osmosed water ad libitum. All the procedures followed were approved by the institutional ethical clearance committee and the animals were handled with utmost care and humanity.
Experimental protocol
After acclimatization, the rats were randomly separated into five groups each groups consisting of 6 rats. Group I rats are considered as the control group, Group II rats are nephrotoxicity induced rats, Group III and IV are high and low dose of AG-AO pretreated rats, respectively, and the Group IV rats are AG-AO drug control rats. Group I rats were treated orally with normal saline (3 ml/kg/day) for 10 days whereas Group II nephrotoxicity induced rats were treated with normal saline (3 ml/kg/day) for 10 days and on 7th day, a single injection of cisplatin (8 mg/kg; i.p.) was given. Group III & IV AG-AO rats were treated orally with 50 mg/kg/day and 100 mg/kg/day for 10 days and on the 7th day, a single injection of cisplatin (8 mg/kg; i.p.) was given. Group V drug control rats were orally treated with 50 mg/kg/day of AG-AO for a period of 10 days. After the treatment period, the rats were euthanized by cervical dislocation procedure and the blood samples were collected using heart puncture. In a sterile plain and anticoagulant coated tubes, the blood from the jugular veins was collected and serum and plasma were separated respectively. The kidney tissue was surgically excised from the rats and washed with ice-cold saline, dried using tissue paper and stored immediately in −80 °C for further analysis.
Nephrotoxicity markers estimation
The ideal biomarkers of nephrotoxicity creatinine, urea and serum albumin were estimated colorimetrically using commercially available kits. Urea, a key component helps in protein catabolism, water reabsorption in nephrons were assessed using the urea assay kit (K375-100). The procedure was followed as per the manufacturer’s protocol, and urea in the sample was measured at 570 nm. Creatinine used to measure the glomerular filtration rate of the kidney was estimated using the BioVision creatinine assay kit (K625-100). Creatinine present in the sample was converted to creatine by the creatininase and it is further converted to sarcosine measured at the wavelength of 570 nm. Serum Albumin which maintains the vascular permeability, nitric oxide, plasma osmotic pressure was estimated using Albumin BCG assay kit (K554-100) (BioVision, USA). Albumin combines with bromocresol green to form a chromophore which is measured at a wavelength of 620 nm.
Proteinuria, the total amount of protein present in the 24 h urine was calculated with the random urine protein creatinine ratio. Random urine protein was estimated with the protocol of Iwata and Nishikaze [Citation27]. The protein in urine samples reacts with benzethonium chloride to form turbidity which was measured at an absorbance of 660 nm. Urine Creatinine was estimated using the Jaffe’s reaction principle, creatinine produced orange colour when it reacts with picric acid which was measured at 492 nm. The levels of proteinuria were calculated by dividing the values of 24 h urine protein concentration by creatinine concentration.
Oxidative stress markers estimation
The oxidative stress induced by cisplatin was evaluated by estimating the levels of lipid peroxidation induced in the kidney tissue of control and treated rats. Thiobarbituric acid reactive substances (TBARS) are reactive substances which combine with the malondialdehyde secondary product of lipid peroxidation to form a pink chromogen measured at the wavelength of 532 nm [Citation28].
The status of antioxidant enzymes in the nephrotoxicity induced and AG-AO pretreated rats were assessed by measuring the levels of superoxide dismutase (SOD), Catalase (CAT), and reduced glutathione (GSH) were measured in kidney tissue homogenate prepared with phosphate-buffered saline, pH 7.4. Superoxide dismutase (EC.1.15.1.1) activity to inhibit the auto-oxidation of pyrogallol was measured using the method of Marklund and Marklund [Citation29] and the values were expressed as Enzyme concentration required to inhibit the chromogen produced by 50% in one min under standard condition. Catalase activity (EC. 1.11.16) was estimated using the procedure of Sinha [Citation30], and the values were expressed as µmole of hydrogen peroxide decomposed/min. The levels of reduced glutathione were estimated using the procedure of Ellman [Citation31], based on the colour intensity produced by sulfhydryl groups present in reduced glutathione combines with 5,5’-dithio 2-nitro benzoic acid. The values are expressed µg of reduced glutathione formed/min.
Cytokines estimation
The levels of cytokine tumor necrosis factor α (TNFα, MBS355371) and interleukin 1β (IL-1β, MBS774854) were estimated using the ELISA kits purchased from MyBiosource, USA. The procedure was followed as per the manufacturer’s protocol, the test principle was based on sandwich enzyme-linked immune-sorbent assay principle. The serum samples were added to the ELISA plates coated with anti-TNFα and IL-1β, respectively, followed by the addition of biotin-conjugated antibodies. The colour change produced by the TMB substrates was measured at the absorbance of 450 nm and the levels of TNFα and IL-1β were calculated.
Nitric oxide levels were estimated using the nitrate reductase colorimetiric assay method (K262-200), BioVision, USA. Nitrate was converted to nitrite using nitrate reductase which was measured using Griess reagent form deep purple colour. The colour intensity was measured at the wavelength of 540 nm and the levels of nitric oxide were calculated which directly proportional to the amount of azochromophore produced.
Inflammatory marker estimation
The inflammatory marker serum lactate dehydrogenase was measured using the colorimetric LDH assay kit (K311-400). Stable cytoplasmic enzyme LDH oxidized lactate to pyruvate which forms formazan crystals with tetrazolium salts measured at 500 nm.
Nuclear factor κB-p65 (MBS9501524) was measured using the ELISA kit purchased from MyBioSource, USA. The procedure was followed as per the manufacturer’s protocol and the levels of NF-kB p65 was measured at the wavelength 450 nm.
Apoptotic marker estimation
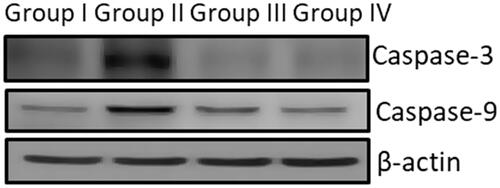
Apoptotic markers Bcl2 family proteins and p53 were estimated using commercially available ELISA kits procured from MyBiosource, USA. BCL2 Associated X Protein (BAX, MBS730995), BCL2 (MBS2881713), p53 (MBS057617). The HRP conjugated polyclonal antibodies specific for Bax, Bcl2 and p53 bind to the respective antigens present in the sample which are immobilized on the ELISA plate precoated with antibodies. The substrate was added to each well and the colour change was measured at the wavelength of 450 nm and the levels of each antibody were calculated using plotted standard curve. The caspase 9 and caspase-3 protein expression was analyzed by immunoblot method using the primary antibodies (SC-7272 AND SC-56076)(Invitrogen, USA) dilution of 1:1000 at 4 °C overnight and quantified using an ECL kit (Millipore, Billerica, MA, USA).
Histopathological analysis
The kidney tissue excised from the control and experimental rats were subjected to histopathological analysis. The excised kidney tissue was fixed with 10% formalin solution and then subjected to a series of hydration and dehydration reactions with xylene and ethanol. Then the tissue is embedded with paraffin and sectioned into thin slices of about 5-micron thickness. The tissue sections were stained with hematoxylin and eosin stain. The sections were viewed under a microscope and photographed to analyze the histopathological changes in control and experimental rats.
Statistical analysis
The results expressed were the mean ± SD of 6 rats present in each group. All the experiments were done in triplicates and the results were statistically analyzed with one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using GraphPad Prism software. p < .05 were considered to statistically significant for all the experiments.
Results
Characterization of AG-AO nanoparticles
UV-Visible spectroscopic analysis of AG-AO nanoparticles
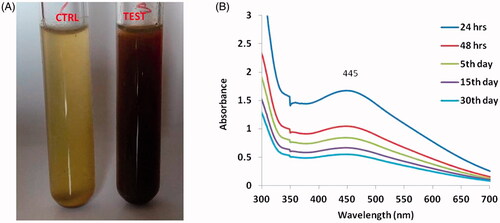
The synthesis of silver nanoparticles was observed through the colour change in the solution form light brown to dark brown (). depicts the surface Plasmon resonance peak of AG-AO solution exhibited at different duration of the time period. A sharp peak was observed at 445 nm and the intensity of the band decreased as the duration of incubation time increased.
Figure 1. The colour change before and after addition of Alpinia officinarum rhizome extract to silver nitrate solution (A). The UV–visible spectrum absorption pattern at different duration of time period (B) of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO).
Fourier-transform infrared spectroscopy analysis of AG-AO nanoparticles
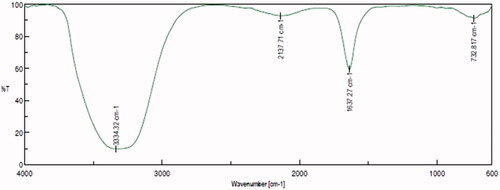
FTIR spectrum analysis depicting the functional groups present in the biosynthesized AG-AO nanoparticles are shown in . Peaks were observed between 600–3500 cm−1. A small blunt peak was observed at 732 cm−1, 2137 cm−1 which may be due to the aromatic C–H bending and asymmetric C–H stretching of alkanes present in AG-AO. The sharp beak observed at 1637 cm−1 and a blunt huge peak at 3334 cm−1 were due to the C=O and N–H stretching of amine present in AG-AO.
Morphological characterization of AG-AO nanoparticles
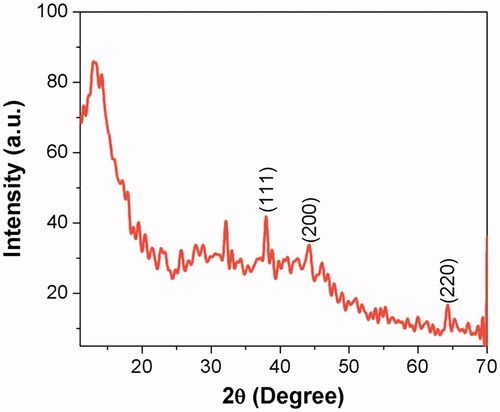
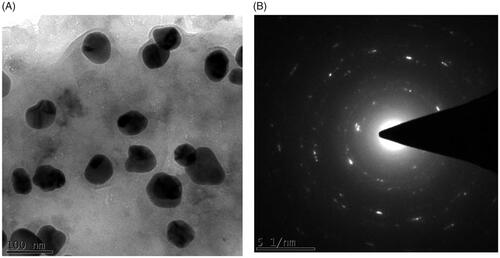
The morphology of the biosynthesized silver nanoparticles was assessed with XRD, TEM and SAED analysis. The X-ray diffraction pattern of AG-AO obtained at 2ϴ degree is depicted in . Peaks were obtained at 45 to 65° which originated from the planes 111, 200 and 220 confirming the face centred cubic crystalline structure of AG-AO. The crystalline structure was further confirmed with the SAED image () which shows a circular arrangement from the lattice reflection. Transmission Electron Microscopic images of AG-AO nanoparticles also correlates with the SAED results which are polydispersed crystalline in nature and an average size of 100 nm ().
AG-AO nanoparticles against nephrotoxicity
Effect of AG-AO on nephrotoxicity markers
The cisplatin-induced nephron damage was detected using the biomarkers urea, creatinine and albumin. The urea and creatinine levels were significantly decreased in AG-AO nanoparticle pretreated Group III & IV rats compared to the nephrotoxicity induced Group II rats. The urea and creatinine levels of 100 mg/kg bwt AG-AO pretreated rats were significantly equal to the levels of control group rats. No significant differences were observed between the Group I control and Group V drug control rats.
Albumin the major serum protein which involves the transportation of hormones, fatty acids, regulates the pH and oncotic pressure were drastically decreased to 1.36 ± 0.98 in nephrotoxicity induced Group II rats whereas in the AG-AO pretreated rats were increased in dose-dependent manner. No much difference was observed in the levels of serum albumin in between control and drug control rats. The levels of protein in urine also significantly decreased in both Group III & Group IV compared to the nephrotoxicity induced Group II rats ().
Table 1. Nephroprotective effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) against nephrotoxicity markers urea, creatinine, serum albumin and proteinuria.
Effect of AG-AO on oxidative stress markers
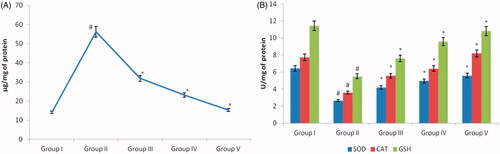
Oxidative stress-induced due to the imbalance between the oxidants and antioxidants is the key culprit for the damage of biomolecules. Free radicals act on lipids present on the tissue leading to lipid peroxidation eventually tissue damage. The end product of lipid peroxidation induced by cisplatin was estimated and depicted in . Compared to nephrotoxicity induced Group II rats the levels of lipid peroxidation were significantly decreased in both low and a high dose of AG-AO nanoparticle treated rats. Both the enzymatic antioxidants SOD, catalase and the non-enzymatic antioxidants reduced glutathione were significantly decreased in nephrotoxicity induced Group II rats compared to the control rats (). The Group III and Group IV rats show significantly increased levels of antioxidants compared to the nephrotoxicity induced Group II rats. No significant difference was observed between the control and drug control AG-AO nanoparticle alone treated rat’s antioxidant status.
Figure 5. Antioxidant effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) against oxidative stress induced by cisplatin nephrotoxicant. The levels of malonaldehyde (MDA), end product of lipid peroxidation were estimated in the kidney tissue of control and experimental rat (A). (B) Depicts the levels of antioxidant SOD, catalase and reduced glutathione in the kidney tissue of control and experimental rats. The values of SOD were expressed as Enzyme concentration required to inhibit the chromogen produced by 50% in one min under standard condition. The values of CAT were expressed as µmole of hydrogen peroxide decomposed/min. The values of GSH were expressed as µg of reduced glutathione formed/min, respectively. The values depicted in the table are the mean ± SD of six rats in each group. *p ≤ .05, #p ≤ .01 considered to be statistically significant.
Effect of AG-AO on cytokines
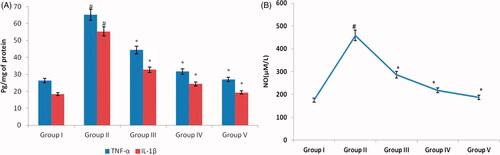
Oxidative stress induced by cisplatin activates the transcription factor NF-κB which in turn induces the production of TNF-α and IL1β. depicts the levels of nitric oxide, one of the major oxidant and the cytokines TNF-α and IL1β levels in control and experimental rats. Compared to the rats with induced nephrotoxicity with cisplatin, the AG-AO nanoparticle pretreated rats shown decreased levels of nitric oxide, TNF-α and IL1β. No significant difference was observed between the control and 50 mg/kg bwt AG-AO nanoparticle alone treated rats.
Figure 6. Nephroprotective effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) against cytokines TNFα, IL1β (A) and nitric oxide (B). The levels of TNFα, IL1β in kidney tissue of control and experimental rats were measured using commercially available ELISA kit. The levels of nitric oxide measured using colorimetric assay kit. The colour intensity developed was measured using ELISA plate reader and the values are illustrated in the figure. The values depicted in the figure are the mean ± SD of six rats in each group. *p ≤ .05, #p ≤ .01 considered to be statistically significant.
Effect of AG-AO on inflammatory markers
depicts the levels of inflammatory markers in the control and experimental rats. Inflammation is the key process of most of the nephrotoxic drugs to induce toxicity in the kidney. The levels of both lactate dehydrogenase and NFκB.p65 were significantly increased in nephrotoxicity induced Group II rats (2732.19 ± 237.93, 7.73 ± 2.43, respectively) compared to the Group I control rats (1968.72 ± 176.24, 1.54 ± 0.62, respectively).On the contrary, these levels were significantly decreased in Group III & Group IV rats when compared with Group II rats. There was no significant difference observed between the Group I control (1968.72 ± 176.24, 1.54 ± 0.62), and AG-AO nanoparticle alone treated rats (1943.44 ± 173.43, 1.96 ± 0.89).
Table 2. Antiinflammation effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) against inflammatory markers serum LDH and NF-kappaB p65.
Effect of AG-AO on apoptotic markers
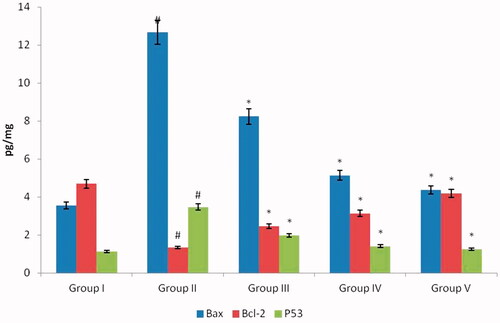
Apoptosis, programmed cell death triggered through various factors like radiation, toxic injury, etc., reports suggest cisplatin induces cell death through an apoptotic mechanism. and represents the levels of proapoptotic proteins Bax, p53, Caspase-3, Caspase-9 and antiapoptotic protein Bcl2 in control and experimental rats. Compared to the nephrotoxicity alone induced rats the AG-AO nanoparticles treated rats shown decreased levels of pro-apoptotic protein and increased level of antiapoptotic protein Bcl2 level. No significant difference was observed between the control and drug control AG-AO nanoparticle alone treated rats.
Figure 7. Antiapoptotic effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) against proapoptotic and antiapoptotic proteins. The levels of proapoptotic proteins Bax, p53 and antiapoptotic protein Bcl2 in kidney tissue of control and experimental rats were measured using commercially available ELISA kit. The colour intensity developed was measured using ELISA plate reader and the values are illustrated in . The values depicted in the figure are the mean ± SD of six rats in each group. *p ≤ 0.05, #p ≤ 0.01 considered to be statistically significant.
Effect of AG-AO on kidney histoarchitecture
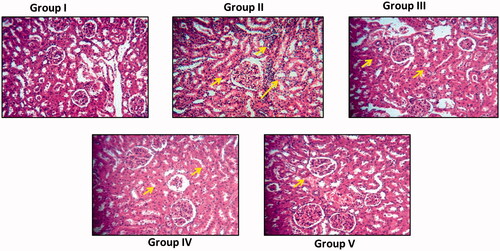
represents the kidney histology of control and experimental rats. Nephrotoxicity alone induced rat’s kidney tissue () shows increased cortex and medullary damage compared to the AG-AO nanoparticle pretreated rats (). Tubular atrophy, necrosis was observed in nephrotoxicity induced rat’s kidney whereas it is significantly decreased in high dose AG-AO nanoparticle pretreated rat’s kidney. Interstitial edema and inflammation were significantly reduced in AG-AO nanoparticle pretreated rat’s kidney compared to the nephrotoxicity induced rat’s kidney. No significant difference in the kidney histoarchitecture was observed between the control and AG-AO nanoparticle alone treated rats.
Figure 9. Nephroprotective effect of silver nanoparticles synthesized from Alpinia officinarum (lesser galangal) traditional Chinese medicinal plant (AG-AO) on histopathological changes in kidney tissue of cisplatin induced nephrotoxicity model. The control and experimental rat’s kidney tissue were processed for histological analysis and sectioned into slices of 5 micron thickness. The sectioned slides were stained with haematoxylin and eosin stains. The stained slides were viewed under light microscope and photographed. The experiments were performed in triplicates.
Discussion
Cisplatin, a platinum-based persuasive anticancer drug approved by FDA long back in the year 1980, are still in use in the field of cancer treatment. Even though various other safer platinum-based drugs were discovered recently, their potency is not comparable to the cisplatin [Citation32]. Usage of cisplatin was limited due to its nephrotoxicity during long-duration medication and also high dosage causes side effects like nausea, vomiting, etc., Cisplatin generates free radicals which causes oxidative stress leading to decreased glomerular filtration rate [Citation8,Citation33]. Patients prescribed cisplatin have decreased glomerular filtration rate this is due to the accumulation of cisplatin on to the tubular epithelial cells of proximal kidney tubes [Citation34]. In the present study, the cisplatin alone treated rats shows increased levels of urea, creatinine and the levels of protein in urine also significantly increased which may be due to the cisplatin accumulation in the proximal tubular epithelial cells of the kidney.
In the present study, the physical characterization of AG-AO nanoparticles satisfies the ideal nanodrug. The 445 nm absorption band confirms the surface plasmon resonance property of synthesized silver nanoparticles which was reported previously [Citation35,Citation36]. FTIR spectrum of AG-AO nanoparticles 3347 and 1639 cm−1 depicts the presence of hydroxyl group and binding of the C=O functional group with the silver nanoparticles [Citation37]. The TEM, XRD and SAED analysis also confirms the crystalline nature and spherical structure of AG-AO which classifies it as an efficient nanodrug. Both high and low dose of AG-AO nanoparticles treated rats decreased the level of urea and creatinine. The serum albumin levels were increased in the AG-AO nanoparticles treated rats, this may be due to the flavonol galangin present in A. officinarum which protects the nephrons from oxidative stress and protects the kidney from cisplatin-induced renal damage [Citation38].
Cisplatin alone treatment increased the levels of lipid peroxidation and decreased the levels of antioxidants SOD, catalase and the reduced glutathione in the kidney tissue. This is due to the organic cation transporters present in the proximal tubes of the kidney which effectively uptake the cisplatin from the circulation to the kidney leading to accumulation of cisplatin in the kidney [Citation39]. In vivo cisplatin treatment disrupts the mitochondrial membrane thereby decreases the uptake of mitochondrial calcium and also the antioxidant defense system [Citation40]. Increased oxidative stress induced by cisplatin leads to a reduction in antioxidant levels, cisplatin reacts with the thiol group present in the enzymatic antioxidant glutathione thereby reduces the antioxidant defense mechanism [Citation41]. This may be the reason for the reduction of antioxidant levels in the cisplatin alone treated rats kidney tissue
The two key mechanisms of cisplatin to induce nephrotoxicity are oxidative stress and inflammation [Citation42]. Inflammation in the renal tissue was mediated by the polypeptides cytokines like interferons [Citation43], tumour necrosis factor, interleukins, etc. interleukins [Citation44–46]. Numerous studies have reported cisplatin triggers the activation of TNFα, thereby, initiating the inflammatory response in the renal tissue [Citation47,Citation48]. TNFα deficient mice were resistant to the cisplatin-induced nephrotoxicity [Citation49]. In the present study, the cisplatin alone treated rat increased levels of TNFα and IL1β which may be due to the oxidative stress induced by cisplatin triggered the activation of TNFα.
Nitric oxide levels were increased in the cisplatin alone treated rats this may be due to the inhibitory effect of cisplatin on endothelial nitric oxidase synthase which protects the renal tissue from ischemic shock [Citation50,Citation51]. The levels of transcription factor nuclear factor-kappa B were also increased in the cisplatin alone treated rats which confirms the inflammation induction of cisplatin in the renal tissues. Holditch et al. [Citation52] also reported cisplatin increases in the levels of free radicals, which, in turn, activates pro-inflammatory cytokines leading to inflammation. Cisplatin induces apoptosis in distal renal nephrons via metabolizing into highly charged electrophilic byproducts [Citation53]. NF-κB family major inducers of apoptosis consists of eight proteins among which Rel A (p65) plays a dual role both apoptotic and antiapoptotic protein [Citation54]. In the present study, the increase in the levels of NF-κB Rel A (p65) activated the proapoptotic protein and inhibited the antiapoptotic protein levels.
Apoptosis is initiated and executed by various proteins, B cell lymphoma 2 are the key proteins which balance the survival and death of the cells [Citation55]. Bcl-2 family proteins comprising both proapoptotic and antiapoptotic protein regulates the apoptosis by controlling mitochondrial function [Citation56]. The activation of proapoptotic proteins Bid, Bad and Bax further activate proteases caspases which leads to apoptosis. Cisplatin alone treated rat increased the levels of proapoptotic protein Bax and decreased the levels of anti-apoptotic protein Bcl2 leading to apoptosis. P53 and caspases play a vital role in the suppression of antiapoptotic genes thereby controlling the cell proliferation [Citation57]. The levels of tumour suppressor gene p53 are also increased in the cisplatin, which confirms the induction of apoptosis [Citation58]. Histopathological analysis of cisplatin-induced kidney tissue clearly depicts the induction of apoptosis by cisplatin generated free radicals.
The levels of cytokines, inflammatory markers and apoptotic protein levels were drastically reduced in AG-AO pretreated rats compared to cisplatin alone treated rats. Diaryheptanoids present in the rhizomes of A. officinarum inhibits the nitric oxide production in the RAW 264.7 mouse macrophage cells treated with LPS [Citation59]. Phenolic hydroxyl groups donate hydrogen and they possess immense antioxidant property [Citation60–62]. Alpinia officinarum contains a huge amount of phenolic compounds [Citation63] which would have scavenged the free radicals generated by cisplatin and protected the renal tissue from inflammation and apoptosis.
Conclusion
Our overall results conclude cisplatin induces nephrotoxicity through the generation of free radicals, thereby, inhibiting the antioxidant defense system. Cisplatin provokes the inflammatory cytokines and proapoptotic protein leading to induction of nephritis. Silver nanoparticles biosynthesized from A. officinarum, a traditional medicinal plant used in the Chinese and Ayurvedic medicine effectively scavenged the free radicals and protected the renal tissue from cisplatin. Therefore, it confirms with further studies, AG-AO nanoparticle can be used as a supplementary drug to cisplatin medicated cancer patients.
Disclosure statement
No potential conflict of interest .
References
- Miller RP, Tadagavadi RK, Ramesh G, et al. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel). 2010;2:2490–2518.
- Campbell NP, Kindler HL. Update on malignant pleural mesothelioma. Semin Respir Crit Care Med. 2011;32:102–110.
- Goffin J, Lacchetti C, Ellis PM, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260–274.
- Ismaili N, Amzerin M, Elmajjaoui S, et al. The role of chemotherapy in the management of bladder cancer. Prog Urol. 2011;21:369–382.
- Santos NAG, Rodrigues MAC, Martins NM, et al. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–1250.
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007.
- Atessahin A, Karahan I, Yilmaz S, et al. The effect of manganese chloride on gentamicine-induced nephrotoxicity in rats. Pharmacol. Res. 2003;48:637–642.
- Yao X, Panichpisal K, Kurtzman N, et al. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124.
- Perše M. Večerić-Haler Ž. Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed Res Int. 2018;12:1462802.
- Volarevic V, Djokovic1 B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biome Sci. 2019;26:25.
- Antunes LM, Darin JD, Bianchi NL. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001;43:145–150.
- Atessahin A, Yilmaz S, Karahan I, et al. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 2005;212:116–123.
- Silva CR, Greggi Antunes LM, Bianchi M. Antioxidant action of bixin against cisplatin-induced chromosome aberrations and lipid peroxidation in rats. Pharmacol. Res. 2001;43:561–566.
- Naziroglu M, Karaoğlu A, Aksoy AO. Selenium and high dose Vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 2004;195:221–230.
- Lim TK. Edible medicinal and non-medicinal plants: modified stems, roots, bulbs, Vol. 12. London (UK): Springer; 2002. p. 178.
- Daniel M. Medicinal plants: chemistry and properties. Enfield (UK): Science Publishers; 2006. p. 63.
- Zhang BB, Dai Y, Liao ZX, et al. Three new antibacterial active diarylheptanoids from Alpinia officinarum. Fitoterapia 2010;81:948–952.
- Dan LI, Wei QU, Ling ZH, et al. A new dimeric diarylheptanoid from the rhizomes of Alpinia officinarum. Chin J Nat Med. 2014;12:139–141.
- Köse LP, Gülcin I, Gören AC, et al. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Industrial Crops and Products. 2015;74:712–721.
- Afifi M, Almaghrabi OA, Kadasa NM. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:153573.
- Abdul H, Sivaraj R, Venckatesh R. Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.- lamiaceae leaf extract. Mater. Lett. 2014;131:16–18.
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Eng Biotechnol. 2016;14:195–202.
- He Y, Ma FYZ, Zhang H, et al. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017;7:39842.
- Rivera-Rangel RD, González-Muñoz MP, Avila-Rodriguez M, et al. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Coll Surf A: Physicochem Eng Asp. 2018;536:60–67.
- Meva FE, Mbeng JO, Ebongue CO, et al. Stachytarpheta cayennensis aqueous extract, a new bioreactor towards silver nanoparticles for biomedical applications. JBNB. 2019;10:102–119.
- Jain D, Kumar Daima H, Kachhwaha S, et al. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomater Bios. 2009;4:3557–3563.
- Iwata J, Nishikaze O. New micro-turbidimetric method for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1979;25:1317–1319.
- Yagi K. Lipid peroxides and human disease. Chem Physiol Lipids. 1987;45:337–351.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77.
- Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol. 2016;102:37–46.
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61.
- Daugaard G, Abildgaard U. Cisplatin nephrotoxicity. A review. Cancer Chemother Pharmacol. 1989;25:1–9.
- Gavade NL, Kadam AN, Suwarnkar MB, et al. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus jujuba leaf extract. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2015;136:953–960.
- Selvi BC, Madhavan J, Santhanam A. Cytotoxic effect of silver nanoparticles synthesized from Padina tetrastromatica on breast cancer cell line. Adv Nat Sci: Nanosci Nanotechnol. 2016;7:035015.
- Ali HM, Abo-Shady A, Sharaf Eldeen HA, et al. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem Cent J. 2013;7:53.
- Huang YC, Tsai MS, Hsieh PC, et al. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol. 2017;329:128–139.
- Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–1484.
- Arany I, Kaushal GP, Portilla D, et al. Cellular mechanisms of nephrotoxicity. In: Broe MED, Porter GA, Bennett WM, Deray G, editors. Clinical Nephrotoxins. New York (NY): Springer; 2008. p. 155–170.
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265–7279.
- Lee S, Moon SO, Kim W, et al. Protective role of L-2-oxothiazolidine-4-carboxylic acid in cisplatin-induced renal injury. Nephrol Dial Transplant. 2006;21:2085–2095.
- Baron S, Tyring SK, Fleischmann WR Jr, et al. The interferons. Mechanisms of action and clinical applications. JAMA 1991;266:1375–1383.
- Horii Y, Iwano M, Hirata E, et al. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int Suppl. 1993;39:S71–S75.
- Wada T, Yokoyama H, Tomosugi N, et al. Detection of urinary interleukin-8 in glomerular diseases. Kidney Int. 1994;46:455–460.
- Finn W, Porter G. Urinary biomarkers and nephrotoxicity. In: de Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical nephrotoxins, 2nd ed. Dordrecht (Netherlands): Springer; 2003. p. 621–655.
- Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004;65:490–499.
- Dong Z, Atherton SS. Tumor necrosis factor-alpha in cisplatin nephrotoxicity: a homebred foe? Kidney Int. 2007;72:5–7.
- Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis incisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–F618.
- Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf). 2006;187:433–446.
- Yamasowa H, Shimizu S, Inoue T, et al. Endothelial nitric oxide contributes to the renal protective effects of ischemic preconditioning. J Pharmacol Exp Ther. 2004;312:153–159.
- Holditch SJ, Brown CN, Lombardi AM, et al. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. IJMS. 2019;20:3011.
- Rosenberg B. Cisplatin: its history and possible mechanism of action. In: Prestayko AW, Crooke ST, Carter SK, editors. Cisplatin: current status and new developments. New York (NY): Academic Press; 1980. p. 9–20.
- Li RY, Zhang WZ, Yan XT, et al. Arginyl-fructosyl-glucose, a major maillard reaction product of red ginseng, attenuates cisplatin-induced acute kidney injury by regulating nuclear factor κB and phosphatidylinositol 3-kinase/protein kinase B signaling pathways. J Agric Food Chem. 2019;67:5754–5763.
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441.
- Meng YC, Jiang HX, Zhang JH, et al. Activation of hepatocyte growth factor-induced apoptosis in hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi. 2012;20:698–702.
- Fridman JS, Lowe W. Control of apoptosis by p53. Oncogene 2003;22:9030–9040.
- Chen X, Wei W, Li Y, et al. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chemico-Biol Interact. 2019;308:269–278.
- Matsuda H, Ando S, Kato T, et al. Inhibitors from the rhizomes of Alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg Med Chem. 2006;14:138–142.
- Ly TN, Yamauchi R, Shimoyamada M, et al. Isolation and structural elucidation of some glycosides from the rhizomes of smaller galanga (Alpinia officinarum Hance). J Agric Food Chem. 2002;50:4919–4924.
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247.
- Nimse SB, Pal D. Free radicals, natural antioxidants and their reaction mechanisms. RSC Adv. 2015;5:27986–28006.
- Mayachiew P, Devahastin S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT Food Sci Technol. 2008;41:1153–1159.