Abstract
Osteoporosis-related bone fracture and falls have a severe impact on patients’ daily lives. Osteoblasts are bone-building cells that play a vital role in bone formation and remodeling. Imbalanced osteoblast differentiation could lead to osteoporosis. GPR39 is an orphan G protein-coupled receptor that mediates metabolic pathways. In this study, we show that GPR39 is expressed in MC3T3-E1 cells. Osteoblast differentiation culture media induces GPR39, suggesting that GPR39 is a differentiation-responsive factor. Activation of GPR39 using its selective agonist TC-G 1008 induces alkaline phosphatase (ALP), osteocalcin (OCN), and type I collagen (Col-I) expression, and increases cellular ALP activity and calcium deposition, implying that GPR activation promotes cells toward osteoblast differentiation. Treatment with TC-G 1008 also increases Runx-2 expression and AMPK activation. However, the inhibition of AMPK by Compound C abolished TC-G 1008-mediated ALP, OCN, and Col-I induction, and reduces ALP activity and cellular calcium deposition as well as Runx-2 induction. These data indicate that TC-G 1008-mediated GPR39 activation involves AMPK-mediated Runx-2 induction. In summary, our study uncovers a new role of GPR39 activation in osteoblast differentiation, implying that GPR39 could be a promising therapeutic target for osteoporosis.
Introduction
Osteoporosis is a common disease characterized by weakened bone structure and low bone mass. Osteoporosis-related bone fracture and fall in aged people are severe threats to lives of patients. Often, osteoporosis goes undiagnosed due to its silent nature. The disease is usually recognized after the occurrence of a fall or facture, but by this time, it is too late to implement preventive measurements [Citation1]. Osteoporosis occurs when the body fails to control the balance between bone formation and resorption. In healthy conditions, bone undergoes constant modeling and remodeling to absorb mature tissue and synthesize new tissue [Citation2]. The formation of new bone tissue is predominantly dependent on the function of osteoblasts. In adults, osteoblasts and adipocytes come from the same type of progenitor cells—mesenchymal stem cells (MSC). The fate of MSC is dependent on many local environmental factors as well as transcriptional regulation. Runx-2 is the key regulator in determining the direction of osteoblast differentiation from MSC [Citation3]. As osteoblasts reach terminal differentiation, they secrete different matrix proteins to replenish the bone matrix, such as collagen type 1 (Col-1), osteocalcin (OCN), and alkaline phosphatase (ALP) [Citation4]. Col-1 forms the collagen fiber and serves as the scaffold for bone tissue. ALP generates calcium phosphate to be deposited in mature osteoblasts and contributes to bone mineralization, while OCN is the most abundant protein involved in regulating mineralization [Citation5]. The age-associated reduction in the functional capacity of osteoblasts is one of the major contributors to bone loss. In aged bone, the replenishment of old osteoblasts is unable to meet the body’s needs for bone remodeling. Therefore, understanding the mechanism that controls osteoblast regeneration is fundamental to the prevention and treatment of osteoporosis [Citation6].
G protein-coupled receptors (GPCRs) mediate various key biological processes in eukaryotes. GPCRs are also highly important drug targets and medications that target GPCRs account for about 34% of available drugs [Citation7]. Among the more than 800 GPCRs, a small portion of them lack endogenous ligands. These are referred to as orphan GPCRs. Orphan GPCRs are highly regarded as a novel class of drug targets. GPR39 is a conserved orphan GPCR that has been shown to be involved in zinc ion transport as well as other aspects of metabolic regulation [Citation8]. Recently, a new compound named TC-G 1008 (also named as GPR39-C3) was developed based on 2-pyridylpyrimidines and has been shown to be highly specific in binding to GPR39, making it a powerful tool to investigate the function of this receptor in diverse cell types [Citation9,Citation10]. GPR39 has been shown to be expressed in different osteoblast cell lines [Citation11]. In the present study, we sought to investigate the control mechanism behind osteogenic differentiation. Thanks to the successful development of the potent GPR39 agonist TC-G 1008, we are able to pursue a deeper understanding of the involvement of GPR39 in bone. Here, we explored the role of GPR39 signaling in osteoblast differentiation using an in vitro cell culture and differentiation model MC3TC-E1 cells.
Materials and methods
Cell culture, treatment
We purchased murine MC3T3-E1 cells and human SHSY-5Y cells from ATCC. These cells were grown in DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin. Human bone marrow-derived mesenchymal stromal cells (BM-MSCs) were isolated from fresh bone marrow aspirates from 11- to 16 year-old donors with informed consent as previously described [Citation12]. Mononuclear adherent cells were washed and collected. All experimental protocols were reviewed and approved by the Institutional ethics committee of Shandong University. To induce osteoblast differentiation, MC3T3-E1 cells were switched to osteogenic medium containing BMP-2 for 14 days. To obtain samples from the different time points, normal or differentiated cell cultures were harvested for further analysis on days 0, 3, 7 and 14. We purchased GPR39 agonist TC-G 1008 from Sigma-Aldrich and used a 10 µM working concentration. The AMPK inhibitor Compound C was from Sigma-Aldrich and dissolved in culture media at a final concentration of 10 µM.
RT-PCR and real-time PCR
The MC3T3-E1 cells cultured in the different media and for the different time lengths were collected for analysis of osteoblast gene expression. To obtain the total cell RNA, a PolyATtract® mRNA Isolation System (Promega) was used to extract RNA from whole cell lysate in accordance with the manufacturer’s instructions. A semi-quantitative PCR approach was used to determine the mRNA expression of GPR39. cDNA was synthesized with 1 µg of RNA using a SuperScript II kit (Invitrogen). The reaction mixture was then diluted 1:20 with ddH2O. A pair of GPR39 specific primers were applied to synthesize a product from the diluted cDNA mix. The PCR product was then electrophoresed by 1.5% agarose gel, and the relative intensity of bands was quantified by Image J software (NIH). SYBR-based real-time PCR experiments were performed on the an ABI system (Vii, ABI) to profile the expression of Runx-2, ALP, OCN, COL-I, and GAPDH. The final results were quantitated using the standard ΔΔCt relative quantification method with GAPHD as the housekeeping control.
Western blot
The whole-cell lysates of MC3T3-E1 cells were prepared with RIPA buffer. Then, 20 μg of protein mixture was run on 10% SDS-PAGE and transferred to PVDF membranes. Samples were blocked with 5% dry milk, followed by a 2-h incubation with each primary antibody and a 1-h incubation with HRP-linked secondary antibody at RT. Chemiluminescent protein bands were visualized with an ECL reagent.
Alkaline phosphatase (ALP) activity
To measure ALP activity, the triplicates of differentiated MC3T3-E1 cells were seeded. ALP activity was measured using a kit (ABCAM, ab83369) following the product’s manual. Briefly, the samples and pNPP substrate (p-nitrophenyl phosphate) were mixed and reacted for 60 min, and the reaction was stopped with stop solution. The microplate was then read at 405 nM wavelength, and ALP activity was calculated based on the plot of standard samples.
Calcium deposition assay
Briefly, cells grown in a 6-well plate were stained with Alizarin Red S at 37 °C overnight. Fifty images of each group were taken using a fluorescent microscope at random locations. The images were quantitated with Image J software (NIH). The final data were obtained by comparing to non-differentiated cells at each condition.
Statistical analysis
All experimental data were collected based on the mean (±SD) of each replicate. The means of two groups with normal distribution was tested using a two-tailed, unpaired t-test. The mean difference between multiple groups was tested by ANOVA. p < .05 was considered to be statistically significant.
Results
GPR39 is fairly expressed in pre-osteoblast MC3T3-E1 cells
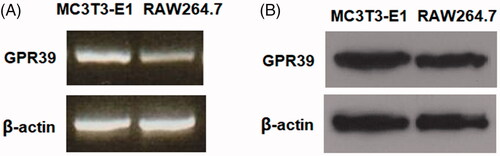
We first assessed whether GPR39 is expressed in MC3T3-E1 cells. To estimate the abundance of GPR39, RAW264.7 cells were chosen as a reference, as they are known to have a high expression level of GPR39. As determined by RT-PCR, the expression of GPR39 in MCCT3-E1 cells was reasonably comparable to its level in RAW264.7 cells (). Using western blot, we confirmed that GPR39 is relatively highly expressed at the protein level in MCCT3-E1 cells (). Human bone marrow-derived mesenchymal stromal cells (BM-MSCs) have been identified as a source of pluripotent cells possessing high self-renewal and multipotent properties. Therefore, we further investigated whether GPR39 is expressed in human BM-MSCs with human SHSY-5Y cells as a reference [Citation13]. RT-PCR analysis and western blot results indicate that GPR39 could be detected in human BM-MSCs (Supplementary Figure 1).
GPR39 is induced during osteoblast differentiation
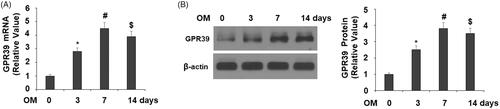
To explore the role of GPR39 in osteoblast differentiation, we assessed the temporal expression profile of GPR39 in cultured MC3TC-E1 cells. The transcript of GPR39 was induced during a 14-day differentiation period, which reached a peak of 4.5-fold at day 7 as compared to non-differentiated cells (). We observed a similar induction trend at the protein level, with peak induction of 4-fold at day 7 ().
GPR39 agonist TC-G 1008 promotes osteoblast differentiation
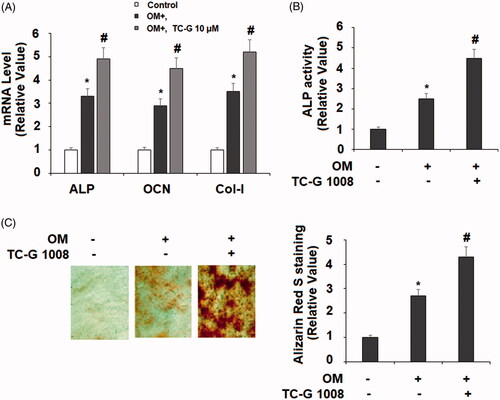
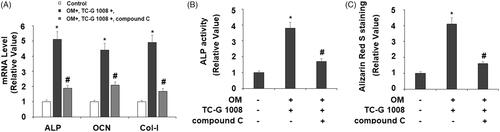
Next, we set out to explore the function of GPR39 in osteoblast differentiation. To activate GPR39 in cultured MC3T3-E1 cells, the selective agonist TC-G 1008 was added to the osteogenic medium. We assessed the influence of GPR39 activation on the phenotype of differentiated osteoblasts. Compared to its absence group, treatment with TC-G 1008 for 14 days induced higher mRNA transcripts of ALP, OCN, and Col1-I (), higher ALP activity () and more calcium deposition, as revealed by Alizarin Red S staining (). Based on these measurements, we got the primary conclusion that the activation of GPR39 promotes MC3T3-E1 towards osteoblast differentiation.
Figure 3. TC-G 1008 stimulated the differentiation and mineralization of MC3T3-E1 cells. Cells were incubated with osteogenic medium (OM) with or without GPR39 agonist TC-G 1008 (10 μM) for 14 days. (A) mRNA levels of ALP, OCN, and Col-I; (B. ALP activity; (C. Alizarin Red S staining (*, #, p < .01).
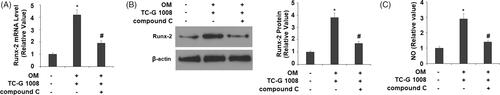
TC-G 1008 increases Runx-2 expression
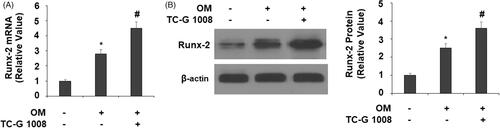
Since GPR39 activation positively influenced osteoblast differentiation, we hypothesized that it would also increase the expression of Runx-2, the master regulator of osteoblast differentiation. Indeed, our experiments confirmed this hypothesis. At the mRNA level and compared to its absence group, TC-G 1008 resulted in significantly higher mRNA and protein expression of Runx-2 ().
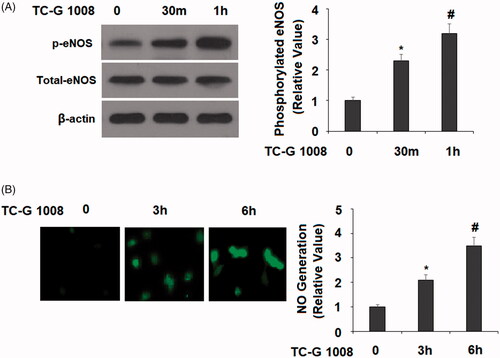
TC-G 1008 promotes eNOS phosphorylation and nitric oxide production
Nitric oxide (NO) has been shown to be involved in the process of bone formation. In osteoblast cells, NO is produced by endothelial nitric oxide synthase (eNOS). We examined the activation status of eNOS and NO contents in our time-course experiment. The results show that TC-G 1008 treatment for 30 and 60 min increased induction of eNOS phosphorylation by about 2- and 3-fold, respectively (). Meanwhile, TC-G 1008 treatment resulted in about 2- and 3.5-fold higher NO production, respectively ().
Figure 5. TC-G 1008 increased the phosphorylation of eNOS and the generation of nitric oxide (NO). (A). Cells were stimulated with TC-G 1008 (10 μM) for 0.5, 1 h. Phosphorylated eNOS was measured; (B) cells were stimulated with TC-G 1008 (10 μM) for 3, 6 h. Generation of NO was measured by DAF FM DA (*, #, p < .01).
TC-G 1008 promotes AMPK activation
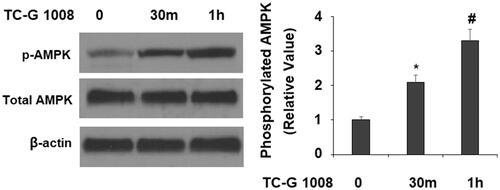
AMP-activated protein kinase (AMPK) has been implicated in local energy control and osteoblast differentiation. We questioned whether the expression of Runx-2 promoted by TC-G 1008 would affect AMPK activation. In the same time-course experiment, TC-G 1008 treatment for 30 and 60 min increased the phosphorylation of AMPK by about 2- and 3-fold, respectively, indicating that this is indeed the manner in which TC-G 1008 activated AMPK ().
Inhibition of AMPK activation attenuates TC-G 1008-mediated osteoblast differentiation and mineralization
We then investigated the consequences of the blockage of AMPK on TC-G 1008-mediated osteoblast differentiation. In the same 14-day treatment experiment, we added compound C to block AMPK activation and assessed osteoblast signature genes, ALP activity, and calcium deposition. Compared to the TC-G 1008 treatment group, the addition of compound C reduced the expression of ALP, OCN, and Col-I by about half or more respectively (), also suppressed half of ALP activity () and more than half of deposited calcium (), suggesting blockage of AMPK partially abolished the effect of TC-G 1008.
Figure 7. Treatment with the AMPK inhibitor compound C suppressed the effects of TC-G 1008 on the differentiation and mineralization of MC3T3-E1 cells. Cells were incubated with osteogenic medium (OM) and TC-G 1008 (10 μM) with or without compound C (10 μM) for 14 days. (A) mRNA levels of ALP, OCN, and Col-I; (B) ALP activity; (C) Alizarin Red S staining (*, #, p < .01).
Inhibition of AMPK activation attenuates TC-G 1008-mediated runx-2 and NO induction
Finally, we evaluated whether the inhibition of AMPK activation would affect TC-G 1008-mediated Runx-2 expression. Compared to the compound C-free group, inactivation of AMPK by compound C reduced Runx-2 mRNA and protein expression by more than half (). Therefore, blockage of AMPK attenuated TC-G 1008-mediated Runx-2 induction. Importantly, we also found that co-treatment with compound C inhibited TC-G 1008- induced production of NO ().
Inhibition of eNOS activation attenuates TC-G 1008-mediated osteoblast differentiation and mineralization
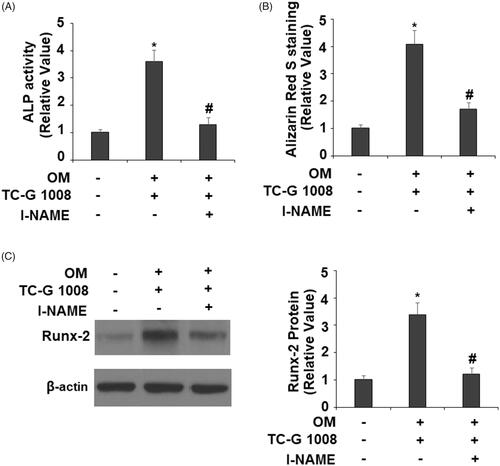
Activation of eNOS plays a critical role in regulating the mineralization of osteoblasts [Citation14]. To further confirm the involvement of eNOS in the effects of TC-G 1008-mediated osteoblast differentiation and mineralization, l-NAME (1 mM), an eNOS inhibitor, was used. The results indicate that the presence of l-NAME significantly abolished TC-G 1008-induced increase in ALP activity () and Alizarin Red S staining (). Notably, inhibition of eNOS reduced TC-G 1008-mediated expression of Runx-2 (). These findings suggest that eNOS is involved in TC-G 1008-induced reactions in MC3T3-E1 cells.
Figure 9. Treatment with the eNOS inhibitor l-NAME suppressed the effects of TC-G 1008 on the differentiation and mineralization of MC3T3-E1 cell. Cells were incubated with osteogenic medium (OM) and TC-G 1008 (10 μM) with or without l-NAME (1 mM). (A). ALP activity; (B). Alizarin Red S staining (C). Expression of RUNX-2 was measured by western blot analysis (*, #, p < .01).
Discussion
TC-G 1008, a newly synthesized compound based on 2-pyridylpyrimidines, has been shown to increase the level of GLP-1 [Citation9]. Recent research reveals that the administration of TC-G 1008 exerts an anti-depressive effect in animal experiments [Citation15]. These evidences indicate that TC-G 1008-mediated GPR39 activation could have different roles in various cell types, but these studies all agree that TC-G 1008 is a highly specific agonist of the GPR39 receptor. In our study, we utilized this agonist as an efficient approach to investigate the involvement of the GPR39 pathway in osteoblast differentiation.
MC3T3-E1 has been established as a well-recognized model of osteoblast differentiation and bone matrix mineralization in vitro [Citation16]. In our study, we investigated the involvement of GPR39 signaling in the process of osteoblast differentiation. We confirmed that GPR39 is abundantly expressed at both the mRNA and protein levels in MC3T3-E1 cells. To profile its expression from the pre-osteoblast state to terminal differentiation, we monitored the expression of GPR39 at various time points. Our data show that GPR39 was consistently induced after day 3, day 7, and day 14 of osteoblast culture, with peak induction at day 7. These data suggest that GPR39 is a responsive factor to osteoblast stimuli, implying its possible involvement in osteoblast differentiation. To test our assumption, we assessed the effect of t TC-G 1008-mediated receptor activation on the expression of osteoblast signature genes and the cellular mineralization profile. Our data reveal that TC-G 1008 not only increased the expression of three key osteoblast signature genes (ALP, OCN, and Col-I) but also raised ALP activity and cellular calcium deposition, thereby indicating that activation of the GPR39 pathway promotes the differentiation of pre-osteoblast cells toward osteoblasts.
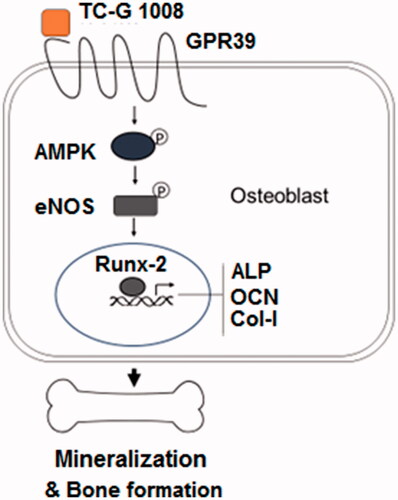
To further explore the molecular mechanisms, we assessed the expression level of the master osteoblast regulator Runx-2. Our experiment demonstrated that TC-G 1008 promoted Runx-2 expression at both the mRNA and protein levels, indicating that another upstream signaling mechanism may be involved. The terminal differentiation of preosteoblast cells is dependent on both transcriptional regulation and local stimulatory factors such as growth factors, second message molecules, and mechanical forces. Therefore, we tested another possible regulatory mechanism by GPR39 activation and finally pinned down one important pathway involved in osteoblast differentiation—NO signaling. During bone formation, the generation of NO results from mechanical and hormone stimulation. NO has been shown to promote osteoblast differentiation and is a pro-survival factor in mature osteoblasts [Citation17,Citation18]. In our study, TC-G 1008-mediated GPR39 activation induced significantly higher generation of NO as well as eNOS expression. In osteoblasts, NO is constitutively produced by eNOS. Our experiment confirmed that TC-G 1008 rapidly induced eNOS activation, suggesting that GPR39 activation promoted type 3 nitric synthase activity and thus induced higher NO generation. Furthermore, our data show that GPR39 activation also rapidly induced an increase in AMPK activation (<1 h). To confirm its role in GPR39-mediated osteoblast control, we used the AMPK inhibitor compound C in differentiation experiments. Our results show that compound C not only attenuated GPR39-mediated signature gene expression (ALP, OCN, and Col-I) but also weakened osteoblast terminal differentiation, as demonstrated by reduced ALP activity and lower calcium deposition. Compound C even partially blocked GPR39-mediated Runx-2 induction. These data imply that AMPK activity is essential to osteoblast differentiation mediated by GPR39 activation. Previous research has reported that AMPK-mediated Runx-2 induction promotes osteoblast differentiation in MC3T3-E1 cells [Citation19]. Our inhibition experiment confirmed that AMPK activity is necessary for TC-G 1008-induced osteoblast differentiation. Moreover, AMPK activation is known to regulate NO production and eNOS expression [Citation20]. Although we have yet to determine the exact role of AMPK cross-talk, NO production, and Runx-2 induction in osteoblast differentiation, our study suggests that AMPK is essential for GPR39-mediated osteoblast differentiation. A representative schematic of the molecular mechanism is shown in . In agreement with our findings, a recent study showed that GPR39 knockout mice had weak bones as a result of abnormal bone composition, while cultured GPR39 null osteoblasts show low collagen matrix and abnormal matrix deposition [Citation21]. Therefore, GPR39-mediated osteoblast differentiation appears to be vital both in vitro and in vivo, indicating its significant role in controlling bone formation. Looking back into the literature, a study has shown that another orphan GCPR, GPR48, regulates osteoblast differentiation, and bone formation in the animal development stage [Citation22]. Therefore, orphan GCPRs may represent a newly uncovered regulatory mechanism for normal bone formation and remodeling.
In conclusion, our study provides ample evidence that GPR39 signaling is involved in the regulatory control of osteoblast differentiation. Activation of GPR39 with its agonist TC-G 1008 may have clinical implications in the prevention and treatment of osteoporosis.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Miller PD. Management of severe osteoporosis. Expert Opin Pharmacother. 2016;17:473–488.
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145.
- Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41–51.
- Katsimbri P. The biology of normal bone remodelling. Eur J Cancer Care. 2017;26:e12740.
- Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–448.
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287.
- Hauser AS, Misty A, Mathias RA, et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–842.
- Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Int J Mol Sci. 2018;19:439.
- Peukert S, Hughes R, Nunez J, et al. Discovery of 2-Pyridylpyrimidines as the First Orally Bioavailable GPR39 Agonists. ACS Med Chem Lett. 2014;5:1114–1118.
- Shimizu Y, Koyama R, Kawamoto T. Rho kinase-dependent desensitization of GPR39; a unique mechanism of GPCR downregulation. Biochem Pharmacol. 2017;140:105–114.
- Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, et al. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12
- Kim SS, Choi JM, Kim JW, et al. cAMP induces neuronal differentiation of mesenchymal stem cells via activation of extracellular signal-regulated kinase/MAPK. Neuroreport. 2005;16:1357–1361.
- Abramovitch-Dahan C, Asraf H, Bogdanovic M, et al. Amyloid β attenuates metabotropic zinc sensing receptor, mZnR/GPR39, dependent Ca2+ , ERK1/2 and Clusterin signaling in neurons. J Neurochem. 2016;139:221–233.
- Kanazawa I, Yamaguchi T, Yano S, et al. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab. 2009;296:E139–46.
- Młyniec K, Starowicz G, Gaweł M, et al. Potential antidepressant-like properties of the TC G-1008, a GPR39 (zinc receptor) agonist. J Affect Disord. 2016;201:179–184.
- Sudo H, Kodama HA, Amagai Y, et al. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198.
- Saura M, Tarin C, Zaragoza C. Recent insights into the implication of nitric oxide in osteoblast differentiation and proliferation during bone development. Scientific World J. 2010;10:624–632.
- Kalyanaraman H, Schall N, Pilz RB. Nitric oxide and cyclic GMP functions in bone. Nitric Oxide. 2018;76:62–70.
- Chen Z, Peng IC, Sun W, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505.
- Jang WG, Kim EJ, Lee KN, et al. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun. 2011;404:1004–1009.
- Jovanovic M, Schmid FN, Guterman-Ram G, et al. Perturbed bone composition and integrity with disorganized osteoblast function in zinc receptor/Gpr39-deficient mice. FASEB J. 2018;32:2507–2518.
- Luo J, Zhou W, Zhou X, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756.