Abstract
Diabetic nephropathy (DN) is one of the major diabetic complications that lead to end-stage renal failure. Angiopoietin-like protein-4 (ANGPTL-4) has been reported to be dysregulated in diabetes mellitus and diabetic complications. However, the role of ANGPTL-4 in glomerular mesangial cells (MCs) during DN remains unclear. In the present study, we evaluated the role of ANGPTL-4 in MCs in response to high glucose (HG) condition and the potential mechanism. The results proved that ANGPTL-4 expression is significantly increased in HG-stimulated MCs. Knockdown of ANGPTL-4 suppressed HG-induced cell proliferation of MCs. The production of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6 were decreased in ANGPTL-4 knocked down MCs. Inhibition of ANGPTL-4 markedly suppressed the expressions of extracellular matrix (ECM) proteins, collagen IV (Col IV) and fibronectin (FN), in HG-stimulated MCs. Furthermore, ANGPTL-4 knockdown inhibited the HG-induced activation of NF-κB signaling pathway in MCs. Collectively, knockdown of ANGPTL-4 suppressed HG-induced cell proliferation, inflammatory response, and ECM accumulation inhibiting NF-κB signaling pathway in MCs. These findings suggested that ANGPTL-4 might be a therapeutic target for the prevention and treatment of DN.
Introduction
Diabetic nephropathy (DN) is one of the major microvascular complications of diabetes mellitus and often leads to end-stage renal failure [Citation1]. DN contributes to cardiovascular morbidity and mortality and causes heavy financial and health‐care burdens globally [Citation2]. Therefore, reduction of the incidence and morbidity of which is an urgent biomedicine issue. It is evident that hyperglycaemia in diabetic patients induces inflammatory and profibrotic reactions, ultimately resulting in gradual and non-stoppable scarring process of renal glomerulus, which is known as glomerulosclerosis [Citation2–4]. It is well known that mesangial cells (MCs) proliferation and extracellular matrix (ECM) production are believed to be correlated closely with progression of DN [Citation3,Citation5,Citation6]. Thus, regulating the function of MCs may be of great clinical importance for intervention in progressive DN in diabetes.
Angiopoietin-like protein-4 (ANGPTL-4), a member of angiopoietin-like family, plays important roles in numerous diseases, such as cancers, diabetes mellitus, and diabetic complications [Citation7–11]. It has been reported that ANGPTL-4 level is significantly increased in the vitreous and serum of patients with proliferative diabetic retinopathy (PDR) [Citation7]. In addition, the protein and mRNA levels of ANGPTL-4 are significantly upregulated in the diabetic rats and high glucose (HG)-exposed human retinal microvascular endothelial cells (HRMECs). ANGPTL-4 might be a potent modulator of increased inflammation, permeability, and angiogenesis during PDR [Citation8]. These findings suggest that ANGPTL-4 may be an effective therapeutic strategy and diagnostic screening biomarker for PDR. Furthermore, it was reported that ANGPTL-4 expression is upregulated in DN rat kidneys, which most likely associated with the heavy proteinuria. Moreover, ANGPTL-4 expression is positively correlated with the amount of 24-h urine protein, creatinine and the kidney weight index, whereas negatively correlated with total cholesterol [Citation12]. However, the role of ANGPTL-4 in DN remains unclear. In the present study, we evaluated the function of ANGPTL-4 in DN and the potential mechanism in vitro.
Materials and methods
Cell culture and treatment
A rat mesangial cell line, HBZY-1 cells, was purchased from Chinese Center for Typical Culture Collection (Wuhan, China). The HBZY-1 cells were cultured in DMEM (Hyclone, Logan, UT, USA) supplemented with 10% FBS, 2 mM glutamine, 1% penicillin/streptomycin. HBZY-1 cells were treated with indicated concentrations of glucose after incubation in DMEM containing 0.1% FBS for 24 h. All cells were maintained in an incubator containing 5% CO2 at 37 °C. The cells in normal glucose group (NG) were treated with 5 mM glucose. The cells in high glucose group (HG) were treated with 40 mM glucose.
SiRNA ANGPTL-4 and transfection
HBZY-1 cells (1 × 105 cells/well) were seeded in 6-well plates and then transfected with 100 nM specific small interfere RNA (siRNA) targeting ANGPTL-4 (si-ANGPTL-4) or negative control siRNA (si-NC) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Forty-eight hours after transfection, transfection efficiency was measured using qRT-PCR and western blot.
Cell proliferation assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Japan) as described in the manufacturer’s protocol. Briefly, HBZY-1 cells (5 × 103 cells/well) were seeded into 96-well plates and cultured under serum-deprived conditions for overnight. Afterwards, cells wereincubated under indicated glucose conditions at 37 °C for 48 h. The CCK-8 solution was added and incubated for another 4 h. The optical density was measured at 450 nm using a microplate reader (Bio-Tek, Winooski, VT, USA).
Real-time quantitative PCR (qRT-PCR)
Cell total RNA was extracted from HBZY-1 cells using TRIZOL reagent (Invitrogen) according to the manufacturer’s directions. Then, 1 μg of total RNA was used for the qRT-PCR with a One-Step RT-PCR Kit (TaKaRa Bio, Shiga, Japan) using an iQ5 RT-PCR Detection system (Bio-Rad Laboratories, Hercules, CA, USA). Primers for rat ANGPTL-4, F 5′-CGA TGG GAC TCA AGC ACG GTG CGC-3′; R 5′-TCT AAA GCA CAC CAG GCC TAT AAT-3′; β-actin, F 5′-CAC GAT GGA GGG CCC GGA CTC ATC-3′; R 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′.
Western blot analysis
The HBZY-1 cells were lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing PMSF protease inhibitors. Protein concentrations of lysates were determined using the BCA protein assay kit (Beyotime). Equal amounts of proteins (50 μg) were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). After blocking in 5% non-fat milk buffer at room temperature for 1.5 h, the membranes were incubated overnight at 4 °C with indicated primary antibodies against ANGPTL-4 (1:2000; ab196746; Abcam, Cambridge, MA, USA), p65 (1:1000; #8242; Cell Signaling Technology, Boston, MA, USA), p-p65(1:2000; #3037; Cell Signaling Technology), p-IκBα (1:2500; ab133462; Abcam), collagen IV (Col IV; 1:3000; ab6586; Abcam), fibronectin (FN; 1:1500; ab2413; Abcam) and β-actin (1:2000; ab227387; Abcam). After washing for three times, the membranes were incubated with goat anti-rabbit IgG (1:2500; ab6721; Abcam) for 1.5 h at 37 °C. Then the bands were developed using the ECL reagents (Thermo Fisher Scientific, Waltham, MA, USA). The intensity of each band was quantified by Image J software (National Institutes of Health, NIH, Bethesda, MD, USA).
ELISA
After different treatments, supernatants were collected for the measurement of TNF-α, IL-1β, IL-6, Col IV and FN levels using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as mean ± SD. The data were analyzed using SPSS software 14.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses were performed using student’s t test or one-way analysis of variance. For all the tests, p < .05 was considered statistical significance.
Results
ANGPTL-4 is up-regulated HG-stimulated MCs
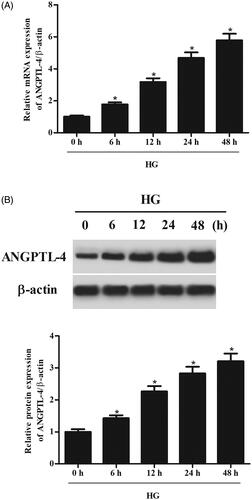
We first evaluated whether the expression of ANGPTL-4 could be altered in MCs under HG condition. The results of qRT-PCR showed that the mRNA level of ANGPTL-4 was markedly increased after HG stimulation in a time-dependent manner (. Western blot analysis indicated that the protein level of ANGPTL-4 was significantly induced by HG stimulation in MCs (.
Figure 1. HG stimulation caused increase in ANGPTL-4 expression in MCs. HBZY-1 cells were treated with 5 mM glucose (NG group) or 40 mM glucose (HG group) for different hours. (A) The mRNA level of ANGPTL-4 was measured using qRT-PCR. (B) The protein level of ANGPTL-4 was detected using western blot. *p < .05.
Knockdown of ANGPTL-4 inhibits cell proliferation in MCs induced by HG
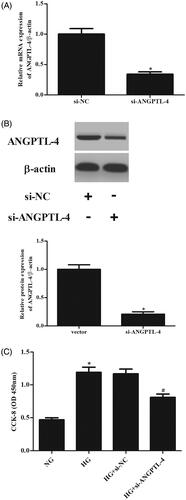
In order to investigate the role of ANGPTL-4 in MCs, si-ANGPTL-4 was transfected into MCs to knock down ANGPTL-4. The data showed that the expressions of ANGPTL-4 at both mRNA and protein levels were dramatically decreased after transfection with si-ANGPTL-4 (. CCK-8 assay proved that cell proliferation of MCs was markedly increased after HG stimulation. However, knockdown of ANGPTL-4 in MCs caused significant decrease in cell proliferation (.
Figure 2. Knockdown of ANGPTL-4 inhibits HG-induced cell proliferation in MCs. HBZY-1 cells were transfected with si-ANGPTL-4 or si-NC, followed by incubation with 5 or 40 mM glucose for 48 h. (A and B) qRT-PCR and Western blot analysis were performed to detect the expression of ANGPTL-4. (C) CCK-8 assay was carried out to measure cell proliferation. *p < .05 compared with NG group; #p < .05 compared with HG + si-NC group.
Knockdown of ANGPTL-4 suppresses inflammatory response in MCs induced by HG
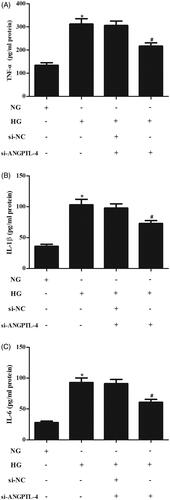
It has been reported that HG results in inflammatory response in MCs. The results of ELISA assay indicated that the secretions of inflammatory cytokines including TNF-α, IL-1β, IL-6 were obviously increased in HG-induced MCs, while the secretions of these inflammatory cytokines were suppressed after transfection with si-ANGPTL-4 ().
Figure 3. Knockdown of ANGPTL-4 suppresses HG-induced inflammatory response in MCs. After transfection with si-ANGPTL-4 or si-NC and the following incubation with 5 or 40 mM glucose for 48 h, the secretions of TNF-α, IL-1β, IL-6 were determined using ELISA (A–C), respectively. *p < .05 compared with NG group; #p < .05 compared with HG + si-NC group.
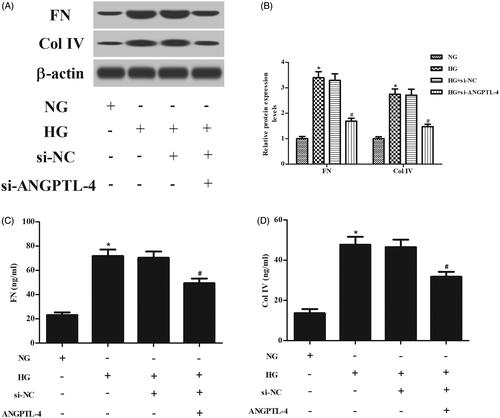
Knockdown of ANGPTL-4 reduces the production of FN and col IV in MCs induced by HG
To explore the effect of ANGPTL-4 knockdown on ECM accumulation in MCs, the ECM proteins including FN and Col IV were determined. Western blot analysis denoted that the protein levels of FN and Col IV were increased in MCs induced by HG, while decreased in MCs transfected with si-ANGPTL-4 (. ELISA revealed that the HG-induced production of FN and Col IV were prevented by knockdown of ANGPTL-4 (.
Figure 4. Knockdown of ANGPTL-4 reduces HG-induced production of FN and Col IV in MCs. HBZY-1 cells were incubated with 5 or 40 mM glucose for 48 h after transfection with si-ANGPTL-4 or si-NC. The protein levels and secretions of FN and Col IV were determined using western blot analysis (A and B) and ELISA (C and D), respectively. *p < .05 compared with NG group; #p < .05 compared with HG + si-NC group.
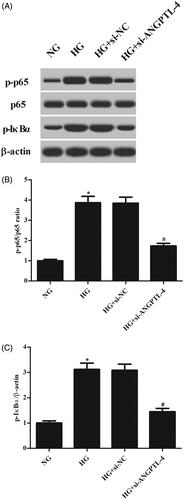
Knockdown of ANGPTL-4 inhibits the activation of NF-κB pathway in HG-treated MCs
To evaluate the activation of NF-κB pathway, expressions of NF-κB p65, p-p65, IκBα, and p-IκBα were measured. Western blot analysis demonstrated that HG stimulation caused significant increases in the expression levels of p-p65 and p-IκBα. However, the increased levels of p-p65 and p-IκBα were attenuated by knockdown of ANGPTL-4, indicating that ANGPTL-4 knockdown suppressed the HG-induced activation of NF-κB pathway in MCs ().
Discussion
Previous studies have demonstrated that dysregulation of ANGPTL-4 is associated with diabetes mellitus and diabetic complications. Li et al. [Citation9] reported that ANGPTL-4 expression is down-regulated in serum of gestational diabetes mellitus (GDM) patients. In vitro experiments prove that down-regulated ANGPTL-4 inhibits apoptosis and promoted proliferation of 3T3-L1 cells. Meanwhile, down-regulated ANGPTL-4 significantly suppresses glucose uptake. Besides, it has been documented that genetic inactivation of ANGPTL-4 improves glucose homeostasis and is associated with reduced risk of type 2 diabetes (T2D) [Citation10]. Thus, we could conclude that ANGPTL-4 participates in the development of diabetes. It has been observed that urinary ANGPTL-4 level is gradually upregulated in both streptozotocin (STZ)-induced diabetic rats and diabetic patients with microalbuminuria and macroalbuminuria [Citation13]. They also proved that the urinary ANGPTL-4 expression is closely associated with albuminuria in the rats and patients with DN, indicating that ANGPTL-4 might be a potential diagnostic and therapeutic biomarker for DN. In the present study, we found that ANGPTL-4 expression was markedly up-regulated HG-stimulated MCs. Therefore, we speculated that ANGPTL-4 might play an important role in MCs in response to HG induction.
In recent years, many researchers have been convinced that inflammation and subsequent ECM expansion play central roles in the progression of DN [Citation14]. The chronic hyperglycemic state results in an increase in advanced glycation end-products (AGEs), which are also potent stimulators of chemokines production, leading to the appearance of inflammation [Citation14]. Besides, AGEs stimulate the glomerular cells to produce transforming growth factor-beta 1 (TGF-β1), which can cause abnormal production of ECM, thereby contributing to glomerular sclerosis and interstitial tubular damage [Citation15,Citation16]. Therefore, inhibiting inflammatory response and ECM production may be effective for the prevention and treatment of DN. ANGPTL-4 was reported to be involved in the progression of diabetic retinopathy (DR). ANGPTL-4 expression is induced by HG both in vivo and in vitro. ANGPTL-4 mediates the production of inflammatory cytokines including IL-1β and IL-6, as well as regulates angiogenesis via the profilin-1 signaling under HG conditions [Citation8]. Furthermore, Li et al. [Citation9] demonstrated that down-regulated ANGPTL-4 results in increased expressions of inflammatory cytokines. Our results showed that knockdown of ANGPTL-4 suppressed HG-induced secretions of TNF-α, IL-1β, IL-6 in MCs. ANGPTL-4 knockdown also inhibited MCs proliferation and ECM accumulation in MCs.
A growing body of evidence indicates that the molecular mechanism for MCs-contributed ECM production in diabetic nephropathy. Wu et al. reported that store-operated Ca(2+) channels could negatively regulate ECM protein expression in MCs [Citation17]. Another study showed that store-operated Ca2+ entry negatively regulated the TGF-β1/Smad3 signaling pathway in glomerular MCs [Citation18]. In addition, NF-κB is an important transcriptional factor that is involved in multiple cellular responses to stimuli [Citation19]. Several lines of evidence support the fundamental role of the transcriptional factor NF-κB in the mesangial cells during the progress of DN [Citation20–22]. The increased AGEs interact through the cellular receptors to favor activation of the transcription factor NF-κB and the protein kinase C system, leading to the appearance of inflammation, growth, and augmentation of synthesis of the ECM [Citation15]. Previous study has demonstrated that inhibition of NF-κB in the kidney using the peroxisome has the ability to improve DN in animal models [Citation23]. Therefore, NF-κB might be a therapeutic target for DN treatment. Guo et al. [Citation24] proved that silencing ANGPTL-4 protects against lipopolysaccharide (LPS)-induced acute lung inflammatory injury via regulating NF-κB signaling pathway. Our data showed that the increased levels of p-p65 and p-IκBα were attenuated by knockdown of ANGPTL-4, indicating that ANGPTL-4 knockdown suppressed the activation of NF-κB pathway in HG-induced MCs.
There existed several limitations in the present study. Firstly, although knockdown of ANGPTL-4 inhibited the activation of NF-κB pathway in HG-treated MCs, the direct regulatory mechanism of ANGPTL-4 on NF-κB pathway in MCs remains to be determined. Secondly, we only evaluated the role of ANGPTL-4 in MCs in response to HG in vitro. An in vivo animal study will be considered in the following studies.
Conclusion
Taken together, the current study proved that ANGPTL-4 expression is increased in MCs under HG condition. Knockdown of ANGPTL-4 suppressed cell proliferation, inflammatory response and ECM accumulation in HG-induced MCs. The effects of ANGPTL-4 were partially mediated by regulating NF-κB signaling pathway.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33:e2841.
- Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176.
- Tung CW, Hsu YC, Shih YH, et al. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton). 2018;23:32–37.
- Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124:139–152.
- Fu J, Lee K, Chuang PY, et al. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308:F287–F297.
- Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Invest. 2015;6:3–15.
- Lu Q, Zou W, Chen B, et al. ANGPTL-4 correlates with vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1281–1288.
- Lu Q, Lu P, Chen W, et al. ANGPTL-4 induces diabetic retinal inflammation by activating Profilin-1. Exp Eye Res. 2018;166:140–150.
- Li M, Yang XJ, Zhang GY, et al. ANGPTL4 participates in gestational diabetes mellitus via regulating Akt pathway. Eur Rev Med Pharmacol Sci. 2018;22:5056–5062.
- Gusarova V, O’Dushlaine C, Teslovich TM, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun. 2018;9:2252–2262.
- La Paglia L, Listi A, Caruso S, et al. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. PPAR Res. 2017;2017:8187235–8187249.
- Xue L, Feng X, Wang C, et al. Benazepril hydrochloride improves diabetic nephropathy and decreases proteinuria by decreasing ANGPTL-4 expression. BMC Nephrol. 2017;18:307–314.
- Ma J, Chen X, Li JS, et al. Upregulation of podocyte-secreted angiopoietin-like-4 in diabetic nephropathy. Endocrine. 2015;49:373–384.
- Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5:393–398.
- Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, et al. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018;2018: 1875870–1875882.
- Pohlers D, Brenmoehl J, Löffler I, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756.
- Wu P, Wang Y, Davis ME, et al. Store-operated Ca (2+) channels in mesangial cells inhibit matrix protein expression. JASN. 2015;26:2691–2702.
- Chaudhari S, Li W, Wang Y, et al. Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells. Am J Physiol Renal Physiol. 2017;313:F729–F739.
- Gilmore T. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684.
- Chen F, Zhu X, Sun Z, et al. Astilbin inhibits high glucose-induced inflammation and extracellular matrix accumulation by suppressing the TLR4/MyD88/NF-kappaB pathway in rat glomerular mesangial cells. Front Pharmacol. 2018;9:1187–1197.
- Liu Z, Han Y, Zhao F, et al. Nobiletin suppresses high-glucose-induced inflammation and ECM accumulation in human mesangial cells through STAT3/NF-kappaB pathway. J Cell Biochem. 2019;120:3467–3473.
- Zhu X, Shi J, Li H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways . Biomed Pharmacother. 2018;106:976–982.
- Fadia K, Nadezhda YG, Honglan P, et al. Local delivery of angiotensin II receptor blockers into the kidney passively attenuates inflammatory reactions during the early phases of streptozotocin-induced diabetic nephropathy through inhibition of calpain activity. Nephron Exp Nephrol. 2010;115:e69–e79.
- Guo L, Li S, Zhao Y, et al. Silencing angiopoietin-like protein 4 (ANGPTL4) protects against lipopolysaccharide-induced acute lung injury via regulating SIRT1 /NF-kB pathway. J Cell Physiol. 2015;230:2390–2402.