Abstract
Cardiovascular complications are the leading cause of mortality and morbidity in type 2 diabetes patients. Diabetes greatly increases the risk of heart disease; therefore, the management of diabetes often involves the prevention of heart disease. DPP-4 inhibitors have been proven to be the effective therapeutic agents of glycaemic control. Recent studies have shown that certain types of DPP-4 inhibitors could also have cardiovascular benefits. In this study, we examined the protective role of the newly developed DPP-4 inhibitor anagliptin in cultured cardiac myocytic cell line H9C2 cells. Our data show that exposure of H9C2 cells to hypoxic conditions induced higher expression of DPP-4, indicating that DPP-4 is a hypoxia-inducible factor. The inhibition of DPP-4 by anagliptin ameliorates hypoxia-induced cytotoxicity and induction of the pro-inflammatory cytokines IL-6 and MCP-1. Anagliptin also suppresses hypoxia-induced oxidative stress as revealed by the detected levels of cellular ROS and reduced GSH. Moreover, anagliptin protects myocytes from hypoxia-associated reduced mitochondrial membrane potential. Mechanistically, we show that anagliptin promotes hypoxia-induced NFR2/HO1 induction but suppresses HMGB1 and MyD88 generation. Collectively, our data indicate that anagliptin-mediated DPP-4 inhibition is a protective mechanism in cardiomyocytes and imply that the DDP-4 inhibitor anagliptin plays dual roles by lowering glucose and protecting cardiomyocytes.
Introduction
The worldwide increase in the prevalence of diabetes mellitus in recent decades has had profound impacts on the health care industry. The number of diabetes patients has continued to grow despite progress being made in the treatment of the disease. In some undeveloped or even developed countries, many diabetic patients have do not receive satisfactory treatment due to poverty or limited access to health care services. If left untreated, the long-time burden of hyperglycaemia often develops into complications such as cardiovascular disorders, neurological diseases, and other organ problems [Citation1]. Among these complications, heart diseases are one of the leading causes of death in diabetic patients. Myocardial infarction resulting from ischaemic heart attack is a major life-threatening event in diabetic patients. Therefore, the treatment of diabetes often involves the management of common complications, such as cardiovascular events [Citation2].
GLP-1 agonists and DPP-4 inhibitors have been widely used to lower blood glucose levels in diabetic patients. DPP-4 inhibitors are also referred to as gliptins and have been used in clinical settings either via direct administration or in combination with other glucose-reducing drugs to treat type 2 diabetes [Citation3]. The mechanism of action of gliptin compounds often differ from one another, and the optimized treatment requires a complete understanding of the pharmacological effect of such agents. In recent studies, several types of DPP-4 inhibitors have been reported to exhibit cardiovascular protection in preclinical animal models and several clinical trials [Citation4–7]. Anagliptin is a newly developed gliptin that was approved for patient use in Japan in 2012. Anagliptin is a type of amino acid amide, which has displayed long-lasting and potent inhibition of DPP-4 [Citation8]. In recent years, several animal studies have shown this agent exerts cardiovascular benefit aside from its glucose-lowering ability. In a murine atherosclerosis model, the treatment of mice with anagliptin reduced aortic plaque burden [Citation9]. In induced vascular injury rats, administration of anagliptin reduces neointimal formation and relieves whole-body inflammation [Citation10]. In a vascular aneurysm model, the treatment of animals by anagliptin significantly reduces the size of intracranial aneurysms and suppresses inflammatory pathway in macrophages [Citation11]. In diabetic mice with induced myocardial infarction, the administration of anagliptin improves heart function [Citation12]. Clinical study indicates anagliptin is a safe and effective drug for the treatment of type 2 diabetes [Citation13]. The retrospective clinical study shows that anagliptin administration decreases blood pressure and reduces cholesterol level [Citation14]. This evidence suggests that anagliptin is a promising DPP-4 inhibitor that exerts cardiovascular protection independent of its glucose-lowing capacity. But the molecular mechanism of its action in cardiac tissue remains largely unknown. Here, we examined the effect of anagliptin in cultured cardiomyocytes.
Materials and methods
Cell culture and treatment
Rat H9C2 cell line in this study was purchased from the American Type Culture Collection (ATCC) (USA). The cells were grown in Dulbecco's Modified Eagle’s Medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and antibiotic regimen (100 units/mL penicillin and 100 μg/mL streptomycin). Cells were pretreated with 10 and 20 μM anagliptin for 6 h before hypoxia treatment.
Hypoxia
To expose the cells to hypoxia, H9C2 cells were fed with fresh glucose-free and serum-free media and then transferred to a tightly sealed culture chamber supplemented with a mixture of 2% O2, 5% CO2, and 94% N2. The hypoxia exposure time in our study was 6, 12, 24, or 48 h, dependent on the specific experiment.
Lactate dehydrogenase (LDH) release
LDH release serves as an indicator of cell death or damage. In brief, the released LDH converts lactate into pyruvate, which can form a formazan product. The measurement of formazan formation is proportionally correlated with LDH release in the medium. We purchased an LDH kit from Sigma-Aldrich to estimate the extracellular LDH level of H9C2 cells. The reaction was performed in accordance with the product manual. The final value of the formazan derivative was read using a microplate reader at the absorption of 490 nm. The relative number of LDH release is calculated based on the curve from standard samples.
Reactive oxygen species (ROS) assay
We measured total cellular ROS by dihydroethidium (DHE) staining. DHE is used to capture the cellular ROS and form fluorescent product. Briefly, H9C2 cells were cultured at 6-well plates to complete confluence; the cells were then stained with 1 µM DHE for 30 min [Citation15]. The morphometric record of stained cells was done on a Leica confocal microscope equipped with a digital camera. The images were quantitated by Image J software (NIH).
Reduced glutathione (GSH)
Reduced GSH is a key antioxidant in the cells. We measured the reduced GSH level from H9C2 cells using a kit from Thermo Fisher Scientific, USA. The cell lysates were added into 96 well plates, and all the reaction steps were performed in accordance with the product manual. The plate was read at 450 nm by a microplate reader. The final value of reduced GSH was calculated based on the curve derived from standard samples.
RT-PCR and real-time PCR
Total RNA from H9C2 cells was isolated using a high pure plasmid kit (Roche, USA). To synthesize cDNA from RNA samples, we used a One-Step Superscript II RT-PCR kit (Bio-Rad, USA). The cDNA was then diluted 10 times with ddH2O, and 1 µL of cDNA was used to amplify DPP-4 transcript. All the transcript levels of other genes including IL-6, MCP-1, HO-1, NRF2, HMGB1, MyD88, and GAPDH (as house keep control) were examined by real-time PCR analysis. The assays were performed by SYBR Green-based approach on Step One Real-Time system (ABI).
Western blot analysis
H9C2 cells were lysed with RIPA buffer. The concentration of total lysate was measured by using a BCA kit (Thermo Fisher). A total of 20 μg samples were separated on a 10% SDS-PAGE gel and transferred onto 0.4 µM PVDF membranes (Bio-Rad). The membranes were blocked with 5% BSA-PBST buffer for 1 h, followed by the incubation with primary antibodies overnight at 4 °C and HRP-linked secondary antibodies for 2 h at RT. Finally, the blots were reacted with Superpico substrate reagent (Pierce, Rockford, IL, USA) and developed on film [Citation16].
Enzyme-linked immunosorbent assay (ELISA)
We measured the production levels of two cytokines IL-6 and MCP-1 in H9C2 cells. The culture supernatants were collected, and protein concentrations in the supernatants were measured using a BCA kit as described above. We purchased IL-6 and MCP-1 ELISA kits from R & D Systems (USA). We followed the same assay procedure as suggested in the product instruction manual.
Measurement of cell viability
Cell viability of H9C2 cells was examined using an MTT assay [Citation17]. H9C2 cells were seeded into 24-well plates and pretreated with anagliptin (10, 20 μM) for 6 h, followed by exposure to hypoxia for 24 h. Then, fresh medium containing 0.8 mg/mL MTT (Sigma-Aldrich, USA) was added to replace the old culture medium. After incubation for 4 h at 37 °C, the purple product was dissolved in DMSO (Sigma-Aldrich, USA). OD value was recorded at 570 nM using a microplate reader to reflect cell viability (Molecular Devices, USA).
Apoptosis analysis via flow cytometry
H9C2 cells were pretreated with anagliptin (10, 20 μM) for 6 h, followed by exposure to hypoxia for 24 h. The percentage of apoptotic H9C2 cells was measured using a commercial kit (Becton-Dickinson, USA). H9C2 cells were stained with FITC-labelled Annexin V (1 mg/mL) and propidium iodide (10 mg/mL) for 15 min. After gentle washes, patterns of apoptotic cells were measured using a FACScan (Becton-Dickinson, USA).
Statistics
The final results are presented as means ± SEM The statistical significance of the experimental data was analysed by analysis of variance (ANOVA). P < .05 was considered statistically significant.
Results
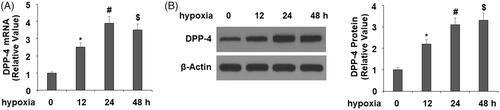
Hypoxia induces DPP-4 expression in H9C2 cardiomyocytes
To examine the expression of DPP-4 in cardiomyocytes, we exposed H9C2 cells in a hypoxia chamber. We monitored the expression of DPP-4 in different hypoxia exposure time. At mRNA level and compared to the normoxia, 12, 24, and 48 h of hypoxia culture all significantly induced 2–4-folds of the DPP-4 mRNA (. At the protein level, hypoxia culture similarly induced 2–3-folds of the DPP-4 protein (.
DPP-4 inhibitor anagliptin ameliorates hypoxia-induced cell death and apoptosis
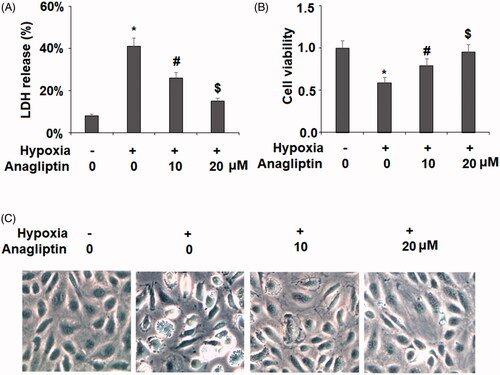
Next, we pretreated H9C2 cells with the DPP-4 inhibitor anagliptin in our hypoxia experiment and examined the effect of the suppression of DPP-4 on cytotoxicity by measuring extracellular LDH release. The experiment showed that hypoxia exposure for 24 h increased LDH release by fivefold, but pretreatment with 10 and 20 μM anagliptin significantly ameliorated hypoxia-induced toxicity, with the higher dose of anagliptin showing a better effect (. Cell viability of H9C2 cells was then measured using MTT assay. The results in indicate that hypoxia resulted in a 38% reduction in cell viability in H9C2 cells, which was prevented by anagliptin in a dose-dependent manner. The morphology of H9C2 cells is shown in .
Figure 2. Anagliptin attenuated hypoxia-induced LDH release in cardiac H9C2 cells. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. (A). LDH release from H9C2 cells; (B). Cell viability was measured by MTT assay; (C). Cell morphology of H9C2 cells (*, #, $, P < .01 vs. previous group).
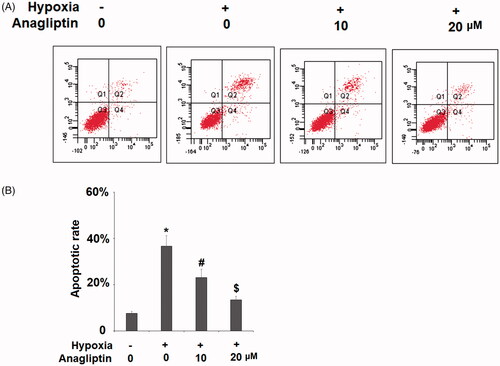
We further examined whether anagliptin possesses a beneficial property against hypoxia-induced apoptosis of H9C2 cells using a flow cytometry assay. Exposure to hypoxia for 24 h resulted in approximately 36.6 ± 4.2% cellular apoptosis. However, the presence of anagliptin (10, 20 μM) significantly ameliorated hypoxia-induced apoptosis in H9C2 cells in a concentration-dependent manner ().
Figure 3. The protective effects of anagliptin against hypoxia-induced apoptosis in H9C2 cells. Cells were pretreated with anagliptin (10, 20 μM) for 6 h, followed by exposure to hypoxia for 24 h. (A). Cell apoptosis was measured by flow cytometric analysis; (B). Quantification of apoptotic cells (*, #, $, P < .01 vs. previous group).
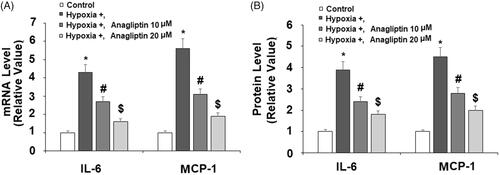
Anagliptin-attenuated hypoxia-induced IL-6 and MCP-1 production
To explore how DPP-4 inhibition protects cardiomyocytes from hypoxia, we measured the production of two key cytokines in our experiment: IL-6 and MCP-1. Our results showed that exposure to hypoxic conditions induced several fold induction of both IL-6 and MCP-1 mRNA, but pretreatment with the two doses of anagliptin significantly attenuated these inductions (. By measuring the cytokine levels in the media, we confirmed that anagliptin had similar inhibitory effects on hypoxia-induced IL-6 and MCP-1 production at the protein level (.
Figure 4. Anagliptin attenuated hypoxia-induced IL-6 and MCP-1 secretion in cardiac H9C2 cells. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. (A) mRNA levels of IL-6 and MPCP-1; (B) Protein levels of IL-6 and MCP-1 (*, #, $, P<.01 vs. previous group).
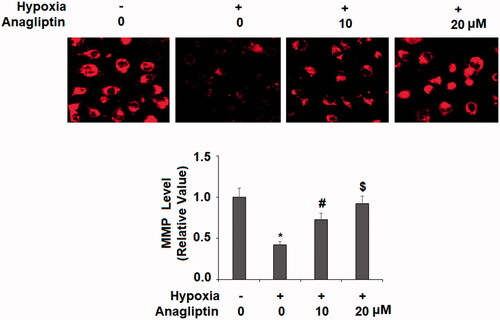
Anagliptin protects against hypoxia-induced mitochondrial membrane potential (MMP) decline
Cardiomyocytes contain a large number of mitochondria. We investigated whether anagliptin would have a protective effect on MMP. The experiment showed that hypoxia exposure caused a decline in MMP of about 50%, while pretreatment with the same two doses of anagliptin protected against hypoxia-induced MMP declines, with the high dose showing near-perfect protection ().
Figure 5. Anagliptin restored hypoxia-induced reduced mitochondrial membrane potential (MMP) in cardiac H9C2 cells. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. Levels of MMP were determined by staining with TMRM (*, #, $, P<.01 vs. previous group).
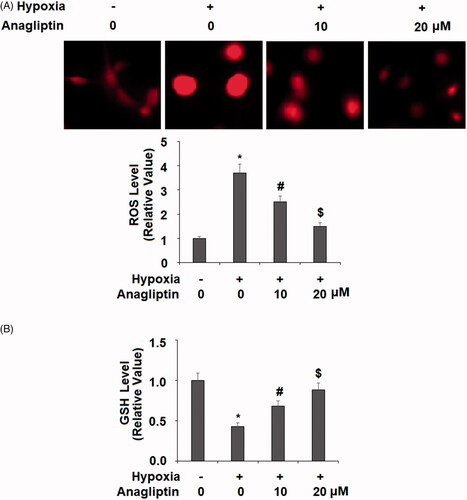
Anagliptin protects against hypoxia-induced oxidative stress
Hypoxia-induced oxidative stress is a major force involved in cardiomyocyte injury. In our next treatment experiment, we measured the levels of total cellular ROS and reduced GSH to determine the effect of anagliptin against oxidative stress. While 24 h of hypoxia induced about fourfold higher total ROS, pretreatment with the two doses of anagliptin significantly mitigated this induction (. At the same time, hypoxia decreased the level of reduced GSH by about 60%. However, the cells pre-incubated with anagliptin had much better preservation of the level of reduced GSH (.
Figure 6. Anagliptin inhibited hypoxia-induced oxidative stress in cardiac H9C2 cells. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. (A) Intracellular ROS was determined by dihydroethidium (DHE) staining; (B) the levels of reduced GSH (*, #, $, P<.01 vs. previous group).
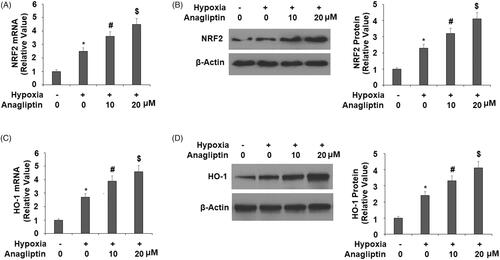
Anagliptin promotes the expression of NRF2 and HO-1
We then explored the potential molecular pathways involved in the effects of anagliptin. We focussed on several protective pathways in cardiomyocytes, including NRF2/HO-1. In the same treatment experiment, hypoxia induced a mild increase in NRF2, but pre-incubation with anagliptin significantly increased both the mRNA and protein expression of NRF2 (). Meanwhile, hypoxia had a similar effect on HO-1 expression, but the presence of anagliptin significantly elevated both the mRNA and protein levels of HO-1 in a dose-dependent manner ().
Figure 7. Anagliptin regulates the expression of NRF2 and HO-1. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. (A) mRNA levels of NRF2; (B) protein levels of NRF2; (C) mRNA levels of HO-1; (D) protein levels of HO-1 (*, #, $, P < .01 vs. previous group).
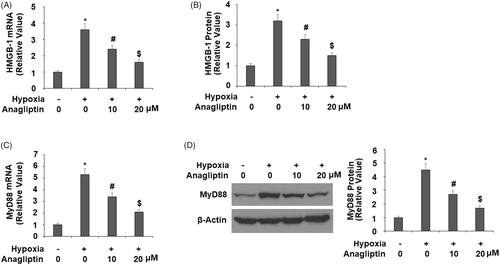
Anagliptin suppresses the expression of HMGB-1 and HO-1
Additionally, we investigated the roles of two immune response factors HMGB1 and MyD88 in anagliptin-mediated protection. In the same experiment, hypoxia induced about 3–4-fold higher HMGB-1 expression, but pre-incubation with anagliptin greatly suppressed the increase in HMGB-1 at both the mRNA and protein levels (). Meanwhile, hypoxia exposure induced 4–5-fold higher MyD88 expression, while pre-incubation with anagliptin dramatically inhibited the increase in MyD88 at both the mRNA and protein levels ().
Figure 8. Anagliptin reduced the expression of HMGB-1 and MyD88. Cells were pretreated with anagliptin (10, 20 μM) for 6 h. Then, cells were subjected to hypoxia for 24 h. (A) mRNA levels of HMGB-1; (B) secretions of HMGB-1 as measured by ELISA; (C) mRNA levels of MyD88; (D) protein levels of MyD88 (*, #, $, P<.01 vs. previous group).
Discussion
In heart tissue, the majority of the energy supply is based on aerobic respiration and TCA cycle. The function of cardiomyocytes is closely associated with an adequate supply of oxygen. To meet the need for large amounts of ATP in order to facilitate heart contractions, cardiomyocytes consume oxygen at a very high rate. This requires a high concentration of oxygen in the heart tissue. Any shortage of oxygen supply will cause hypoxia and hypoxic injury to the heart tissue. Diabetes-associated vascular dysfunction often causes a shortage of blood supply to the heart muscle and ultimately leads to ischaemic heart disease [Citation18].
Since animal studies have already suggested that anagliptin has a cardiovascular protective effect, we investigated the molecular mechanism behind its action in cultured cardiomyocytes H9C2 cells. H9C2 cells mimic the reactions of primary cardiomyocytes to hypoxia and oxidative stress conditions, so they are often used as an ischaemic-reperfusion model in vitro [Citation19]. In our study, we used 2% oxygen to simulate hypoxia exposure and examined the cellular response upon DPP-4 inhibition by anagliptin in hypoxic conditions. We first examined the cardiac DPP-4 expression profile when H9C2 cells were exposed to hypoxia at different time points. Our experiments showed that cardiac DPP-4 was induced at 12, 24, and 48 h of hypoxia exposure, indicating that it is a hypoxia-responsive factor and may play a role in hypoxia-induced cardiomyocyte injury. We demonstrated its effect on several aspects of cellular function. Firstly, the presence of anagliptin ameliorated hypoxia-induced cytotoxicity as the cells treated with anagliptin released less LDH. Secondly, the presence of anagliptin suppressed hypoxia-induced production of major pro-inflammatory cytokines such as IL-6 and MCP-1. Thirdly, anagliptin treatment mitigated hypoxia-induced mitochondrial membrane potential decline. Fourthly, anagliptin showed protective force against hypoxia-induced cellular ROS production and reduced GSH decline. These facts indicate that anagliptin is a potent anti-inflammatory, anti-ROS, and prosurvival agent with the capacity to protect cardiomyocytes from hypoxia-induced injury. Mechanistically, our study demonstrates that anagliptin regulated several key ischemia-associated proteins. Our data show that anagliptin promoted the expression of the transcriptional factor NRF2 and HO-1 in hypoxic conditions but suppressed the induction on MyD88 and HMGB-1. The anti-oxidative factor NRF2 and its direct target HO-1 have been linked to cardiovascular diseases. Both NRF2 and HO-1 have protective roles against cellular oxidative stress. The activation of NRF2 induces HO-1, SOD, and other antioxidant genes, and the expression of these antioxidants relieves oxidative stress-related cardiac injury [Citation20]. HO-1 is a rate-limiting enzyme involved in the cellular degradation of hemeproteins. HO-1 and its metabolic by-products, including carbon monoxide (CO), ion, and bilirubin, play important roles in mediating cellular signalling and mitochondrial function [Citation21]. HO-1 has been considered a promising therapeutic target in the treatment of heart diseases [Citation22]. MyD88 is an adapter protein for toll-like receptor 4 (TLR4) and TLR2. MyD88-mediated signalling is essential for the activation of NFκB and the initiation of the inflammatory response. The functions of MyD88 and HMGB1 are often interlinked. In cardiomyocytes, HMGB1 induction results from hypoxia-induced cell injury and could further activate MyD88-mediated cardiac immune response [Citation23].
Although the complete mechanism of anagliptin remains to be further investigated, one important question is how the inhibition of cardiac DPP-4 by anagliptin mitigates the hypoxia-induced expression of different regulators such as NRF2, MyD88, and HMBG-1. Cardiac NRF2 and its downstream target HO-1 are the major regulatory pathways that control the expression of a series of antioxidant and detoxifying enzymes. The protective effect of the NRF2/HO-1 pathway involves its mechanism on cardiomyocyte survival [Citation24]. A recent study shows that NRF2 activation could directly suppress hypoxia-induced HMGB-1 and MyD88 expression in cardiomyocytes [Citation25]. Therefore, the amelioration of anagliptin on NRF2 and HO-1 could play a major role in the suppression of inflammatory pathways. The amelioration of these cascades of transcription factors by anagliptin could be a direct mechanism through which to suppress cytokine induction and ROS production. Hypoxia-induced ROS production and cytokine production in the heart directly cause necrosis or apoptosis of cardiomyocytes, which consequently results in tissue remodelling and disturbs normal cardiac function. The amelioration of cardiac NRF2 and its downstream regulators by anagliptin as well as its consequence on ROS and cytokine production imply that the administration of anagliptin could have a certain preventive effect against ischaemic heart diseases.
Previous reports have shown that anagliptin also exerts a beneficial effect in vascular endothelial cells and skeletal muscle. Several studies have shown that anagliptin promotes antioxidant gene expression and improves endothelial survival [Citation26–28]. Another study shows that anagliptin treatment improves skeletal muscle glucose uptake and capillary recruitment in pre-diabetic mice [Citation29]. This evidence indicates that anagliptin possesses a pro-survival mechanism in different vascular cells and muscle cells. As described previously, clinical observations indicate that the administration of anagliptin reduces low-density lipoprotein cholesterol in type 2 diabetes patients [Citation14]. A multi-centre randomized clinical trial has been proposed to compare anagliptin and another promising DPP-4 inhibitor, Sitagliptin [Citation30]. It is expected that this trial could shed light on the implication of DPP-4 inhibitors in diabetes-related heart diseases.
In summary, our study provides a molecular mechanism of the cardioprotective effect of the DPP-4 inhibitor anagliptin. Anagliptin has not been approved to be used in countries other than Japan, but it is a very promising drug with distinct cardiovascular benefits. The complete elucidation of its molecular mechanism could pave the road for our understanding of the extent of its clinical benefit. It is appealing that anagliptin possesses the dual roles of lowering glucose and offering a cardiovascular protective effect, and the full evaluation of its cardiovascular benefit would provide a new treatment avenue in the management of type 2 diabetes with cardiovascular complications.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625.
- American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26:s33–s50.
- Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother. 2013;14:2047–2058.
- Nauck MA, Meier JJ, Cavender MA, et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870.
- Paneni F, Lüscher TF. Cardiovascular protection in the treatment of type 2 diabetes: a review of clinical trial results across drug classes. Am J Cardiol. 2017;120:S17–S27.
- Cutshall BT, Twilla JD, Olinger AS, et al. A review on cardiovascular effects of newer hypoglycaemic medications. Ann Med. 2017;49:603–612.
- Xie W, Song X, Liu Z. Impact of dipeptidyl-peptidase 4 inhibitors on cardiovascular diseases. Vascul Pharmacol. 2018;109:17–26.
- Kato N, Oka M, Murase T, et al. Discovery and pharmacological characterization of N-[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl}amino)-2-methylpropyl]-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxamide hydrochloride (anagliptin hydrochloride salt) as a potent and selective DPP-IV inhibitor. Bioorg Med Chem. 2011;19:7221–7227.
- Ervinna N, Mita T, Yasunari E, et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology. 2013;154:1260–1270.
- Li Q, Wu X, Liu Y, et al. The effect of anagliptin on intimal hyperplasia of rat carotid artery after balloon injury. Mol Med Rep. 2017;16:8003–8010.
- Ikedo T, Minami M, Kataoka H, et al. Dipeptidyl peptidase-4 inhibitor anagliptin prevents intracranial aneurysm growth by suppressing macrophage infiltration and activation. J Am Heart Assoc. 2017;6:e004777.
- Sato A, Suzuki S, Watanabe S, et al. DPP4 Inhibition Ameliorates Cardiac Function by Blocking the Cleavage of HMGB1 in Diabetic Mice After Myocardial Infarction. Int Heart J. 2017;58:778–786.
- Nishio S, Abe M, Ito H. Anagliptin in the treatment of type 2 diabetes: safety, efficacy, and patient acceptability. Diabetes Metab Syndr Obes. 2015;8:163–171.
- Chiba Y, Yamakawa T, Tsuchiya H, et al. Effect of anagliptin on glycemic and lipid profile in patients with type 2 diabetes mellitus. J Clin Med Res. 2018;10:648–656.
- Faria RX, Gonzaga DTG, Pacheco PAF, et al. Searching for new drugs for Chagas diseases: triazole analogs display high in vitro activity against Trypanosoma cruzi and low toxicity toward mammalian cells. J Bioenerg Biomembr. 2018;50:81–91.
- Piskozub M, Króliczewska B, Króliczewski J. Ribosome nascent chain complexes of the chloroplast-encoded cytochrome b6 thylakoid membrane protein interact with cpSRP54 but not with cpSecY. J Bioenerg Biomembr. 2015;47:265–278.
- Hosseinimehr SJ, Safavi Z, Kangarani Farahani S, et al. The synergistic effect of mefenamic acid with ionizing radiation in colon cancer. J Bioenerg Biomembr. 2019;51:249–257.
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508.
- Kuznetsov AV, Javadov S, Sickinger S, et al. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta. 2015;1853:276–284.
- Zhou S, Sun W, Zhang Z, et al. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev. 2014;2014:260429.
- Otterbein LE, Foresti R, Motterlini R. Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res. 2016;118:1940–1959.
- Czibik G, Derumeaux G, Sawaki D, et al. Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol. 2014;109:450.
- Chen H, Jiang Z. The essential adaptors of innate immune signaling. Protein Cell. 2013;4:27–39.
- Liang J, Li L, Sun Y, et al. The protective effect of activating Nrf2/HO-1 signaling pathway on cardiomyocyte apoptosis after coronary microembolization in rats. BMC Cardiovasc Disord. 2017;17:272.
- Faridvand Y, Nozari S, Vahedian V, et al. Nrf2 activation and down-regulation of HMGB1 and MyD88 expression by amnion membrane extracts in response to the hypoxia-induced injury in cardiac H9c2 cells. Biomed Pharmacother. 2019;109:360–368.
- Jiang T, Jiang D, Zhang L, et al. Anagliptin ameliorates high glucose-induced endothelial dysfunction via suppression of NLRP3 inflammasome activation mediated by SIRT1. Mol Immunol. 2019;107:54–60.
- Li Q, Li J, Liu Y, et al. Anagliptin prevents apoptosis of human umbilical vein endothelial cells by modulating NOX-4 signaling pathways. Biomed Pharmacother. 2018;103:1623–1631.
- Li Q, Zhang M, Xuan L, et al. Anagliptin inhibits neointimal hyperplasia after balloon injury via endothelial cell-specific modulation of SOD-1/RhoA/JNK signaling in the arterial wall. Free Radic Biol Med. 2018;121:105–116.
- Sato H, Kubota N, Kubota T, et al. Anagliptin increases insulin-induced skeletal muscle glucose uptake via an NO-dependent mechanism in mice. Diabetologia. 2016;59:2426–2434.
- Ueda S, Shimabukuro M, Arasaki O, et al. Effect of anagliptin and sitagliptin on low-density lipoprotein cholesterol in type 2 diabetic patients with dyslipidemia and cardiovascular risk: rationale and study design of the reason trial. Cardiovasc Drugs Ther. 2018;32:73–80.