Abstract
Osteoarthritis (OA) is one of the most characterized joint diseases associated with chondrocyte apoptosis. Juglanin has been reported to have anti-inflammation activity. This study aimed to evaluate the protective anti-inflammatory effects of juglanin in human OA chondrocytes. Human OA chondrocytes were pretreated with juglanin (10, 20 and 40 μM) for 2 h and subsequently stimulated with IL-1β for 24 h. Nitric oxide (NO) production was determined using the Griess method and prostaglandin E2 (PGE2), matrix metalloproteinase-3, -9 and -13 (MMP-3, MMP-9 and MMP-13), TNF-α, and IL-6 were assessed using ELISA. The expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), a disintegrin and metalloproteinase with thrombospondin motifs-4 and -5 (ADAMTS-4 and ADAMTS-5) were detected by qRT-PCR and western blot analysis. NF-κB signalling molecules were detected by western blot analysis. The results showed that juglanin dose-dependently suppressed PGE2, NO, MMP-1, MMP3, MMP13, TNF-α and IL-6 production induced by IL-1β. The expression of COX-2, iNOS, ADAMTS-4 and ADAMTS-5 induced by IL-1β were also suppressed by juglanin pretreatment. Western blot analysis showed that juglanin suppressed IL-1β-induced NF-κB activation. Taken together, we found that juglanin inhibits IL-1β-induced inflammation through the regulation of NF-κB signalling. Juglanin might be used as a therapeutic agent for treating OA.
Introduction
Osteoarthritis (OA) is the most prevalent chronic degenerative joint disease, affecting tens of millions of people around the world [Citation1]. The previous study has reported that OA is an age-related chronic disease, which is characterized by inflammation of the synovium, and finally causes disability frequently [Citation2]. Chondrocytes are the only cells in cartilage and are responsible for the synthesis and turnover of the extracellular matrix, which is crucial for joint function [Citation3]. Inflammatory cytokines such as IL-1β and TNF-α have been reported to play an important role in the pathogenesis of OA via up-regulating inflammatory mediators production and matrix metalloproteinases (MMPs) expression [Citation4,Citation5]. IL-1β initiates the activation of inflammation-related signalling pathways and stimulates the expression of matrix metalloproteinases (MMPs), which results in the destruction of the cartilage matrix [Citation6]. Furthermore, treatment of chondrocytes with IL-1β could stimulate the release of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), leading to the production of nitric oxide (NO) and prostaglandin E2 (PGE2) [Citation7,Citation8]. Therefore, there is a reason to believe that the inhibition of IL-1β and IL-1β induced inflammatory mediators may attenuate the progression of OA.
Juglanin is a natural compound belonging to flavonoids, it is extracted from crude “Polygonum aviculare”, exhibiting inhibitory activity against the inflammation response as well as cancer growth [Citation9,Citation10]. Juglanin has been explored in human breast cancer development and progression through apoptosis and autophagy, which was related to intracellular reactive oxygen species (ROS) accumulation [Citation11]. Furthermore, juglanin may exert inhibitory effects against lipopolysaccharide-induced cytokine production in RAW 264.7 macrophages, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6 [Citation12]. However, the effect of juglanin on inflammatory response in human OA chondrocytes remains unclear. Thus, the objective of this study was to evaluate the effect of juglanin on inflammatory response in chondrocytes exposed to interleukin-1β (IL-1β).
Materials and methods
Reagents and antibodies
Juglanin (purity > 98%), dimethylsulfoxide (DMSO), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and recombinant human IL-1β were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Primary antibodies against iNOs, COX-2, p65, p-p65, IκBα, ADAMTS-4, and ADAMTS-5 were acquired from Abcam (Cambridge, MA, USA). ELISA kits of TNF-α, IL-6, MMP-1, MMP-3 and MMP-13, were purchased from Amersham (Little Chalfont, UK).
Primary human OA chondrocyte culture
Tissue collection was according to the terms of the Medical Ethical Committee of the Huaihe Hospital of Henan University and following the guidelines of the Declaration of Helsinki. Human cartilage samples were obtained from OA patients (aged 45–60 years, five women and four men) who underwent total knee replacement surgery at the Huaihe Hospital of Henan University. Primary chondrocytes were isolated from articular cartilage as described previously [Citation13]. Briefly, cartilage was harvested from non-lesional areas and further minced. The tissues were digested with 0.25% trypsin for 30 min. After that, the tissues were further digested by 2 mg/mL collagenase II in DMEM with antibiotics for 6 h at 37 °C. The cells were suspended in DMEM containing 10% fetal bovine serum (FBS), 100 units/mL of penicillin and 100 mg/mL of streptomycin and cultured at 37 °C with 5% CO2. Cells between passages 1–3 were used in this study.
Cell viability assay
MTT assay was used to measure the effect of juglanin on cell viability. Briefly, chondrocytes were seeded in a 96-well plate at a density of 6 × 103/well. Then the cells were treated with various concentrations of juglanin for 24 h and stimulated with or without IL-1β (10 ng/mL) for 24 h. Then the cells were incubated with 20 μL MTT (5 mg/mL) for an additional 4 h. The supernatant was removed and the cells were dissolved in DMSO (150 μL/well). The optical densities of the samples were measured at 490 nm on a micro-plate reader (Bio-Rad, Hercules, CA, USA).
NO measurement
Chondrocytes were treated with juglanin 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The NO concentration in surpernatant was determined using the Griess reagent (Sigma) according to the manufacturer’s instructions. The assays were performed in duplicate.
qRT-PCR
RNA isolation was performed by using the RNeasy mini kit (QIAGEN, Valencia, CA, USA). The Quantitect reverse transcription kit (QIAGEN, USA) was used to synthesize the subsequent cDNA. Real-time PCR was performed using SYBR Green Master Mix (QIAGEN, USA). Samples were normalized to internal control β-actin. Primer sequences used for real-time PCR are listed in .
Table 1. Primer sequences used in qRT-PCR experiments.
Western blot
The chondrocytes at different conditions were lysed by RIPA buffer with protease and phosphatase inhibitors. Protein concentration was determined by the DC Bio-Rad Laboratories protein reagent (Bio-Rad, USA). Total 20 μg cell lysates or nuclear extracts were immobilized by PAGE gel. The separated protein mixes were then transferred to PVDF membranes and blotted against their specific antibodies. The following antibodies were used: antibodies against iNOS, COX-2, ADAMTS-4, ADAMTS-5, p65, p-p65, IκBα and β-actin (dilution 1:1000). The immunoreactive bands were visualized using an ECL System (Amersham Biosciences, Piscataway, NJ).
ELISA
Chondrocytes were pretreated with juglanin for 2 h and then stimulated with IL-1β (10 ng/mL) for 24 h. The levels of PGE2, IL-6, TNF-α, MMP-1, MMP-3, and MMP-13 in the culture medium were measured using ELISA kits according to the manufacturer’s instructions.
Statistical analysis
All data are presented as the mean ± SEM Statistical analysis was assessed by one-way ANOVA (Dunnett’s t-test) and Tukey’s multiple comparison tests. Differences were considered significant when the values of p were < .05.
Results
Juglanin reversed IL-1β-induced reduction in cell viability
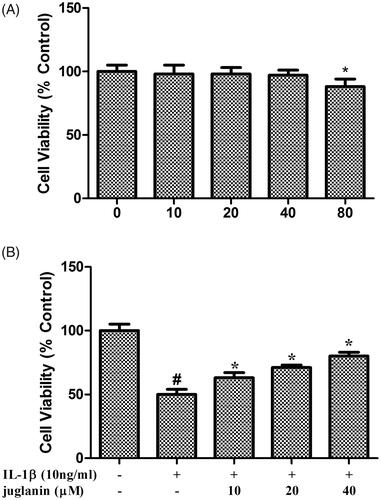
First, we assessed the effect of juglanin on chondrocyte viability. As shown in , treatment with different concentrations (0, 10, 20, 40 and 80 μM) of juglanin for 24 h did not affect the viability of the chondrocytes. Then, we investigated the protective effects of juglanin against IL-1β-induced apoptosis in chondrocytes. Chondrocytes treated with juglanin and IL-1β showed higher cell viability than the IL-1β-induced group (.
Figure 1. Juglanin reversed IL-1β-induced reduction in cell viability. (A) Cells were treated with 0, 10, 20, 40 and 80 μM of juglanin in serum-free medium for 24 h. Cell proliferation was measured with the MTT assay. (n = 3). *p < .05 compared to control group. (B) The cells were pre-cultured in serum-free medium in the presence or absence of juglanin (0, 10, 20 and 40 μM) for 24 h, and then stimulated with 10 ng/mL IL-1β for a further 24 h (n = 3). Cell proliferation was measured with the MTT assay. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
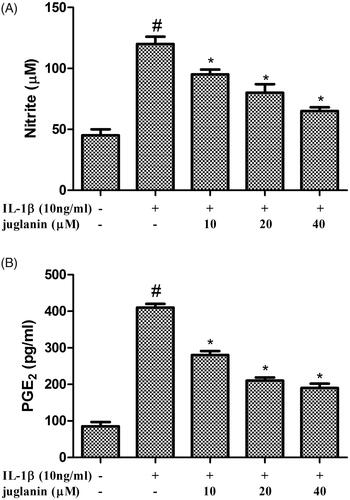
Juglanin reduced NO and PGE2 production in IL-1β-induced human OA chondrocytes
In order to assess the effect of juglanin on NO and PGE2 production in chondrocytes in response to IL-1β, chondrocytes were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. The NO production in the cell suspension increased after IL-1β treatment, while IL-1β-induced NO production was suppressed in a dose-dependent manner by juglanin (. IL-1β stimulation also significantly increased the PGE2 level; however, juglanin reduced IL-1β induced PGE2 release in a dose-dependent manner (.
Figure 2. Juglanin decreased iNOS and COX-2 expression in IL-1β-induced human OA chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. NO concentration in the culture medium was determined by the Griess reaction (A). PGE2 concentration was determined by an Enzyme-linked immunosorbent assay (ELISA) kit (B). Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
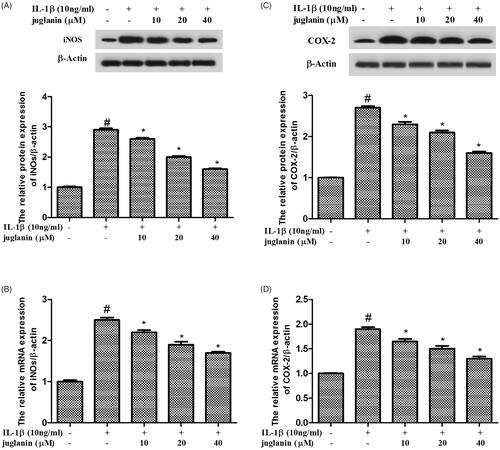
Juglanin decreased iNOS and COX-2 expression in IL-1β-induced human OA chondrocytes
Human OA chondrocytes were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h, then stimulated with 10 ng/mL IL-1β for 24 h, followed by Western blot analysis and qRT-PCR. As shown in , we found that IL-1β markedly increased the expression of iNOs and COX-2 compared to the control group. The enhanced expressions of these mediators induced by IL-1β were dose-dependently suppressed by juglanin pretreatment.
Figure 3. Juglanin decreased iNOS and COX-2 expression in IL-1β-induced human OA chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. The expression of these proteins and mRNA were assessed by Western blot and qRT-PCR. Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
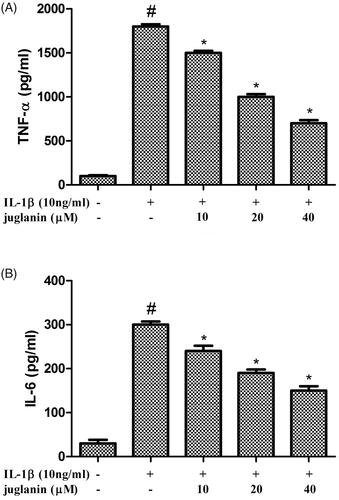
Juglanin inhibited the production of TNF-α and IL-6 in IL-1β-stimulated human OA chondrocytes
Chondrocytes were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. The IL-6 and TNF-α levels were investigated using an ELISA kit. As shown in , the results showed that the level of IL-6 and TNF-α in the supernatant increased significantly after IL-1β treatment. However, juglanin dose-dependently suppressed IL-1β-induced IL-6 and TNF-α production.
Figure 4. Juglanin inhibited the production of TNF-α and IL-6 in IL-1β-stimulated human OA chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. TNF-α and IL-6 concentrations were determined by ELISA kits. Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
Juglanin decreased the production of MMP-1, MMP-3 and MMP-13 in IL-1β-stimulated chondrocytes
MMP-1, MMP-3, and MMP-13 play critical roles in degrading cartilage. In this study, the effects of juglanin on IL-1β-induced MMP-1, MMP-3 and MMP-13 expression in chondrocytes were detected by ELISA. The results showed that the expression of MMP-1, MMP-3 and MMP-13 increased significantly after IL-1β treatment. However, juglanin inhibited IL-1β-induced MMP-1, MMP-3 and MMP-13 expression in a dose-dependent manner ().
Figure 5. Juglanin decreased production of MMP-1, MMP-3 and MMP-13 in IL-1β-stimulated chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. MMP-1, MMP-3 and MMP-13 concentrations were determined by ELISA kits. Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
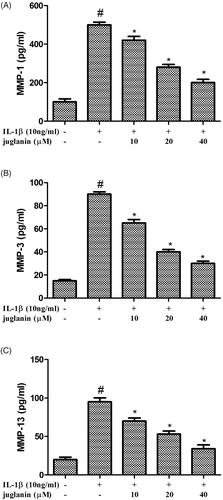
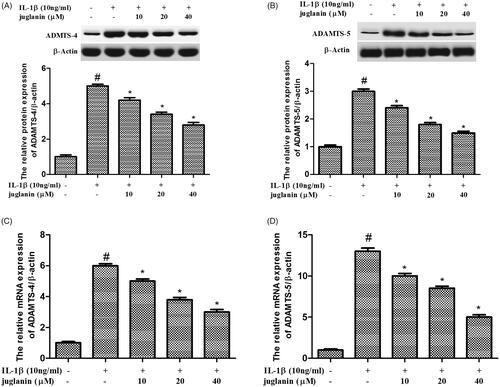
Juglanin suppressed the expression of ADAMTS-4 and ADAMTS-5 in IL-1β-stimulated chondrocytes
The inflammation and synthesis of catabolic factors have been identified as key players in OA pathogenesis. Therefore, the protein levels of these mediators including ADAMTS-4 and ADAMTS-5 were measured. Chondrocytes were pretreated with different concentrations (10, 20 and 40 μM) of juglanin for 2 h, then stimulated with 10 ng/mL IL-1β for 24 h. IL-1β upregulated the expression ADAMTS-4 and ADAMTS-5 compared to the control. The enhanced expressions of these mediators induced by IL-1β were dose-dependently suppressed by juglanin pretreatment ().
Figure 6. Juglanin suppressed the expression of ADAMTS-4 and ADAMTS-5 in IL-1β-stimulated chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. The expression of these proteins and mRNA were assessed by Western blot and qRT-PCR. Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
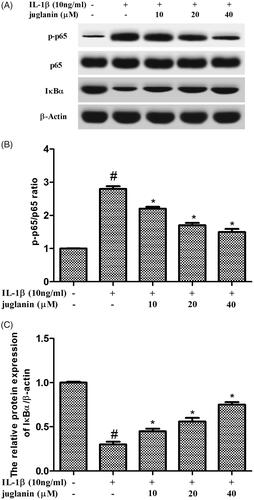
Juglanin prevented IL-1β-induced NF-κB activation in chondrocytes
To further investigate the mechanism underlying the inhibitory effect of juglanin, Western blot analysis was performed to study changes in NF-κB signalling pathway. As shown in ), IL-1β significantly promoted the expression of p-p65, reduced the expression of IκBα. In contrast, juglanin exhibited a dramatic inhibitory effect on IL-1β-induced phosphorylation of p65 as well as degradation of IκBα. The results indicated that IL-1β promoted the activity of NF-κB, while juglanin reduced the activity of NF-κB.
Figure 7. Juglanin prevented IL-1β-induced NF-κB activation in chondrocytes. The cells were pretreated with various concentrations of juglanin (10, 20 and 40 μM) for 2 h before subsequent IL-1β stimulation for 24 h. The expression of these proteins was assessed by Western blot. Data are expressed as mean ± SEM. All experiments were repeated three times. #p < .05 compared to control group. *p < .05 compared to IL-1β treatment group.
Discussion
OA is the most common joint diseases that usually caused pain and led to disability in order adults [Citation14]. Juglanin, a natural compound extracted from the crude Polygonum aviculare, was reported to possess anti-inflammatory activity. In this study, we found that juglanin inhibited IL-1β-induced inflammatory response by activating NF-κB in human osteoarthritis chondrocytes. Juglanin has potential as a therapeutic drug in the treatment of OA.
IL-1β, an important inflammatory cytokine, plays an important role in cartilage degradation [Citation15]. IL-1β stimulation promotes gene expression and protein secretion of other pro-inflammatory factors and chemokines such as IL-6 and TNF-α, which may induce secondary insults to chondrocytes [Citation16]. Furthermore, treatment of chondrocytes with IL-1β could stimulate the release of iNOS and COX-2, leading to the production of NO and PGE2, which are important inflammatory mediators of OA [Citation17]. In this study, we found that juglanin blocked the production of NO, PGE2, TNF-α and IL-6, and downregulated COX-2 and iNOS expression at both mRNA and protein levels. Our results are consistent with the previous study that juglanin blocked the production of TNF-α and IL-6 in lipopolysaccharide-induced RAW 264.7 macrophages [Citation12].
Furthermore, IL-1β has also been reported to induce MMP-1, MMP-3, MMP-13 and ADAMTS-4 in human tendon cells20 and promote MMP-1 and MMP-2 expression in human aortic valve myofibroblasts [Citation18]. Many studies suggested that inhibition of MMP production could inhibit the progression of cartilage degradation [Citation19]. Thus, targeting MMPs represents a promising strategy for potential therapy of OA. In the current study, we demonstrated that juglanin inhibited IL-1β-induced MMP-1, MMP-3 and MMP13 expression in human OA chondrocytes. These results indicate that juglanin has anti-inflammatory effects against OA.
ADAMTS is also an important protein hydrolysate in OA [Citation14], especially ADAMTS-4 and ADAMTS-5, which are responsible for cleaving aggrecan. Several studies have demonstrated that IL-1β raises ADAMTS levels in OA patients. In this study, we found that juglanin inhibited IL-1β-stimulated ADAMTS-4 and ADAMTS-5 production at both mRNA and protein levels in human OA chondrocytes. Thus we speculated that juglanin may exert anti-inflammation action by decreasing the production and activation of ADAMTS in the progression of OA.
NF-κB is one of the most important transcription factors that regulate the expression of iNOS, MMPs and COX-2 [Citation20]. IκB-a is a cellular protein which inhibits the NF-κB activation by masking the nuclear localization signals of NF-κB proteins and keeping them sequestered in an inactive state in the cytoplasm [Citation21]. To investigate the anti-inflammatory mechanism of juglanin, the effects of juglanin on IL-1β-induced NF-κB activation were detected in this study. Our results indicated that juglanin markedly suppressed the expression of p-p65, promoted the expression of IκBα in IL-1β-stimulated chondrocytes. These results indicated that the anti-inflammatory effects of juglanin on chondrocytes are likely realized through the NF-κB pathway.
In conclusion, our study is the first to evaluate the potential effects of juglanin on human OA chondrocytes. Our results demonstrate that juglanin inhibits IL-1β-induced inflammation via the regulation of NF-κB signalling, and suggest that juglanin may be a potential therapeutic agent for OA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
- Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23:1966–1971.
- He W, Cheng Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;97:607–615.
- Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220.
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:1.
- Scuruchi M, D'Ascola A, Avenoso A, et al. Serglycin as part of IL-1beta induced inflammation in human chondrocytes. Arch Biochem Biophys. 2019;669:80–86.
- Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427:S27–S36.
- Ahmed S, Rahman A, Hasnain A, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002;33:1097–1105.
- Huang XY, Duan QY, Liu JX, et al. Determination of a novel diarylheptanoid (Juglanin B) from green walnut husks (Juglans regia L.) in rat plasma by high-performance liquid chromatography. Biomed Chromatogr.. 2010;24:307–311.
- Yang HH, Hwangbo K, Zheng MS, et al. Inhibitory effects of juglanin on cellular senescence in human dermal fibroblasts. J Nat Med. 2014;68:473–480.
- Zhou GY, Yi YX, Jin LX, et al. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother. 2016;81:318–328.
- Schwarz S, Sauter D, Wang K, et al. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80:177–182.
- Cheng AW, Stabler TV, Bolognesi M, et al. Selenomethionine inhibits IL-1beta inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary human chondrocytes. Osteoarthr Cartil. 2011;19:118–125.
- Fan Z, Bau B, Yang H, et al. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52:136–143.
- Xu Y, Dai GJ, Liu Q, et al. Sanmiao formula inhibits chondrocyte apoptosis and cartilage matrix degradation in a rat model of osteoarthritis. Exper Therapeutic Med. 2014;8:1065–1074.
- Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42.
- Roman-Blas JA, Contreras-Blasco MA, Largo R, et al. Differential effects of the antioxidant n-acetylcysteine on the production of catabolic mediators in IL-1beta-stimulated human osteoarthritic synoviocytes and chondrocytes. Euro J Pharmacol. 2009;623:125–131.
- Choi YH, Bae JK, Chae HS, et al. Isoliquiritigenin ameliorates dextran sulfate sodium-induced colitis through the inhibition of MAPK pathway. Int Immunopharmacol. 2016;31:223–232.
- Mengshol JA, Mix KS, Brinckerhoff CE. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull’s-eye or missing the mark? Arthritis Rheum. 2002;46:13–20.
- Pan T, Chen R, Wu D, et al. Corrigendum to “Alpha-Mangostin suppresses interleukin-1beta-induced apoptosis in rat chondrocytes by inhibiting the NF-kappaB signaling pathway and delays the progression of osteoarthritis in a rat model” [Int. Immunopharmacol. 52 (2017) 156-162]. Int Immunopharmacol. 2018;65:559–560.
- Chen Q, Wu S, Wu Y, et al. MiR-149 suppresses the inflammatory response of chondrocytes in osteoarthritis by down-regulating the activation of TAK1/NF-kappaB. Biomed Pharmacother. 2018;101:763–768.
