Abstract
Nimbolide, a triterpenoid isolated from flower of neem tree possess various therapeutic properties. The objective of the study was to assess the anti-arthritic activity of nimbolide in arthritis induced rats. Nimbolide (20 mg/kg per day) was given orally to arthritic rats induced with Complete Freund’s Adjuvant and changes in paw volume, body weight, organ indices (thymus and spleen), arthritic score, biochemical parameters and proinflammatory cytokines levels were determined. Histopathological analysis was also performed. Western blot analysis was also performed. Rats treated with nimbolide displayed marked reduction in arthritic score, organ indices, volume of paw, edema formation, along with substantial enhancement in body weight. Histopathological findings showed significant reduction in destruction of joints and inflammation following nimbolide treatment. The protective action of arthritic rats treated with nimbolide was also substantiated by molecular and biochemical studies. The results of the study show that nimbolide treatment has markedly enhanced health and reduced inflammation via lessening the proinflammatory cytokines expression in arthritic rats. Hence, nimbolide may be used as a potent therapeutic drug in treating rheumatoid arthritis.
Introduction
Rheumatoid arthritis (RA), a chronic, autoimmune disease and the causative factors that are responsible for development of disease are unknown. Approximately about 0.75% in India and 1% adult population in the world are affected with this disease which creates a major health problem worldwide [Citation1]. The pathological features of the patients who suffer from this disease display joint pain, joint inflammation, synovial tissue proliferation which leads to damage and disability of joints [Citation2]. Disease prevalence is mostly reported in male population than females in developed countries. The disease onset occurs mainly between age groups of 30–55, even though it can occur at any age [Citation3].
The mechanism by which joint destruction occurs in RA includes increased expression of cytokines and transcription factors. Interleukins namely IL-6, tumour necrosis factor (TNF)-α, IL-1β and IL-1 are the cytokines involved in progression of arthritis [Citation4]. IL-6 promotes inflammation through stimulation of growth in blood vessels. TNF-α, amplifies inflammation via stimulation of synovial fibroblasts that expresses cellular adhesion molecules and increase the leukocytes migration into the joints resulting in damage. IL-1 helps in bone resorption, cartilage destruction and in turn can modify the production of nitric oxide (NO) and prostaglandin (PGE2). PGE2 can stimulate pain receptors and induce fever [Citation5]. Thus, the imbalance which occurs between proinflammatory and anti-inflammatory state has resulted in synovial membrane inflammation and damage in the joints [Citation6].
Four variety of drugs are available for treatment of RA which includes steroid hormones, biological agents, immunosuppressant, anti-rheumatic drugs and anti-inflammatory drugs [Citation7–10]. The anti-arthritic drugs even though have various potent benefits but criteria such as high costs, side effects and their efficacy towards specific target site limits their clinical use [Citation11]. Reported side effects of RA include immune deficiency, gastrointestinal tract disorders, hormonal disturbances and complications in cardiovascular system [Citation12]. The beneficial compounds against RA are studied using various animal models to identify their therapeutic benefits which shares disease similarity with that of humans. Among other models of arthritis in rodents, Complete Freund’s Adjuvant (CFA) induced model displays various similarities with that of human arthritis which makes it most suitable model for inducing arthritis [Citation13]. Thus, the therapeutic approach for arthritis demands the drug to be economical, long life and minimal or less side effects. The drugs should also inhibit inflammation and proinflammatory cytokines expression thereby preventing damage of joints.
Previous literature evidences have reported that neem (Azadirachta indica A. Juss) is, a native of Indian subcontinent. The people of this region show high esteem to the tree region. In India, the tree is found in Andhra Pradesh, Karnataka and Tamil Nadu and it is frequently naturalized in sub-tropical and tropical regions of India. The leaves, flower, bark, fruit, root and seed of the plant have been proved to be beneficial in treating various diseases and used in industrial products as well. Leaves of the plant can be useful in treating eczema, diabetes and reducing fever. Barks are used in making toothbrush and roots helps to heal diseases and repellent against insects [Citation14]. This is eco-friendly and numerous researches has been made in this tree worldwide. The neem has numerous therapeutic properties such as antifungal, antifeedant, antibacterial, repellent, antidiabetic, antiulcer, antitumour, pesticide, anti-inflammatory, antimalarial, hypolipidemic, hepatoprotective, hypoglycemic and therefore commercially exploitable [Citation15].
Various therapeutic principles are isolated from various parts of the neem plant all of which gives the bitter nature to neem oil. The seeds of neem comprise tignic acid which gives distinguishing aroma to the oil [Citation16]. All these active principles contain numerous pharmacological properties.
Neem and its active principles have been testified in several disease pathologies, even though the action of nimbolide on inflammation of joints and mechanism through which it reduces inflammation is still unclear. Therefore, the study was carried out to examine the efficiency of nimbolide against joint inflammation which was induced by Freund’s adjuvant and to understand its potent ability in reducing the proinflammatory cytokines expression.
Materials and methods
Drugs and chemicals
Nimbolide, CFA and diclofenac sodium was purchased from Sigma Chemical Co., St. Louis, USA. ELISA kits for cytokines and Diagnostic kits for estimation of liver marker enzymes were procured from R&D Systems, Minneapolis, MN, USA. Antibodies such as cox-2, iNOS, Nf-kb, P-IkBα and IKKα were obtained from Thermo Scientific, USA. All other chemicals used for the experiments were freshly prepared and of analytical grade.
Animal procurement and maintenance
Male albino rats (150–200 g) of wistar stain were kept under standard animal house conditions under relative humidity and temperature. They are provided with free access to water and standard pellet diet. Prior 7 days to the start of the experiment, the rats were adjusted to the animal house environment. Experimental procedures used for the study were approved and the guidelines are followed strictly throughout the experimental period.
Experimental design
It consisted of four groups (n = 6) of six animals each.
Group I: Control rats were administered with DMSO orally
Group II: Rats were administered with single dose of 0.1 mL of CFA into right hind paw intradermally to induce arthritis
Group III: CFA induced arthritic rats treated with nimbolide (20 mg/kg per day) orally
Group IV: Arthritic rats induced with CFA were treated with Diclofenac Sodium (5 mg/kg per day) orally.
On 1st day, excluding control group, all the other groups of rats were given single dose of 0.1 mL of CFA into the right hind footpad intradermally. The rats of nimbolide groups were given nimbolide (20 mg/kg) and rats of diclofenac sodium were administered with diclofenac sodium (5 mg/kg per day) by oral gavage.
Diclofenac Sodium: Standard Drug. Both nimbolide and Diclofenac Sodium was dissolved in DMSO.
Arthritic rats treated with nimbolide showed increase in paw volume and the arthritic score throughout the experimental period. The biochemical parameters such as ALP, SGPT and SGOT, lipid peroxidation and antioxidant status were carried out. Estimation of cytokines were also performed. The tissues were homogenized and stored at −80 °C for western blot analysis. Animal tissues were collected, stored, and histological analysis was performed. Hind paw volume, organ indexes and body weight were calculated after sacrifice.
Measurement of body weight and hind paw volume
Hind paw volume of rats was measured on initial day (0th day) before CFA injection and subsequently for different time period till 25th day using plethysometer. The hind paw volume can be measured by subtracting final paw volume from the initial volume. Body weight was also measured from animals from 0th day till the end of the experimental period. Body weight can be calculate using difference between initial and final weight of animals [Citation17].
Organ indices measurement
After the end of the experimental period (i.e. on 25th day), the thymus and spleen were removed from the animals immediately and the organ weight was measured [Citation18]. The organ weight of the animals was calculated as the ratio of wet weight (mg) of spleen and thymus to the body weight of rats (g) [Citation19].
Measurement of arthritic score
The severity of arthritis in paw of animals was evaluated and then it was graded from 0 to 4. Grade 0 indicates absence of swelling; grade 1 denotes mild swelling or erythema in one of the fingers in paw; grade 2 shows swelling in one or more fingers of paw; grade 3 displays swelling of wrist or ankle; grade 4 specifies severe arthritic swelling in fingers and wrist. Score 8 is the highest arthritic score fixed for rats which are induced with CFA [Citation20].
Measurement of biochemical parameters
After the end of the experimental period (i.e. on 25th day), the rats were sacrificed and the blood was drawn from animals and serum was separated. The serum was used for estimation of liver marker enzymes such as SGOT, SGPT and ALP using standard laboratory protocols [Citation21].
Measurement of proinflammatory cytokines
The blood of experimental animals was collected and serum was separated by clotting at room temperature for 30 min [Citation22]. The protein concentration of serum proinflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-10 was evaluated using ELISA kits and the procedure was carried out according to manufacturer’s instructions.
Measurement of serum antioxidant and prooxidant activity
Malondialdehyde levels was estimated by the method of Devasagayam and Tarachand [Citation23] in serum of arthritic rats. The colour intensity was read at 532 nm and the result was expressed in nmol/mg protein. Antioxidant activities of superoxide dismutase (SOD), catalase and reduced glutathione was determined using standard protocols [Citation24–26].
Histological analysis
The ankle joints removed from arthritic rats are engrossed in formaldehyde (10%) for 24 h followed by immersion in 5% formic acid. Then the tissues are processed, sectioned and embedded at 5 m thickness. The sections were stained in Haematoxylin and Eosin stain and viewed under a light microscope which gives the localization of inflammatory cells present and destruction of joints [Citation27].
Western blot analysis
Proteins were separated by SDS-Polyacrylamide Gel Electrophoresis as described by Laemmli [Citation28]. About 30–40 µg of protein was separated. After protein separation the gel is transferred to PVDF membrane. The PVDF were blocked with skimmed milk powder (5%) for 12 h at room temperature. Following blocking, the blocking solution was decanted and the membrane was briefly rinsed in Tris Buffer Solution-Tween20 (TBS-T) and incubated for overnight in TBS containing suitably diluted primary antibody for cox-2, iNOS, Nf-kb, P-IkBα and IKKα. Subsequently after primary incubation the membrane was washed with TBS-T and further it was incubated with HRP-conjugated secondary antibody. The protein expression of cox-2, iNOS, Nf-kb, P-IkBα and IKKα were detected by chemiluminescence and corresponding densitometry analysis was performed. β-actin was loaded as the internal reference gene.
Statistical analysis (check with the statistical analysis)
Values are expressed as mean ± standard error mean (SEM) (n = 6) and analysed by one-way analysis of variance (ANOVA) followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group; Significance at p values <.05 and p values <.01 as given in tables and figures.
Results
Effect of nimbolide on organ indices, body weight and hind paw volume
The body weight of CFA induced rats was markedly decreased when compared with control rats. Treatment with nimbolide (20 mg/kg per day) showed marked increase in body weight in CFA when compared with CFA alone induced group (). The organ indices were increased significantly in CFA induced rats than control rats. Standard drug treated group also displayed significant reduction in organ indices when compared with group II animals ().
Figure 1. Effect of nimbolide on body weight, organ indexes, hind paw volume and arthritis index score in CFA induced rheumatoid arthritis rats. (a) Body weight, (b) organ indices, (c) hind paw volume, (d) arthritis index score. Group I: Control group; Group II: Arthritis induced group; Group III: Arthritis induced + nimbolide group and Group IV: Arthritis induced + Diclofenac Sodium. Values expressed as mean ± SEM (n = 6) and analysed by one-way ANOVA followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group.
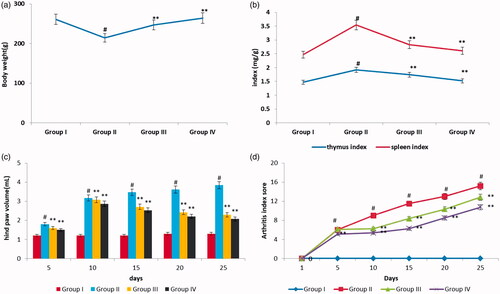
Hind paw volume of group II rats was increased markedly than group I. Nimbolide treated and diclofenac sodium treated arthritic rats showed decrease in paw volume comparable with CFA induced rats (). CFA induced rats resulted in increase in arthritic score index compared with control animals. CFA induced rats administered with nimbolide and diclofenac sodium displayed decline in arthritic score in comparison to that of group II animals.
Arthritic rats showed marked rise in arthritic index score with that of control rats. Nimbolide treated rats portrayed clear decrease in arthritic score with that of group II rats. Diclofenac sodium treated rats showed also displayed reduction in arthritic score when compared with CFA induced rats ().
Effect of nimbolide on biochemical parameters
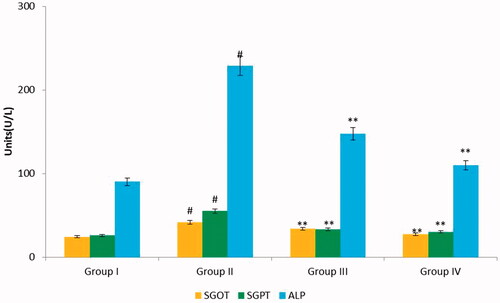
The cellular integrity in CFA induced arthritic rats was assessed by determining the activities of liver marker enzymes such as SGOT, SGPT and ALP as shown in . Assessment of liver marker enzymes in serum of various experimental groups dictates that the levels of these enzymes were elevated in CFA induced arthritic rats (Group II). There was a marked decrease in the activities of the marker enzymes in nimbolide and diclofenac sodium treated animals. The result thus signifies that the impact of arthritis is CFA induced group is severe and supplementation of nimbolide and diclofenac sodium has significantly brought down the activities of the enzymes in group III and group IV rats when compared to group II rats.
Figure 2. Effect of nimbolide on SGOT, SGPT and ALP parameters in CFA-induced arthritis in rats. Units: SGOT, SGPT and ALP- U/L. Group I: Control group; Group II: Arthritis-induced group; Group III: Arthritis induced + nimbolide group and Group IV: Arthritis induced + Diclofenac Sodium. Values expressed as mean ± SEM (n = 6) and analysed by one-way ANOVA followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group.
Effect of nimbolide on cytokines
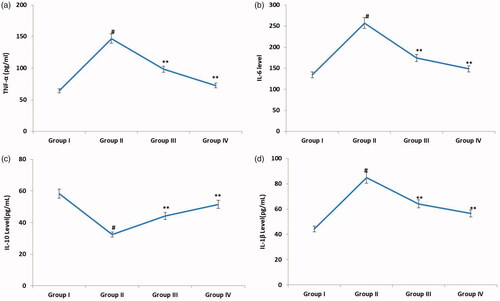
displays the serum levels of proinflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-10 in various experimental groups. Assessment of serum levels of cytokines in various experimental groups revealed that there was a significant increase in serum levels of TNF-α, IL-6, IL-1β and IL-10 in group II rats when compared with control animals. Upon supplementation with nimbolide and diclofenac sodium, the serum levels of TNF-α, IL-6, IL-1β and IL-10 were markedly brought down in group III and group IV rats, when compared to the untreated counterparts.
Figure 3. Effect of nimbolide on the levels of TNF-α, IL-6, IL-10 and IL-1β in CFA-induced rheumatoid arthritis rats. Units: TNF-α, IL-6, IL-10 and IL-1β- pg/mL. Group I: Control group; Group II: Arthritis induced group; Group III: Arthritis induced + nimbolide group and Group IV: Arthritis induced + Diclofenac Sodium. Values expressed as mean ± SEM (n = 6) and analysed by one-way ANOVA followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group.
Effect of nimbolide on lipid peroxides and antioxidant status
shows the impact of nimbolide on the levels of MDA in serum of various experimental rats. Assessment of MDA levels in various experimental groups dictate that MDA levels were elevated in arthritis induced animals. On nimbolide and diclofenac sodium supplementation, the MDA levels were markedly brought down in group III and group IV rats in comparison with CFA alone administered rats.
Figure 4. Effect of nimbolide on oxidative stress in CFA-induced rheumatoid arthritis rats. (a) MDA level (b) Antioxidant enzyme activities of SOD, catalase and GSH. Units: SOD, CAT and GSH- U/mg protein. Group I: Control group; Group II: Arthritis induced group; Group III: Arthritis induced + nimbolide group and Group IV: Arthritis induced + Diclofenac Sodium. Values expressed as mean ± SEM (n = 6) and analysed by one-way ANOVA followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group.
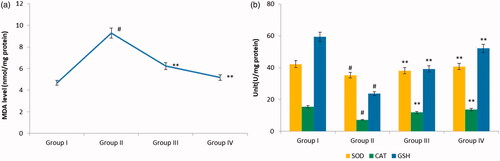
Status of oxidative stress in CFA induced animals can be evaluated by measuring the levels of antioxidants. CFA induced animals exhibited marked decrease in levels of antioxidant enzymes when compared with control rats. Treatment groups showed marked improvement in the activities of antioxidant enzymes when compared with CFA alone administered rats ().
Histopathological analysis
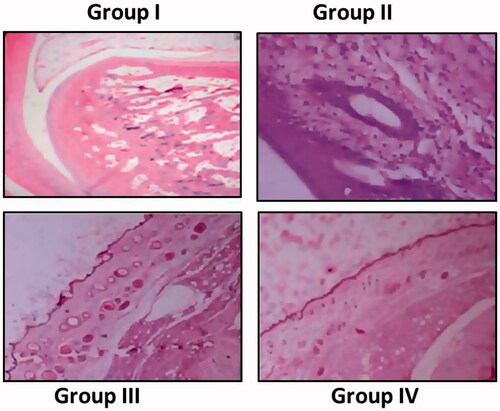
represents the changes observed in the hind paw of the experimental groups. Group I show regular histological patterns of hind paw of the control group. Abnormalities in the hind paw was observed in Group II rats which showed edema, infiltrated inflammatory cells accumulation, degeneration of cartilage and bone marrow destruction. Nimbolide treated rats (Group III) showed less inflammatory signs such as improved bone marrow, absence of edema, and less cellular infiltration. Diclofenac sodium (Group IV) showed marked reduction of inflammation, no of destruction in cartilages and bone marrow found to be normal with minimal cellular infiltrates.
Effect of nimbolide on protein expression of iNOS, Nf-kb, cox-2, P-IkBα and IKKα
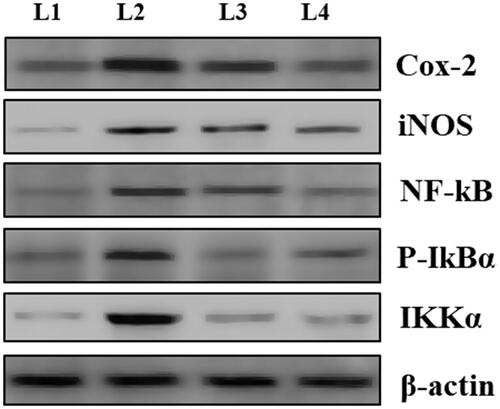
display the nimbolide effect on protein expression of iNOS, Nf-kb, cox-2, P-IkBα and IKKα in control and experimental animals. Results from the study showed that there was a marked upregulation in the protein levels of cox-2, iNOS, Nf-kb, P-IkBα and IKKα in group II rats in comparison to group I animals. Nimbolide supplementation brought down the levels of iNOS, P-IkBα, Nf-kb, cox-2, and IKKα to near normalcy in treated rats to that of their respective untreated counter parts. Diclofenac sodium treated RA rats showed even low levels of protein expression than nimbolide treated rats and group IV rats displayed near normalcy values when compared with that of normal control animals.
Figure 6. Effect of nimbolide on protein expression of cox-2, iNOS, Nf-kb, P-IkBα and IKKα in CFA-induced rheumatoid arthritis rats. L1-Group I: Control group; L2-Group II: Arthritis induced group; L3-Group III: Arthritis induced + nimbolide group and L5-Group IV: Arthritis induced + Diclofenac Sodium. Values expressed as mean ± SEM (n = 6) and analysed by one-way ANOVA followed by Tukey’s Kramer test. #p < .05 as compared to NC group. *p < .01 as compared to CFA group.
Discussion
RA is disease causing inflammation of the joints which affects around 1% of the adult population worldwide in which the causative factor remains unknown [Citation29,Citation30]. It not only affects joints but it also contains close association with immune and other organ systems. Therapeutic approach required for developing drugs for RA demands biochemical and pathological characteristics of arthritis [Citation30]. Arthritis induced with CFA is proved to be beneficial in evaluating the novelty of drug for treating RA in humans. CFA induced models is used commonly for assessing the anti-arthritic potency and anti-inflammatory of drugs used for treatment against RA [Citation31]. CFA induced arthritis model of rats shares numerous similarities with that of RA in humans which makes it more appropriate model for the study [Citation32].
CFA administered intradermally into rat paw shows local inflammatory reaction and chronic systemic reaction and it also exhibits strong reactivity to heat shock proteins and proteoglycans of cartilage. The local reactions following CFA induction subsides around 3–4 days however the chronic phase can prolong from 2 weeks to several months [Citation33]. Inflammatory mediators which are released following CFA induction include cytokines, prostaglandins, lysosomal and hydrolytic enzymes. Body weight change in the animals induced with CFA acts as a vital factor for evaluating the response of the drug and the duration of the disease towards inflammation [Citation34]. Significant changes in body weight noted in the CFA induced rats is in concurrence with the earlier study of Mondal et al. [Citation35] which showed that inflammation resulting from arthritis might have affected the body weight in CFA induced animals than control animals. Improvement in body weight was seen in animals treated with nimbolide which is due to its anti-inflammatory efficacy of the neem extracts [Citation36].
The drugs used for treating CFA induced model of rats are assessed using changes in arthritic index score, body weight and swelling of the hind paw volume. CFA induced animals show significant weight loss and body weight restoration following nimbolide administration which is closely related with upregulated expression of cytokines.
Immune organs, spleen and thymus which plays an important role by serving as a reservoir for antibody storage [Citation37]. The weight of these organs is used for evaluating the immunomodulatory efficacy of the drugs used for treating RA [Citation38]. The organ indices were increased in arthritic animals whereas nimbolide treatment significantly decreased the indices of these organs. The antiarthritic and immunostimulatory effect of nimbolide from neem has downregulated the organ indices in arthritis induced rats.
Besides these changes, animals induced with arthritis was also found to show high arthritic index score and paw swelling [Citation39]. Paw swelling indicates the measure of intensity of inflammation in CFA induced rats and reduction in paw swelling following treatment indicates the antiarthritic activity of the drug [Citation40]. The findings of the study displayed increase in paw volume of the group II rats following inflammation to a significant extent which is in unison with previous findings. Nimbolide treatment significantly decreased the paw volume in group II rats. Inflammation results in increased accumulation of monocytes and granulocytes in the first phase. Macrophages activation in turn activate the proinflammatory cytokines such as TNF-α and IL-6 [Citation41]. Arthritic index score is also increased to significant extent in CFA induced rats [Citation42] and nimbolide treatment reduced the arthritic index score.
Serum of RA induced rats showed increase in the activities of liver marker enzymes. Increase in the levels of the liver enzymes depicts damage in liver architecture and release of the enzymes into circulation. Estimating the levels of biomarkers in serum is important feature of arthritis which reflects the hepatic and renal damages [Citation21]. The study indicates significant increase in levels of marker enzymes of liver in group II which reflects the damage in liver cells [Citation43]. Nimbolide treatment significantly lowered the levels of liver marker enzymes in rats induced with CFA thus proving its efficacy in protecting liver against arthritis. Literature evidences of Banwra et al. [Citation44] showed that neem leaf aqueous extract was shown to be protective in preventing liver damage which correlates with our present observation.
The factors which are closely associated with RA is found to disturb the state of equilibrium between proinflammatory and anti-inflammatory molecules [Citation45]. Cytokines are the key factors that are involved in stages of inflammatory changes [Citation43]. Cytokines are elevated markedly during initial phase of inflammation [Citation46]. IL-12 and TNF-α were highly activated in inflammatory cells [Citation47]. IL-6 plays a main role in RA pathogenesis in humans and in other associated inflammatory changes [Citation48]. The observations of the study reported marked increase in cytokines levels in group II rats which is in correlation with earlier evidences. Nimbolide treatment has reduced the levels of these cytokines markedly. The results of the study correlate with literature evidences which shows that nimbolide present in neem leaf extract has inhibited inflammation which is facilitated by TNF-α via ROS levels reduction [Citation36].
Lipid peroxidation results from oxidative damage of membrane lipids which forms MDA [Citation49]. Increased levels of MDA in group II rats has altered cellular membranes structure and function which eventually leads to increased ROS production [Citation50]. Antioxidant property of nimbolide has significantly decreased the MDA levels which correlates with the current results [Citation51,Citation52]. CFA administered rats also displayed marked decline in activity of antioxidant enzymes which might be due to accumulation of MDA that might have reduced the levels of CAT through inhibition of protein synthesis [Citation53]. Glutathione depletion results in drastic metabolic effects which leads to increased sensitivity in cells [Citation54]. Nimbolide treatment significantly brought back the activities of antioxidant enzymes which proves the protective effect of nimbolide as an antioxidant against arthritis [Citation55].
In addition to this, arthritis was suppressed by nimbolide (20 mg/kg per day) treatment which was further confirmed by histopathological analysis. Nimbolide treatment reduced the cellular infiltration, edema formation and inflammation in arthritis induced rats. The western blot analysis of cox-2, iNOS, Nf-kb, P-IkBα and IKKα also showed significant upregulation in expression in CFA induced arthritic rats. Nimbolide treatment significantly downregulated the expression of these inflammatory proteins. The anti-inflammatory efficacy of neem extract has decreased the edema and prevented arthritis progression in CFA induced arthritic rats. Thus, the findings of the study propose that nimbolide might prevent the arthritis and may reduce the inflammation and joints destruction in arthritis induced animals.
Conclusion
The observation of the study substantiates that nimbolide was found to be protective against rats induced with CFA. It has reduced body weight, alleviate paw swelling, reduced organ indices, reduce arthritic score and decrease the inflammatory cytokines levels. The study similarly validated the promising anti-arthritic activity of nimbolide which was assessed using biochemical, histopathological and molecular methodologies. Thus, these observations substantiate that nimbolide can be used as an effective drug candidate in arthritis treatment because of its wide range of properties. However, studies should be carried out in future to understand the mechanistic action of nimbolide against arthritis which possibly will contribute to the advancement of effective treatment strategies against RA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mcinnes B, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219.
- Chang X, He H, Zhu L, et al. Protective effect of apigenin on Freund’s complete adjuvant‐induced arthritis in rats via inhibiting P2X7/NF‐kappaB pathway. Chem Biol Interact. 2015;236:41–46.
- Mendoza-Vázquez G, Rocha-Muñoz AD, Guerra-Soto AJ, et al. Artritis reumatoide y dislipidemias. El Residente. 2013;8:12–22.
- Zhu L, Chen T, Chang X, et al. Salidroside ameliorates arthritis‐induced brain cognition deficits by regulating Rho/ROCK/NF‐kappaB pathway. Neuropharmacology. 2016;103:134–142.
- Campbell K, Piccoli DS, Hamilton JA. Stimulation of human chondrocyte prostaglandin E2 production by recombinant human interleukin-1 and tumour necrosis factor. Biochim Biophys Acta. 1990;1051:310–318.
- Chimenti MS, Triggianese P, Conigliaro P, et al. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 2015;6:e1887.
- Lima-Garcia JF, Dutra RC, da Silva K, et al. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293.
- Momohara S, Kawakami K, Iwamoto T, et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21:469–475.
- Pincus T, Cutolo M. Clinical trials documenting the efficacy of low-dose glucocorticoids in rheumatoid arthritis. Neuroimmunomodulation. 2015;22:46–50.
- Atzeni F, Benucci M, Sallì S, et al. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev. 2013;12:575–579.
- Ekambaram S, Perumal SS, Subramanian V. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complement Altern Med. 2010;10:56.
- Bansod S, Kagathara VG, Pujari RR, et al. Therapeutic effect of a poly-herbal preparation on adjuvant induced arthritis in wistar rats. Int J Pharm Pharm Sci. 2011;3:186–192.
- Ghosh S, Mehla RK, Sirohi SK, et al. The effect of dietary garlic supplementation on body weight gain, feed intake, feed conversion efficiency, faecal score, faecal coliform count and feeding cost in crossbred dairy calves. Trop Anim Health Prod. 2010;42:961–963.
- Ragasa CY, Nacpil ZD, Natividad GM, et al. Tetranortriterpenoids from Azadirachta indica. J Phytochem. 1997;46:555–558.
- Mousa MAA, El-Ashram AMM, Hamed M. Effect of neem leaf extract on freshwater fishes and Zooplankton community. Proceedings of the 8th International Symposium on Tilapia in Aquaculture; 2008 Oct 12–14; Cairo, Egypt.
- Sharma P, Tomar L, Bachwani M, et al. Review on neem (Azadirechta indica): thousand problem one solution. Int Res J Pharm. 2011;2:97–102.
- Lee DY, Choo BK, Yoon T, et al. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;121:28–34.
- Hu F, Hepburn R, Li Y, et al. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J Ethnopharmacol. 2005;100:276–283.
- Zhang YQ, Xu W, Li H, et al. Therapeutic effects of total alkaloids of Tripterygium wilfordii Hook f. on collagen-induced arthritis in rats. J Ethnopharmacol. 2013;145:699–705.
- Paval J, Kaitheri SK, Potu BK, et al. Anti-arthritic potential of the plant Justicia gendarussa Burm F. Clinics. 2009;64:357–362.
- Mythilypriya R, Shanthi P, Sachdanandam P. Salubrious effect of Kalpaamruthaa, a modified indigenous preparation in adjuvant induced arthritis in rats: a biochemical approach. Chem Biol Interact. 2008;173:148–158.
- Zheng CJ, Zhao XX, Ai HW, et al. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine. 2014;21:838–846.
- Devasagayam TP, Tarachand U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun. 1987;145:134–138.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78.
- Patil MVK, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomed Aging Pathol. 2012;2:6–15.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010;36:385–404.
- Bax M, van Heemst J, Huizinga TW, et al. Genetics of rheumatoid arthritis: what have we learned? Immunogenetics. 2011;63:459–466.
- Lin B, Zhao Y, Han P, et al. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund’s adjuvant induced arthritis in rats. J Ethnopharmacol. 2014;155:8248–8255.
- Tag HM, Kelany OE, Tantawy HM, et al. Potential anti-inflammatory effect of lemon and hot pepper extracts on adjuvant-induced arthritis in mice. J Basic Appl Zool. 2014;67:149–157.
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
- Naik SR, Wala SM. Arthritis, a complex connective and synovial joint destructive autoimmune diseases: animal models of arthritis with varied etiopathology and its significance. J Postgrad Med. 2014;60:309–317.
- Mondal P, Das S, Mahato K, et al. Evaluation of anti-arthritic potential of the hydro-alcoholic extract of the stem bark of Plumeria rubra in Freund’s complete adjuvant-induced arthritis in rats. IJPSR. 2016;7:3675–3688.
- Kausik B, Ishita C, Ranjiit K, et al. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002;82:1336–1345.
- Sundaram R, Naresh R, Shanthi P, et al. Antihyperglycemic effect of iridoid glucoside, isolated from the leaves of Vitex negundo in streptozotocin-induced diabetic rats with special reference to glycoprotein components. Phytomedicine. 2012;19:211–216.
- Zhang LL, Wei W, Yan SX, et al. Therapeutic effects of glucosides of Cheanomeles speciosa on collagen-induced arthritis in mice. Acta Pharmacol Sin. 2004;25:1495–1501.
- Zhang X, Dong Y, Dong H, et al. Investigation of the effect of phlomisoside F on complete Freund’s adjuvant-induced arthritis. Exp Ther Med. 2017;13:710–716.
- Rajendran R, Krishnakumar E. Anti-arthritic activity of Premna serratifolia Linn., wood against adjuvant induced arthritis. Avicenna J Med Biotechnol. 2010;2:101–106.
- Yu Y, Xiong Z, Lv Y, et al. In vivo evaluation of early disease progression by X-ray phase-contrast imaging in the adjuvant-induced arthritic rat. Skeletal Radiol. 2006;35:156–164.
- Ingawale DK, Patel SS. Hecogenin exhibits anti-arthritic activity in rats through suppression of pro-inflammatory cytokines in Complete Freund’s adjuvant-induced arthritis. Immunopharmacol Immunotoxicol. 2018;40:59–71.
- Hackett TL, Holloway R, Holgate ST, et al. Dynamics of proinflammatory and anti-inflammatory cytokine release during acute inflammation in chronic obstructive pulmonary disease: an ex vivo study. Respir Res. 2008;9:47.
- Bhanwra S, Singh J, Khosla P. Effects of Azadirachta indica (Neem) leaf aqueous extracts on paracetamol induced liver damage in rats. Physiol Pharmacol. 2000;44:64–68.
- Ferraccioli G, Bracci-Laudiero L, Alivernini S, et al. Interleukin-1 b and Interleukin-6 in arthritis animal models: roles in the early phase of transition from acute to chronic inflammation and relevance for human rheumatoid arthritis. Mol Med. 2010;16:552–557.
- Zuo J, Xia Y, Li X, et al. Therapeutic effects of dichloromethane fraction of Securidaca inappendiculata on adjuvant-induced arthritis in rat. J Ethnopharmacol. 2014;153:352–358.
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440.
- Ho CY, Weng CJ, Jhang JJ, et al. Diallyl sulfide as a potential dietary agent to reduce TNF-α- and histamine-induced proinflammatory responses in A7r5 cells. Mol Nutr Food Res. 2014;58:1069–1078.
- Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–215.
- Halliwell B, Aeschbach R, Löliger J, et al. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617.
- Arivazhagan S, Balasenthil S, Nagini S. Modulatory effects of garlic and neem leaf extracts on N-methyl-N?-nitro-N-nitrosoguanidine (MNNG)-induced oxidative stress in Wistar rats. Cell Biochem Funct. 2000;18:17–21.
- Arivazhagan S, Balasenthil S, Nagini S. Garlic and neem leaf extracts enhance hepatic glutathione and glutathione dependent enzymes duringN-methyl-N?-nitro-N-nitrosoguanidine (MNNG)-induced gastric carcinogenesis in rats. Phytother Res. 2000;14:291–293.
- Fahmy SR, Hamdi S. Antioxidant effect of the Egyptian freshwater Procambarus clarkii extract in rat liver and erythrocytes. Afr J Pharm Pharmacol. 2011;5:776–785.
- Limón-Pacheco JH, Hernández NA, Fanjul-Moles ML, et al. Glutathione depletion activates mitogen-activated protein kinase (MAPK) pathways that display organ-specific responses and brain protection in mice. Free Radic Biol Med. 2007;43:1335–1347.
- Rao AD, Devi KN, Thyagaraju K. Isolation of antioxidant principle from Azadirachta seed kernels: determination of its role on plant lipoxygenases. J Enzyme Inhib. 1998;14:85–86.