Abstract
PAX8 is identified as a regulator in the pathogenesis of human tumours and an indicator of the prognosis for patients. However, the role of PAX8 on proliferation in gastric cancer have not been studied. This study was aimed to explore the expression pattern of PAX8 in gastric cancer, and investigate the effect of PAX8 on the proliferation of gastric cancer cells. PAX8 and SOX13 were identified to be synchronously up-regulated in primary gastric cancer in human gastric cancer tissues and the gastric cancer datasets of TCGA, and gastric cancer patients of combined high PAX8 and SOX13 expression showed poor prognosis. Furthermore, SOX13 can mediate PAX8 and its targeted genes, Aurora B and Cyclin B1, expression in AGS and MGC803 cell lines. Flow cytometry and EdU incorporation assays showed that silencing PAX8 can block the cell cycle of gastric cancer cell in G1 phase and SOX13 expression can rescue the arrested proliferative process induced by PAX8 silenced in CCK8 and colony formation assays. Thus, combined SOX13 and PAX8 expression regulate the proliferation of gastric cancer cells, and both SOX13 and PAX8 play an oncogene function in gastric cancer.
Introduction
Gastric carcinoma has been the main cause of cancer-induced death, due to its characteristic of inefficient preclinical screening and rapid pathological progression [Citation1,Citation2]. At present, patients with gastric cancer treated by surgery, chemotherapy and other measures generally show poor prognosis and high tumour recurrence rate [Citation3–5]. As malignant proliferation is one of the characteristics distinguishing tumour cells from normal somatic cells, in recent years, the development and application of molecular drugs targeting cell proliferation have greatly improved the therapeutic effect of gastric cancer and the survival prognosis of patients [Citation6,Citation7]. Therefore, in-depth exploration of the molecular mechanism of gastric cancer cell proliferation may potentially open up new possibilities for developing new gastric cancer treatment and improving patient survival [Citation8].
PAX8 has been reported as a significant transcription factor in embryonic development [Citation9]. More reports shown that silencing of PAX8 expression in embryonic cells can lead to abnormal development of the urinary system organs, including kidney and the lack of PAX8 gene in mice sufficiently leads to endometrial insufficiency [Citation10]. In addition, PAX8 has also been reported to exhibit a high expression pattern in tumour cells with malignant proliferation [Citation11,Citation12]. Staining results indicate that PAX8 expression in ovarian and endometrial cancer tumour tissue is significantly elevated relative to adjacent tissues, particularly in serous tumours such as ovarian cancer and clear cell carcinoma [Citation13,Citation14]. Furthermore, ovarian cancer patients with high expression of PAX8 usually have a high recurrence rate and poor clinical prognosis [Citation15]. However, the role of PAX8 on proliferation and its clinical significance in human gastric cancer has not been reported.
SRY-BOX13 (SOX13) has been shown a key regulator for cell stemness and cell differentiation in normal and cancer tissues by regulating Wnt/β-catenin signalling [Citation16,Citation17]. Since SOX13 shares more than 60% homology with HMG-box, SOX13, usually shown as a transcription factor, can bind to the HMG-box motif of the target gene promoter, and recruit other transcription molecules to form a transcriptional complex [Citation18,Citation19]. In recent years, SOX13 has been found highly expressed in tumours, and cancer patients with a high SOX13 expression often have a poor survival prognosis, making it a potential tumour biomarker for diagnosis and treatment in malignant tumour [Citation20]. However, the role of SOX13 on tumourigenesis in human gastric cancer has not been explored.
In this study, we explained the role of PAX8 and SOX13 on the proliferation and clinical outcome of gastric cancer. Our results confirm the potential role of PAX8 in the progression of gastric cancer, providing a new basis for treatment.
Materials and methods
Cell lines
Gastric cancer cell lines (MKN45, MKN28) were obtained from ATCC (American Type Culture Collection). Gastric cancer cell lines (MGC803, AGS) and GES1 cell lines were purchased from China National Infrastructure of Cell Line Resource (Beijing, China). RPM1636 medium supplemented with 10% FBS (fetal bovine serum, Gibco, Grand Island, NY, USA) were used to culture cells.
Human tissue specimens
After 36 patients with gastric cancer signed informed consent, primary gastric cancer tissues and matched para-cancerous tissues were surgically collected. Part of specimens were stored in liquid nitrogen for subsequent gene expression assays, and the remainder was stored in 10% formaldehyde solution for tissue immunostaining analysis. All patients enrolled in the study were not treated with radiotherapy or chemotherapy, and clinical medical records were recorded. The Ethics Committee of our hospital approved this study.
Immunohistochemistry
The fixed gastric cancer tissue was sliced to a thickness of 3 μm. After the tissue sections were dewaxed-hydrated, heat-induced epitope repair was performed in a pH 9.0 citrate buffer solution. After inactivating the endogenous peroxidase with 3% H2O2, the antibody was incubated with the primary antibody for 2 h at 37 °C. Protein expression in tissues was detected using DAB-labelled secondary antibodies according to the manufacturer's protocol. In order to evaluate protein concentration in tissues, 36 gastric cancer tissues were grouped according to the results of staining, and the high expression group was defined as >30% positive, and the remaining was defined as a low expression group.
Virus packaging and infection
A vector plasmid containing the shRNA targeting PAX8 or SOX13 was co-transfected into HEK293T cells with psPAX2 and PMD2.G plasmids (Addgene, MA, USA). The culture was continued for 48 h, and the supernatant was collected. A virus concentration kit (Ribo, Guangzhou, China) was used to concentrate the virus by centrifugation at 30,000 rpm for 2 h. The cells were infected with the concentrated virus solution and screened with puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 1 week to obtain a cell line stably silencing PAX8 or SOX13.
RNA extraction and qRT-PCR
RNA was extracted with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and the cDNA was reverse transcribed using 2 μg of total RNA using the ReverTra Ace qPCR RT kit, and then quantitative PCR was performed on an ABI 7500 instrument (Applied Biosciences, Foster, CA, USA) using SYBR green fluorescent quantitative PCR mixture (TOYOBO, Osaka, Japan). The primers used in this study are listed in .
Table1. Primers used for quantitative real-time PCR.
Protein extraction and immunoblotting
After losing cells with RIPA buffer (Biotool, Switzerland), the Bradford reagent was used to determine the protein concentration. Eighty micrograms of total protein was used for SDS-PAGE (polyacrylamide gel electrophoresis). The isolated protein was transferred to a nitrocellulose filter (NC) membrane (Millipore, Billerica, MA, USA) by lateral electrophoresis, followed by blocking with 5% skim milk for 1 h and immunoblotting with a specific primary antibody. After incubating with the HRP-labelled secondary antibody for 1 h, the protein was visualized using ECL Substrate (Santa Cruz, CA, USA) and X-ray film.
Cell cycle analysis by flow cytometry
After harvesting the cells with trypsin, the pellets were suspended in 80% methanol solution precooled at –20 °C and fixed overnight at –20 °C. After centrifugation at 5000 rpm for 5 min, the cell pellet was incubated with propidium iodide (50 μg/ml) and RNase A (50 μg/ml) in 500 μl PBS at 37 °C for 30 min in the dark. The cycle distribution of various genotype cells was analyzed on a FACSCalibur system (BD Biosciences, San Jose, CA, USA).
EdU incorporation assay
Various genotype cells were seeded in 96-well plate (3000/well) for 24 h, and the medium was changed to 37 °C pre-warmed medium containing 20 mM EdU (EdU Apollo@594 in vitro imaging kit [Ribo]). After incubating for 2 h, the cells were stained according to the kit's instructions and the staining results were captured with a Leica inverted fluorescence microscope.
Statistical analysis
The experiments were repeated three times independently. Gene expression differences in tissues were compared with paired t tests, and other qRT-PCR results were evaluated using a two-tailed t test. The different distribution of PAX8 and SOX13 in gastric cancer tissue samples was detected by χ2 test. All statistical analyses were performed with SPSS 21.0, and statistical difference was considered at p < .05 (*p < .05, **p < .01, ***p < .001).
Result
Expression patterns of PAX8 and SOX13 were up-regulated in gastric cancer
In order to explore transcription factors that have a carcinogenic role in gastric cancer, we searched for all up-regulated transcription factors in gastric cancer tissues based on the TCGA database. Interestingly, the expression of PAX8 in gastric cancer was higher than that in the normal group (). Although PAX8 has been widely reported in various cancers as a tumour marker in some cancers, the potential role of PAX8 in gastric cancer has not been reported, our team tried to investigate whether PAX8 is involved in gastric cancer pathology. To further verify the expression pattern of PAX8 in the pathological progression of gastric cancer, we divided the gastric cancer samples into four stages and it was found that with the increase of tumour pathological grade, the expression level of PAX8 also showed a sequential increase ().
Figure 1. Expression pattern of PAX8 and SOX13 in gastric cancer. (A) Expression lever of PAX8 in gastric cancer in contrast to normal tissues based on TCGA dataset. (B) PAX8 mRNA expression lever in four different grades of cancer samples based on TCGA dataset. (C) SOX13 co-expressed with PAX8 in gastric cancer based on TCGA dataset. (D and E) PAX8 (D) and SOX13 (E) mRNA expression pattern in 36 pairs of human gastric cancer tissues (Cancer) and adjacent tissues(Normal). (F) SOX13 co-expressed with PAX8 in 36 human gastric cancer tissues. (G and H) Relative mRNA and protein expression level of PAX8 and SOX13 in four gastric cancer cell lines compared to GES1 cells. GAPDH served as endogenous control.
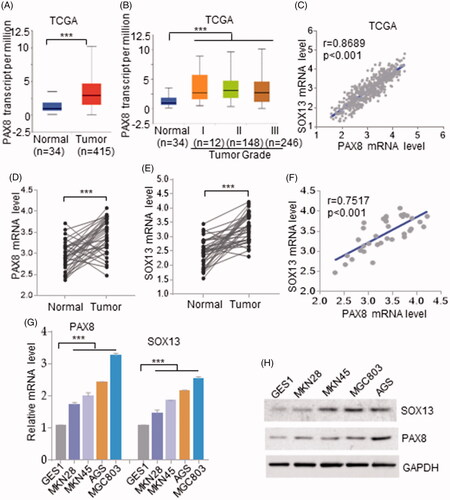
To elucidate why PAX8 was elevated in gastric carcinoma, we analyzed the gene structure of PAX8, and found more HMG-box elements in the PAX8 promoter region, which indicated that up-regulated PAX8 may be associated with a factor containing HMG-box. We then searched for all up-regulated HMG-box domain contained transcription factors in gastric cancer, and found a strong positive correlation between SOX13 and PAX8 in gastric cancer (r = 0.6567, p < .001; ).
Further, we verified the expression pattern of SOX13 and PAX8 in 36 surgically collected tissues. The qRT-PCR results showed that SOX13 and PAX8 mRNA expression patterns in gastric cancer tissues were significantly up-regulated than those in adjacent tissues (). Consistent with the result based on TCGA, spearman correlation analysis showed the expression level of SOX13 is positively correlated with that of PAX8 (r = 0.6567, p < .001; ).
To further validate the SOX13 and PAX8 expression patterns in gastric cancer, SOX13 and PAX8 expression levels in four gastric cancer cell lines was compared to GES1 cells (gastric epithelial cells). Certainly, PAX8 was found to overexpression in gastric cancer cell lines (). In parallel, SOX13 overexpressed in gastric cancer cell lines than that in normal controls, and the expression levels of SOX13 and PAX8 in the AGS and MGC803 cell lines were particularly higher than that in other gastric cancer cell lines (). Further analysis of the expression trends of SOX13 and PAX8 revealed that the cell lines SOX13 significantly up-regulated also showed high expression of PAX8 both at mRNA and protein levels ().
Combined PAX8 and SOX13 expression may predict the overall survival in patients with gastric cancer
To explore the distribution of SOX13 and PAX8 in gastric cancer tissue, we compared immune-histochemical staining results of 36 gastric cancer samples and found that both SOX13 and PAX8 presented in the nucleus of gastric cancer cells (). According to the staining of SOX13 and PAX8 in gastric cancer tissues, 36 cases were divided into 4 groups and the distribution of SOX13 and PAX8 showed a significant difference in the 4 groups (χ2 test, p = .0361; ). For data, 47.22% (17/36) of patients showed both SOX13 and PAX8 up-regulated in gastric cancer tissues, and only 22.22% (8/36) patients showed low levels of PAX8 and SOX13 expression in gastric cancer tissues (). These results indicated that there was a high probability of overexpressing PAX8 when gastric cancer tissues highly expressed SOX13, and it can be indirectly presumed there was a positive expression correlation between SOX13 and PAX8.
Figure 2. Combined PAX8 and SOX13 expression may predict the overall survival in patients with gastric cancer. (A) Immuno-histochemical analysis of PAX8 and SOX13 expressions in 36 gastric cancer samples. (B) Correlation between PAX8 and SOX13 was statistically analyzed. (C and D) Overall survival of patients with gastric cancer was calculated using Kaplan–Meier analysis according to low and high PAX8 (C) or SOX13 (D) staining. The p values were calculated by log-rank test. (E) Overall survival of patients with gastric cancer was calculated according to combined PAX8 and SOX13 staining level.
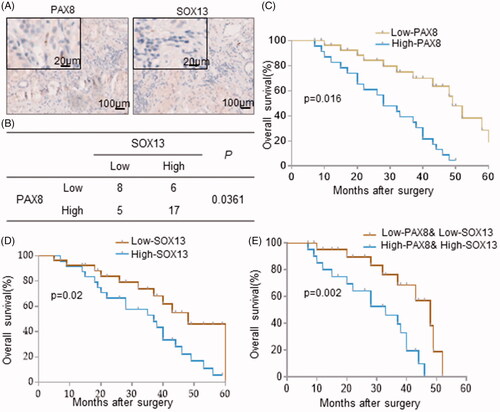
Because the clinical significance of PAX8 is still unclear in gastric cancer, we further analyze the relationship between SOX13 and PAX8 expression pattern with the survival prognosis of patients based on the gastric cancer patients’ medical records. By using the Kaplan-Meier method to map survival curves, the survival prognosis of patients with high expression of PAX8 was found significantly worse than that of low PAX8 expression patients (). Consistently, patients with high expression of SOX13 in tumour tissues also have a poor clinical survival (). By comparing the survival curves of patients with consistent SOX13 and PAX8 expression patterns, it indicated that low SOX13 and PAX8 expression was usually accompanied with a better overall survival compared to patients with up-regulated SOX13 and PAX8 (). These results suggest that SOX13 and PAX8 expression pattern in gastric tumours is positively related to clinical survival, making them a possibility be biological indicators to predict the overall survival of patients.
SOX13 regulated PAX8 expression in gastric cancer
To elucidate the mechanism of up-regulated PAX8 in gastric cancer, we hypothesized that raised PAX8 expression in gastric cancer may be partly caused by up-regulated SOX13. Therefore, we verified whether SOX13 has the potential to regulate PAX8 expression in AGS and MGC803 cell lines that simultaneously express SOX13 and PAX8. We transfected different amounts of SOX13-expressing plasmids in AGS and MGC803 cells. Surprisingly, the results of qRT-PCR and Western blotting both showed an increasing SOX13 expression can result in a stepwise increasing expression pattern of PAX8 at mRNA and protein levels ().
Figure 3. PAX8 expression pattern can be regulated by SOX13 in gastric cancer. (A and B) Relative mRNA and protein expression of PAX8 in SOX13 overexpressed AGS and MGC803cell lines. (C and D) SOX13 can rescue mRNA and protein expression level of PAX8 in AGS and MGC803 cell lines. (E) SOX13 mediated PAX8 targeted genes expression in AGS and MGC803 cell lines.
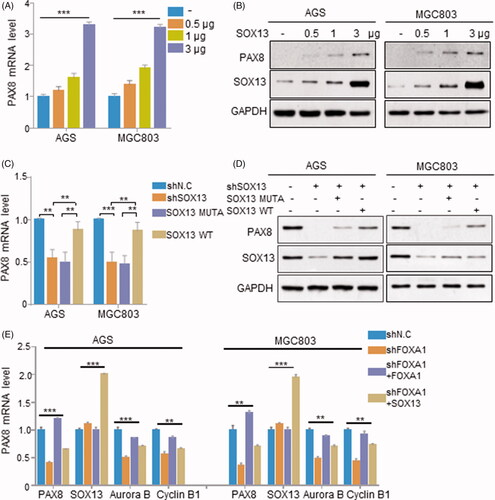
Then, for analyzing the expression correlation between SOX13 and PAX8 at the endogenous level, we constructed cell lines stably knocking down SOX13 based on AGS and MGC803 cell lines. Furthermore, since existed studies have shown that SOX transcription members regulated targeted gene expression by binding to the targeted DNA sequence through its HMG-box domain, we constructed SOX13 mutant (SOX13 ins6), could not bind to the target gene DNA sequence, by inserting six amino acids into the HMG-box domain. The results showed that silencing SOX13 indeed resulted in a lower PAX8 expression (). However, after we restored the expression of wild type SOX13 in the SOX13 silenced cell lines, PAX8 mRNA and protein levels were recovered to some extent, but when we introduced SOX13 mutant (SOX13 ins6) in those cell lines, it could not recuse the expression of PAX8 (). These results indicated that SOX13 can be a regulator to affect the expression of PAX8 in gastric cancer.
Previous studies showed that PAX8 can regulate the progression of mitosis via mediating cell cycle-associated genes expression in tumour, such as Aurora B and Cyclin B1. Since the above results indicated SOX13 has the potential to regulate PAX8 expression in gastric cancer cells, we attempted to verify whether SOX13 can modulate Aurora B and Cyclin B1 via mediating PAX8 expression. We found that in AGS and MGC803 cells, stably silencing PAX8 also down-regulated Aurora B and Cyclin B1 in gastric cancer, and when PAX8 was restored, Aurora B and Cyclin B1 could also be rescued (). Consistent with this, when SOX13 was introduced in PAX8 silenced cell lines, Aurora B and Cyclin B1 expression were also restored to some extent, and the expression of PAX8 in the cells was also increased (). These results indicate that PAX8 dose regulate Aurora B and Cyclin B1 expression in gastric cancer cells, and SOX13 can also affect PAX8 downstream target genes expression via mediating PAX8 expression.
SOX13 dependent PAX8 expression promotes cell proliferative progress in gastric cancer
Due to PAX8 has been shown to regulate Aurora B and Cyclin B1 expression in gastric cancer, we next explored the effect of PAX8 on the cell cycle progression of gastric cancer cells. Flow cytometry was used to detect changes in cell cycle distribution resulted by knocking down PAX8, and found that down-regulated PAX8 can cause a G1-phase block in mitosis of gastric cancer cells, but overexpression of SOX13 can abolish the G1-phase arrest caused by silencing PAX8 in gastric cancer cells (). In parallel, the results of EdU incorporation showed that knockdown of PAX8 decreased Edu-positive cells, whereas overexpression of SOX13 reversed this trend (). These results indicate that PAX8 combined with SOX13 regulates cell cycle progression in gastric cancer.
Figure 4. SOX13 dependent PAX8 expression promotes cell proliferation in gastric cancer. (A and B) Flow cytometry analysis of cell cycle distribution in PAX8 silenced AGS and MGC803 cell lines and controls. (C and D) EdU incorporation assay showed the percentage of in S-phase in PAX8 silenced AGS and MGC803cells. (E) CCK8 assay showed cells viability in PAX8 silenced AGS and MGC803 cells. (F and G) The clone formation assay in PAX8 silenced AGS and MGC803 cells and controls.
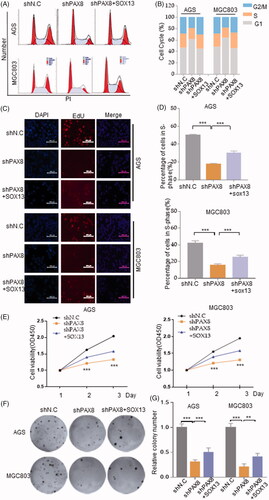
Since unregulated cell viability is one of the key characteristic of the exuberant proliferation of cancer cells, we next studied the effect of PAX8 on the cell viability of gastric cancer cells. By plotting the cell growth curve, a significant decrease in cell viability was observed to result by knocking down PAX8 compared to the control groups, but it was significantly increased after overexpression of SOX13 (). Consistently, knockdown of PAX8 significantly reduced the formation of colons, while overexpression of SOX13 allowed more clones formed relative to the PAX8 silenced group (). Therefore, the above results can be inferred that PAX8 has the ability to promote the proliferation of gastric cancer cells, and SOX13 can regulate the proliferation of gastric cancer cells by regulating the expression of PAX8.
Discussion
For a long time, in addition to the traditional treatment of gastric cancer, exploring for methods to inhibit the malignant proliferation of tumour cells has become one of the main directions for the innovative treatment of gastric cancer [Citation21, Citation22]. Therefore, researches on molecules and signal transduction pathways participating in the regulation of gastric cancer cell proliferation is still one of the hot areas of recent research on gastric cancer [Citation23, Citation24]. Studies have shown that PAX8 is usually up-regulated in digestive system tumours and urinary system tumours, and PAX8 has been identified as a diagnostic marker for ovarian cancer and renal cell carcinoma [Citation25, Citation26]. Recent studies have shown that PAX8 has a direct or indirect effect on cell growth and survival [Citation12]. PAX8 is one of the factors involved in the regulation of cell differentiation in rat thyroid cells, while inhibition of PAX8 expression in rat thyroid cells leads to a significant decrease in cell proliferation [Citation27]. Secondly, PAX8 was found to be highly expressed in colorectal and glioma cell lines, and has the function of activating telomerase to maintain immortalization of cells [Citation28]. Although these results all reveal the potential of PAX8 to regulate cell cycle progression, there is little evidence that PAX8 regulates gastric cell progression.
In this study, we demonstrated for the first time that PAX8 plays an oncogenic function in the pathological development of gastric cancer. Several of our studies showed PAX8 promoted human gastric cancer cell proliferation. First, PAX8 expression was significantly elevated in human primary gastric cancer tissues and cell lines than that in normal tissues or cells. Secondly, gastric cancer patients with high expression of PAX8 have poor prognosis. Third, knockdown of PAX8 in gastric cancer cells can down-regulate Aurora B and Cyclin B1. Furthermore, down-regulation of PAX8 can significantly cause cell cycle arrest and a decrease in gastric cancer cell proliferation. These results confirm that the signalling pathway mediated by PAX8 in gastric cancer can be used as a new direction for the treatment of gastric cancer.
The SOX transcription members are normally expressed in embryonic tissues and stem cells in vivo, to maintain stemness and control differentiation direction [Citation29, Citation30]. In recent years, it has been found that HMG-box-related transcription factors such as SOX10 and SOX12 are essential for tumour cell survival and growth [Citation31]. For example, SOX10 can synergize with GPM6B and COL9A3 to stimulate EGFR-stimulated proliferation of breast tumour cells, making it a biomarker for diagnosing basal-like breast cancer and a prognostic serum marker [Citation31]. SOX12 is significantly up-regulated in colon cancer and also a biomarker for poor prognosis [Citation32]. Further, SOX12 knockdown can significantly inhibit colon cancer cells proliferation [Citation33]. However, studies on the role of SOX13 in tumours have not been reported. In this study, SOX13 and PAX8 expression in gastric cancer was found to be positive correlated, and highly regulated in tumours than adjacent tissues. Interestingly, SOX13 regulates the expression of PAX8 in gastric cancer cells, and overexpression of SOX13 promotes the expression of PAX8-regulated cell cycle-related genes Aurora B and Cyclin B1. Consistent with this, overexpression of SOX13 can restore cell cycle arrest caused by PAX8 knockdown by restoring the expression of Aurora B and Cyclin B1. These results indicate that SOX13 is involved in the regulation of gastric cancer cell cycle with the role of oncogene in gastric cancer. Overall, our findings reveal an unrecognized function of SOX13 in tumours and look forward to discovering more signalling pathways SOX13 involved.
In conclusion, we conclude that PAX8 is overexpressed in primary gastric cancer tissues and cells, which contributes to gastric cancer cell proliferation and tumour formation. SXO13 and PAX8 have similar expression patterns in gastric cancer, and both show oncogene effects in the formation of gastric cancer and clinical prognosis of patients. Furthermore, SOX13 can indirectly contribute to tumourigenesis by regulating the expression of PAX8. However, it is necessary to further explore the regulatory network of PAX8 in gastric cancer, to explore the significance of PAX8 in the treatment of gastric cancer.
Acknowledgements
We sincerely appreciate Dr. Yan Wei for supporting the experiments and revising the manuscript.
Disclosure statement
We declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Bijlsma MF, Sadanandam A, Tan P, et al. Molecular subtypes in cancers of the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2017;14:333–342.
- Balakrishnan M, George R, Sharma A, et al. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19:36.
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568.
- Ding K, Tan S, Huang X, et al. GSE1 predicts poor survival outcome in gastric cancer patients by SLC7A5 enhancement of tumor growth and metastasis. J Biol Chem. 2018;293:3949–3964.
- Zheng J, Rutegard M, Santoni G, et al. Prediabetes and diabetes in relation to risk of gastric adenocarcinoma. Br J Cancer. 2019;12:1147-1152.
- Kankeu Fonkoua L, Yee NS. Molecular characterization of gastric carcinoma: therapeutic implications for biomarkers and targets. Biomedicines 2018;6:32.
- Cidon EU, Ellis SG, Inam Y, et al. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers 2013;5:64–91.
- Kim TJ, Lee H, Min YW, et al. Diabetic biomarkers and the risk of proximal or distal gastric cancer. J Gastroenterol Hepatol. 2016;31:1705–1710.
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18:41–47.
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90.
- Mittag J, Winterhager E, Bauer K, et al. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007;148:719–725.
- Li CG, Nyman JE, Braithwaite AW, et al. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30:4824–4834.
- Laury AR, Hornick JL, Perets R, et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34:627–635.
- Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826.
- Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571.
- Melichar HJ, Narayan K, Der SD, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science (New York, N.Y.). 2007;315:230–233.
- Marfil V, Moya M, Pierreux CE, et al. Interaction between Hhex and SOX13 modulates Wnt/TCF activity. J Biol Chem. 2010;285:5726–5737.
- Kido S, Hiraoka Y, Ogawa M, et al. Cloning and characterization of mouse mSox13 cDNA. Gene 1998;208:201–206.
- Roose J, Korver W, de Boer R, et al. The Sox-13 gene: structure, promoter characterization, and chromosomal localization. Genomics. 1999;57:301–305.
- He Z, Ruan X, Liu X, et al. FUS/circ_002136/miR-138-5p/SOX13 feedback loop regulates angiogenesis in Glioma. J Exp Clin Cancer Res. 2019;38:65.
- Bass AJ, Laird PW, Shmulevich I, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;5:202–209.
- Chen YL, Cheng KC, Lai SW, et al. Diabetes and risk of subsequent gastric cancer: a population-based cohort study in Taiwan. Gastric Cancer 2013;16:389–396.
- Liu Y, Sethi NS, Hinoue T, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33:721–735.e728.
- Duraes C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464:367–378.
- Posenato I, Calio A, Segala D, et al. Primary seminal vesicle carcinoma. The usefulness of PAX8 immunohistochemical expression for the differential diagnosis. Human Pathol. 2017;69:123–128.
- Kar SP, Adler E, Tyrer J, et al. Enrichment of putative PAX8 target genes at serous epithelial ovarian cancer susceptibility loci. Br J Cancer. 2017;116:524–535.
- Koumarianou P, Gomez-Lopez G, Santisteban P. Pax8 controls thyroid follicular polarity through cadherin-16. J Cell Sci. 2017;130:219–231.
- Hung NA, Eiholzer RA, Kirs S, et al. Telomere profiles and tumor-associated macrophages with different immune signatures affect prognosis in glioblastoma. Mod Pathol. 2016;29:212–226.
- Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013;12:15–30.
- Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development (Cambridge, England). 2013;140:4129–4144.
- Ivanov SV, Panaccione A, Nonaka D, et al. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451.
- Huang W, Chen Z, Shang X, et al. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology (Baltimore, Md.). 2015;61:1920–1933.
- Duquet A, Melotti A, Mishra S, et al. A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT-TCF pathway modulators TMED3 and SOX12. EMBO Mol Med. 2014;6:882–901.
