Abstract
Background
Myocardial ischemia is the main reason for ischemic heart disease. Baicalin is a plant-derived flavonoid with cardio-protective activity. Herein, we tested the influences of baicalin on cardiomyocytes H9c2 apoptosis aroused by hypoxia stimulation.
Methods
Firstly, H9c2 cells were subjected to hypoxia and/or baicalin exposure. Cell viability and apoptosis, along with hypoxia-inducible factor 1α (HIF1α) and Bcl-2/adenovirus E1B 19-KDa interacting protein 3 (BNIP3) expressions were tested respectively. Then, si-HIF1α was transfected into H9c2 cells to probe whether up-regulation of HIF1α attended to the influences of baicalin on hypoxia-stimulated H9c2 cells. Finally, the regulatory effect of nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1) pathway on HIF1α expression was analyzed.
Results
Hypoxia exposure aroused H9c2 cell viability reduction and apoptosis. Baicalin mitigated H9c2 cell viability reduction and apoptosis aroused by hypoxia. Moreover, HIF1α/BNIP3 pathway was further activated by baicalin in hypoxia-exposed H9c2 cells. Silencing HIF1α lowered the functions of baicalin on hypoxia-exposed H9c2 cells. Besides, baicalin enhanced hypoxia-caused activation of Nrf2/HO-1 pathway. Activation of Nrf2/HO-1 pathway was associated with the up-regulation of HIF1α and protective functions of baicalin on hypoxia-exposed H9c2 cells.
Conclusion
Baicalin relieved cardiomyocytes H9c2 apoptosis aroused by hypoxia might be achieved through activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway.
Baicalin mitigates H9c2 cell viability loss and apoptosis aroused by hypoxia;
Baicalin activates HIF1a/BNIP3 pathway in hypoxia-exposed H9c2 cells;
Silencing HIF1α weakens the influences of baicalin on hypoxia-exposed H9c2 cells;
Baicalin promotes Nrf2/HO-1 pathway in hypoxia-exposed H9c2 cells;
Promotion of Nrf2/HO-1 pathway is related to the up-regulation of HIF1α.
Highlights
Introduction
Myocardial ischemia is the main reason for ischemic heart disease, in which the blood supplement to the cardiomyocytes is diminished [Citation1]. Insufficient oxygen supplement will disturb the energy metabolism in cardiomuyocytes, resulting in cardiomyocytes damage and inability to support normal heart function [Citation2,Citation3]. Detailed studies over the past few years have proved that persistent myocardial ischemia is the main reason for ischemic heart disease and sudden death [Citation4,Citation5]. The strategies for exploring the molecular mechanism of cardiomyocytes damage under hypoxia environment and searching effective drugs that can prevent cardiomyocytes from damage are of great importance sense for controlling ischemic heart disease.
Hypoxia-inducible factor 1 (HIF1) is a transcription factor that exerts pivotal regulatory role in oxygen homeostasis by controlling both the delivery and utilization of oxygen [Citation6]. HIF1α is the functional subunit of HIF1 and important to the HIF1 activation under hypoxia condition [Citation7]. Blanco et al. reported that the HIF1α expression was steadily increased in human cardiac fibers after ischemia [Citation8]. Bcl-2/adenovirus E1B 19-KDa interacting protein 3 (BNIP3) is an key downstream factor of HIF1α [Citation9]. It is proved that overexpression of HIF1α could promote the expression of BNIP3 [Citation9]. Peng et al. proved that injection of HIF1α expressing recombinant Adeno-associated virus (rAAV) could amelioratemyocardial ischemia and improve cardiac function in Wistar rats [Citation10].
Baicalin (CAS number: 21967–41-9, ) is a natural flavonoid separated from the roots of Scutellaria baicalensis Georgi with cardio-protective activity under hypoxia condition [Citation11,Citation12]. For example, an experimental study from Xue et al. reported that baicalin could attenuate myocardial ischemic inflammatory injury via suppressing the expression of aryl hydrocarbon receptor (AhR) [Citation13]. Another study from Liu et al. indicated that baicalin could attenuate rat myocardial infarction through modulating mitogen-activated protein kinase (MAPK) pathway [Citation14]. However, there is a relative paucity of literature concerning the influences of baicalin on HIF1α expression under hypoxia condition. More experiments are demanded to probe the possible regulatory activity of baicalin on HIF1α/BNIP3 pathway in cardiomyocytes after hypoxia stimulation.
In the current research, rat cardiomyocytes H9c2 was grown under hypoxia environment to simulate cardiomyocytes damage in myocardial ischemia as previous described [Citation15]. Then, we tested the influence of baicalin on cardiomyocytes damage under hypoxia environment. What′s more, the role of HIF1α/BNIP3 pathway activation in this influence was analyzed.
Materials and methods
Cell culture and treatment
H9c2 cells (GNR-5, Stem Cell Bank, Chinese Academy of Science, Shanghai, China) were grown in DMEM (D0819, Sigma-Aldrich, MO, USA) including 10% (v/v) fetal bovine serum (FBS, F8192, Sigma-Aldrich), 1% (v/v) penicillin-streptomycin solution (15070–063, Gibco, CA, USA) at 37 °C in the incubator (MCO-5AC, Panasonic, Kyoto, Japan) with 5% CO2 and 95% air.
To stimulate cell damage, H9c2 cells were cultured in hypoxia incubator with 94% N2, 5% CO2 and 1% O2 for different times [Citation16]. Baicalin (purity >95%, 572667, Sigma-Aldrich) was diluted into dimethyl sulfoxide (DMSO). H9c2 cells were subjected to 25, 50, 75 or 100 μM baicalin for 24 h.
Cell transfection
si-HIF1α and its negative control (si-NC#1), along with si-nuclear factor E2-related factor 2 (si-Nrf2) and its NC(si-NC#2) were received from GenePharmo Corporation (Shanghai, China). The sequences of si-HIF1α were: 5′-GGAAAGAGAGUCAUAGAAA-3′ (sense) and 5′-UUUCUAUGACUCUCUUUCC-3′ (antisense). The sequences of si-NC#1 were: 5′-GAUCAUACGUGCGAUCAGA-3′ (sense) and 5′-UCUGAUCGCACGUAUGAUC-3′ (sense). The sequences of si-Nrf2 were: 5′-CAGGAGAACUGUACCUGUUU-3′ (sense) and 5′-ACCAGGUACAGUUCUGCUGUU-3′ (antisense). The sequences of si-NC#2 were: 5′-UAGCGACUAAACACAUCAAUU-3′ (sense) and 5′-UUGAUGUGUUUAGUCGCUAUU-3′ (antisense). LipofectamineTM 3000 transfection reagent (L3000-008, Invitrogen, CA, USA) was utilized for transfection. Transfection efficiencies were evaluated by western blotting.
Detection of cell viability
Cell counting kit-8 (CCK-8) assay (C0039, Beyotime Biotechnology) was utilized for test H9c2 cell viability. Briefly, cells were cultivated into 96-well plate (5000 cells/well) and exposed to 25–100 μM baicalin under normoxia/hypoxia condition for 12–48 h. Then, 10 μL kit solution was mixed into the culture medium for 1 h. The absorbance was tested by Microplate reader (PerkinElmer EnSpire, MA, USA) at 450 nm. Cell viability was expressed as the percentages of control.
Assessment of cell apoptosis
Annexin V-FITC/PI apoptosis detection kit (556547, BD Biosciences, NJ, USA) was utilized for test H9c2 cell apoptosis. Briefly, cells were cultivated into 6-well plate (1 × 105 cells/well) and exposed to 75 μM baicalin under normoxia/hypoxia condition for 24 h. Subsequently, cells were gathered in line with the experimental group, rinsed using kit buffer and mixed with 5 μL Annexin V-FITC and 5 μL PI for 15 min at 20–25 °C in the dark. After that, the percentage of apoptotic cells was assessed by flow cytometer (Attune NxT, Life Technologies, CA, USA).
Western blotting
Total proteins were separated using RIPA Lysis Buffer (HY-K1001, MedChem Express, NJ, USA) including protease inhibitors (HY-K0010, MedChem Express). Western blotting was carried out and signals of proteins were analyzed as earlier described [Citation17]. Following antibodies were used: Bcl-2 (ab196495), HIF1α (ab216842), BNIP3 (ab10433), Nrf2 (ab137550), heme oxygenase 1 (HO-1, ab13243, Abcam Biotechnology, MA, USA), p53 (#2524), Bax (#2772), Cleaved-caspase 9 (#9508), Cleaved-caspase 3 (#9665), β-actin (#4967), anti-mouse IgG (H + L) (DyLightTM 800 4X PEG Conjugate) (#5257) and anti-rabbit IgG (H + L) (DyLightTM 800 4X PEG Conjugate) (#5151, Cell Signaling Technology, MA, USA).
Statistical analysis
All experiments were repeated three times. Results were expressed as the mean ± standard deviation (SD). Graphpad 6.0 statistical software was utilized for statistical analysis. p values were computed by ANOVA with Tukey’s post-hoc test. p < .05 was regarded as statistical significance.
Results
Baicalin mitigated H9c2 cell viability reduction and apoptosis aroused by hypoxia
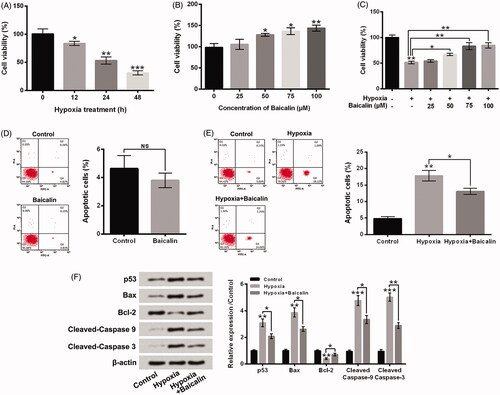
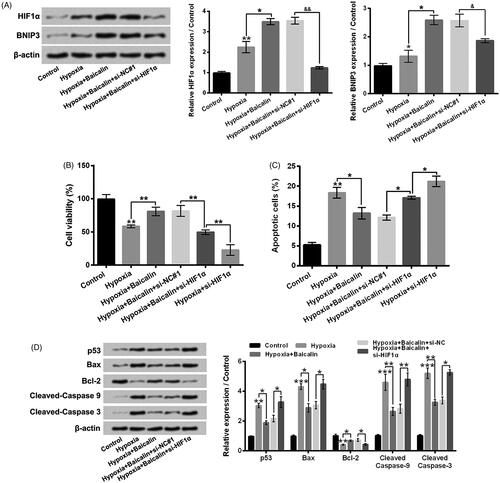
Data in showed that hypoxia exposure notably lowered H9c2 cell viability with a time-dependent pathway (p < .05, p < .01 or p < .001). Hypoxia exposure for 24 h lowered the H9c2 cell viability to 53.31 ± 4.63%, which was picked for subsequent experiments. displayed that 50–100 μM baicalin treatment significantly increased H9c2 cell viability (p < .05 or p < .01). Moreover, presented that 50, 75 or 100 μM baicalin remarkably relieved the cell viability reduction aroused by hypoxia stimulation (p < .05 or p < .01). Baicalin treatment of 75 μM was selected for further experiments. presented that 75 μM baicalin had no obvious influence on cell apoptosis. Hypoxia dramatically elevated cell apoptosis (, p < .001), while baicalin noticeably alleviated the cell apoptosis aroused by hypoxia (p < .05). Besides, hypoxia remarkably raised the p53, Bax, Cleaved-caspase 9 and Cleaved-caspase 3 expressions, but declined the Bcl-2 expression in H9c2 cells (, p < .01 or p < .001). By contrast with single hypoxia exposure group, the p53, Bax, Cleaved-caspase 9 and Cleaved-caspase 3 expressions were lowered, while the Bcl-2 expression was enhanced in hypoxia + baicalin group (p < .05). These results illustrated that baicalin could weaken cardiomyocates H9c2 viability reduction and apoptosis aroused by hypoxia.
Figure 2. Baicalin weakened H9c2 cell apoptosis aroused by hypoxia. H9c2 cells were subjected to hypoxia exposure for 12, 24 or 48 h (A), 25, 50, 75 or 100 μM baicalin incubation for 24 h (B) or 25–100 μM baicalin incubation for 24 h under hypoxia condition (C), respectively. Cell viability was tested. (D) Followed by 75 μM baicalin incubation for 24 h, the H9c2 cell apoptosis was tested. Followed by 75 μM baicalin incubation for 24 h under hypoxia condition, (E) H9c2 cell apoptosis and (F) the p53, Bax, Bcl-2, Cleaved-caspase 9 and Cleaved-caspase 3 expressions were tested respectively. N = 3. *p < .05, **p < .01, ***p < .001. NS: No significance.
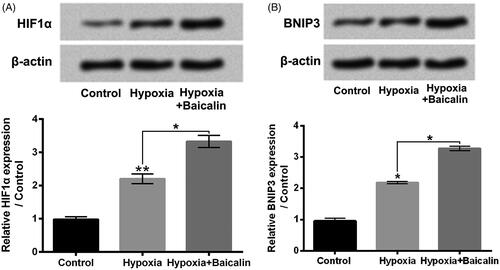
Baicalin further activated HIF1α/BNIP3 pathway in hypoxia-stimulated H9c2 cells
displayed that hypoxia exposure for 24 h obviously enhanced the HIF1α and BNIP3 protein levels in H9c2 cells (p < .05 or p < .01). Besides, 75 μM baicalin further raised the HIF1α and BNIP3 protein levels in hypoxia-exposed H9c2 cells (p < .05). These results illustrated that baicalin could further activated HIF1α/BNIP3 pathway in hypoxia-stimulated H9c2 cells and implied that activation of HIF1α/BNIP3 pathway might be related to the influences of baicalin on H9c2 cell viability reduction and apoptosis aroused by hypoxia.
Silencing HIF1α weakened the influences of baicalin on H9c2 cell viability reduced and apoptosis aroused by hypoxia
showed that si-HIF1α transfection significantly reversed the hypoxia + Baicalin-caused elevation of HIF1α and BNIP3 expressions in H9c2 cells (p < .05 or p < .01). Moreover, by contrast with hypoxia + baicalin + si-NC#1 group, the H9c2 cell viability was notably lowered (, p < .01), while the apoptosis was noticeably enhanced (, p < .05) in hypoxia + baicalin + si-HIF1α group. In addition, by contrast with hypoxia + baicalin + si-NC#1 group, the p53, Bax, Cleaved-caspase 9 and Cleaved-caspase 3 expressions were raised, while the Bcl-2 expression was decreased in hypoxia + baicalin + si-HIF1α group (, p < .05 or p < .01). These above findings illustrated that activation of HIF1α/BNIP3 pathway joined in the influences of baicalin on H9c2 cell viability reduction and apoptosis aroused by hypoxia.
Figure 4. Silencing HIF1α weakened the influences of baicalin on H9c2 cell viability reduction and apoptosis aroused by hypoxia. H9c2 cells were subjected to 75 μM baicalin treatment and/or si-HIF1α transfection under hypoxia condition. (A) The HIF1α and BNIP3 expressions, (B) cell viability, (C) cell apoptosis and (D) the p53, Bax, Bcl-2, Cleaved-caspase 9 and Cleaved-caspase 3 expressions were tested respectively. N = 3. *p < .05, **p < .01 or ***p < .001.
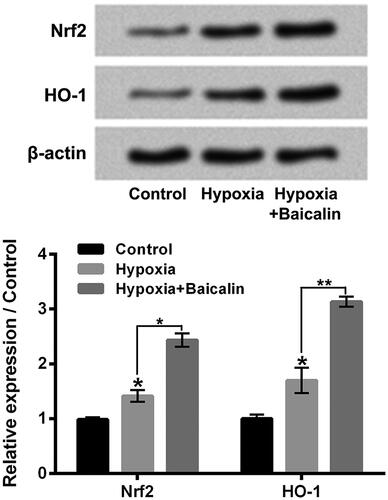
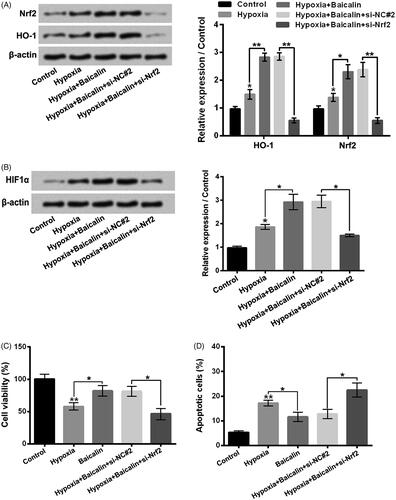
Baicalin enhanced hypoxia-aroused activation of Nrf2/HO-1 pathway
Nrf2/HO-1 pathway was discovered to took part in the regulation of HIF1α expression in cells [Citation18]. So, followed by hypoxia and/or baicalin exposure, the Nrf2/HO-1 pathway in H9c2 cells was tested. As shown in , hypoxia exposure for 24 h notably activated Nrf2/HO-1 pathway in H9c2 cells by enhancing the Nrf2 and HO-1 protein levels (p < .05). Moreover, 75 μM baicalin further enhanced the Nrf2 and HO-1 expressions (p < .05 or p < .01). These results indicated that baicalin could further promote Nrf2/HO-1 pathway inhypoxia-stimulated H9c2 cells.
Promotion of Nrf2/HO-1 pathway attended to the up-regulation of HIF1α and influences of baicalin on hypoxia-stimulated H9c2 cells
displayed that si-Nrf2 transfection obviously lowered the Nrf2 and HO-1 expressions in H9c2 cells (p < .01). In addition, the HIF1α expression was also notably declined in hypoxia + baicalin + si-Nrf2 group, by contrast with hypoxia + baicalin + si-NC#2 group (, p < .05). Besides, showed that si-Nrf2 transfection also weakened the influences of baicalin on H9c2 cell viability reduction and apoptosis aroused by hypoxia. These outcomes illustrated that baicalin exhibited protective influence on hypoxia-stimulated H9c2 cell damage might be via activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway.
Figure 6. Activation of Nrf2/HO-1 pathway attended to the up-regulation of HIF1α and influences of baicalin on hypoxia-stimulated H9c2 cells. H9c2 cells were subjected to 75 μM baicalin treatment and/or si-Nrf2 transfection under hypoxia condition. (A and B) The Nrf2, HO-1 and HIF1α expressions, and (C and D) the cell viability and apoptosis were tested respectively. N = 3. *p < .05, **p < .01.
Discussion
Cardiomyocytes damage caused by hypoxia has been linked to many ischemia heart diseases [Citation19,Citation20]. Herein, we further tested the influences of baicalin on cardiomyocytes H9c2 viability reduction and apoptosis aroused by hypoxia. Specifically, we found that baicalin activated the HIF1α/BNIP3 pathway in hypoxia-exposed H9c2 cells. Up-regulation of HIF1α joined in the influences of baicalin on H9c2 cell viability reduction and apoptosis aroused by hypoxia. Besides, we discovered that baicalin enhanced the hypoxia-caused activation of Nrf2/HO-1 pathway, which attended to the up-regulation of HIF1α in H9c2 cells.
With the idea of “back to nature”, great efforts have been made to explore the pharmacological activity of plant-derived compounds [Citation21,Citation22]. Baicalin is a natural flavonoid compound separated from the roots of Scutellaria baicalensis [Citation13]. Earlier reports proved that baicalin relieved cardiomyocytes damage under hypoxia environment [Citation14,Citation23]. Herein, we further confirmed that baicalin mitigated cardiomyocytes H9c2 viability loss and apoptosis aroused by hypoxia.
HIF1α is only steadily expressed in cells under hypoxia environment, which can activate HIF1 and then influence the expression of multiple genes, including BNIP3, in response to low oxygen condition [Citation6]. Under normoxia environment, HIF1α is rapidly degraded by the ubiquitin-proteasome pathway [Citation24]. In this research, we discovered that hypoxia exposure enhanced the HIF1α and BNIP3 expressions in H9c2 cells, which was identical with the results of experimental study from Blanco Pampin et al. [Citation8], and might be caused by self-protective effects of cardiomyocates [Citation25]. More importantly, we discovered that baicalin treatment further up-regulated the HIF1α and BNIP3 expressions in H9c2 cells under hypoxia condition. Silencing HIF1α by si-HIF1α transfection attenuated the protective influences of baicalin on H9c2 cell viability loss and apoptosis aroused by hypoxia. These findings suggested that baicalin exerted protective activity on cardiomyocytes damage under hypoxia condition could be via up-regulating HIF1α and activating HIF1α/BNIP3 pathway. Besides, considering that baicalin exerts protective activity on myocardial ischemic also by suppressing AhR expression [Citation13], inhibiting 12/15-lipoxygenase [Citation26] and attenuating oxidative stress [Citation27], it is worthy believing that many cellular factors joining in the beneficial influences of baicalin on myocardial ischemic that may form a complex regulatory network.
As a redox-sensitive transcription factor, Nrf2 is important to inhibit oxidative stress in cells [Citation28]. Once activated, Nrf2 can transactivate antioxidant response element (ARE)-driven genes, specially HO-1 [Citation29]. Nrf2/HO-1 pathway has been discovered to exhibit protective roles in many ischemic disorders, including myocardial ischemia [Citation30]. Wang et al. reported that hypoxia stimulation could raise the Nrf2/HO-1 pathway activation in cardiomyocytes [Citation31]. Cheng et al. proved that baicalin could elevate Nrf2/HO-1 pathway activation in rats intestinal tissues under ischemic/reperfusion treatment [Citation32]. Herein, we discovered that hypoxia exposure promoted the Nrf2/HO-1 pathway activation in H9c2 cells by raising the Nrf2 and HO-1 expressions. Baicalin treatment further activated the Nrf2/HO-1 pathway in H9c2 cells via further enhancing the Nrf2 and HO-1 expressions. Besides, previous study demonstrated that Nrf2/HO-1 pathway attended to the modulation of HIF1α expression in cells [Citation18]. Herein, we discovered that knockdown of Nrf2 by si-Nrf2 transfection reversed the baicalin-induced elevation of HIF1α expression under hypoxia condition and also weakened the influences of baicalin on H9c2 cell viability loss and apoptosis aroused by hypoxia. These outcomes illustrated that baicalin activated HIF1α/BNIP3 pathway at least partially through activating Nrf2/HO-1 pathway.
Taken together, this research confirmed that baicalin weakened the hypoxia-aroused cardiomyocytes H9c2 viability reduction and apoptosis might be via activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Baicalin may be a possible preventive and therapeutic compound for alleviating cardiomyocytes damage in myocardial ischemia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Balakumar P, Singh H, Singh M, et al. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res. 2009;60:18–23
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508.
- Hsieh DJ, Huang CY, Pai P, et al. Prolactin protects cardiomyocytes against intermittent hypoxia-induced cell damage by the modulation of signaling pathways related to cardiac hypertrophy and proliferation. Int J Cardiol. 2015;181:255–266.
- Tibaut M, Mekis D, Petrovic D. Pathophysiology of myocardial infarction and acute management strategies. Cardiovasc Hematol Agents Med Chem. 2017;14:150–159.
- Chugh SS, Aro AL. Coronary ischemia: global trigger of sudden cardiac death. Heart Rhythm. 2017;14:88–89.
- Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56.
- Joshi S, Singh AR, Durden DL. MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J Biol Chem. 2014;289(33):22785–22797.
- Blanco Pampin J, Garcia Rivero SA, Otero Cepeda XL, et al. Immunohistochemical expression of HIF-1alpha in response to early myocardial ischemia. J Forensic Sci. 2006;51:120–124.
- Vasagiri N, Kutala VK. Structure, function, and epigenetic regulation of BNIP3: a pathophysiological relevance. Mol Biol Rep. 2014;41:7705–7714.
- Jianqiang P, Ping Z, Xinmin F, et al. Expression of hypoxia-inducible factor 1 alpha ameliorate myocardial ischemia in rat. Biochem Biophys Res Commun. 2015;465:691–695.
- Zhang S, Wang J, Pan J. Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016;23:3696–3703.
- Zhang J, Liu S, Xu B, et al. Study of baicalin on sympathoexcitation induced by myocardial ischemia via P2X3 receptor in superior cervical ganglia. Auton Neurosci. 2015;189:8–15.
- Xue Y, Shui X, Su W, et al. Baicalin inhibits inflammation and attenuates myocardial ischaemic injury by aryl hydrocarbon receptor. J Pharm Pharmacol.. 2015;67:1756–1764.
- Liu X, Gu J, Fan Y, et al. Baicalin attenuates acute myocardial infarction of rats via mediating the mitogen-activated protein kinase pathway. Biol Pharm Bull. 2013;36:988–994.
- Long TY, Jing R, Kuang F, et al. CIRBP protects H9C2 cells against myocardial ischemia through inhibition of NF-kappaB pathway. Braz J Med Biol Res. 2017;50(4):e5861.
- Bijlsma MF, Groot AP, Oduro JP, et al. Hypoxia induces a hedgehog response mediated by HIF-1alpha. J Cell Mol Med. 2009;13:2053–2060.
- Chu Y, Fang Y, Chi J, et al. Astragalus polysaccharides decrease proliferation, migration, and invasion but increase apoptosis of human osteosarcoma cells by up-regulation of microRNA-133a. Braz J Med Biol Res. 2018;51:e7665.
- Zhao R, Feng J, He G. Hypoxia increases Nrf2-induced HO-1 expression via the PI3K/Akt pathway. Front Biosci (Landmark Ed). 2016;21:385–396.
- Lin L, Knowlton AA. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100:1–8.
- Olivas-Chacon CI, Mullins C, Solberg A, et al. Assessment of ischemic cardiomyopathy using cardiovascular magnetic resonance imaging: a pictorial review. J Clin Imaging Sci. 2015;5:37.
- Shan M, Yu S, Yan H, et al. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules. 2017;22:1689.
- Licciardi PV, Underwood JR. Plant-derived medicines: a novel class of immunological adjuvants. Int Immunopharmacol. 2011;11:390–398.
- Jiang WB, Zhao W, Chen H, et al. Baicalin protects H9c2 cardiomyocytes against hypoxia/reoxygenation-induced apoptosis and oxidative stress through activation of mitochondrial aldehyde dehydrogenase 2. Clin Exp Pharmacol Physiol. 2018;45:303–311.
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647.
- Li HL, Gu XH, Li BJ, et al. Characterization and functional analysis of hypoxia-inducible factor HIF1alpha and its inhibitor HIF1alphan in tilapia. PLOS One. 2017;12:e0173478.
- Song L, Yang H, Wang HX, et al. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19:567–580.
- Shao ZH, Vanden Hoek TL, Qin Y, et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H999–H1006.
- Gallorini M, Petzel C, Bolay C, et al. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials. 2015;56:114–128.
- Tong F, Zhou X. The Nrf2/HO-1 pathway mediates the antagonist effect of L-arginine on renal ischemia/reperfusion injury in rats. Kidney Blood Press Res. 2017;42:519–529.
- Zeng X, Li J, Li Z. Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int J Clin Exp Med. 2015;8:14497–14504.
- Wang Y, Che J, Zhao H, et al. Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem Biophys Res Commun. 2018;503:3219–3224.
- Cheng FC, Geng L, Li L, et al. Baicalin attenuates intestinal ischemia/reperfusion injury and alters intestinal expression of Nrf2 and HO-1 in rats. WCJD. 2014;22:1510.