Abstract
Rheumatoid arthritis (RA) is characterized by tumor-like expansion of the synovium and the subsequent destruction of adjacent articular cartilage and bone. The latest studies proved phosphatase and tension homolog deleted on chromosome 10 (PTEN) might contribute to the surviving, proliferation and pro-inflammatory cytokines in RA. The purpose of this study was to explore the function and underlying mechanisms of PTEN in RA pro-inflammatory cytokines and chemokines of fibroblast-like synoviocytes (FLSs). Increased level of PTEN was observed in adjuvant-induced arthritis (AIA) FLSs in comparison to normal rats. Increased concentrations of pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), chemokines (CCL-2 and CCL-3), VCAM-1 and VEGF-α expression were observed in FLSs with PTEN inhibitor bpv or PTEN-RNAi. Moreover, co-incubation FLSs with overexpression vector with PTEN-GV141 reduced the expression of pro-inflammatory cytokines, chemokines, VCAM-1 and VEGF-α in AIA. Interestingly, we also found DNA methylation could regulate PTEN expression and activation of AKT signaling was with a change of PTEN. Altogether, our findings in the present study suggested that PTEN might play a pivotal role during pro-inflammatory cytokines and chemokines of FLSs through activation of AKT signaling pathway. In addition, PTEN expression may be regulated by DNA methylation in the pathogenesis of AIA.
Keywords:
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease of the joints characterized by synovial inflammation, hyperplasia of synovial tissues, synovial pannus, and then destroys adjacent articular cartilage and bone [Citation1,Citation2]. In the meantime, angiogenesis is needed to maintain local inflammation, transport inflammatory cells to the synovitis site and provide nutrients for vascular pannus [Citation3]. Among the various pathological events in the synovium, it has been reported that fibroblast-like synoviocytes (FLSs), are the main cell population of invasive pannus and paly a key role in the local inflammatory response of RA [Citation2,Citation4]. RA FLSs directly invade the extracellular matrix and secrete pro-inflammatory cytokines, chemokines, matrix metalloproteinases (MMPs) and angiogenic factors into synovial fluid, which destroys cartilage and bone and exacerbates joint damage, such as tumor necrosis factor-α (TNF-α) [Citation5], interleukin (IL)-6 [Citation6], CCL-2 [Citation7], MMP-3 [Citation8], VEGF-α [Citation9]. It has been reported that activation of FLSs and immunological dysregulation are major factors in the mechanisms of RA [Citation1,Citation2]. Therefore, inhibition of FLS activation and inflammatory response has positive significance for RA treatment.
Cytokines play critical roles in the onset and progression of RA [Citation10,Citation11]. Several pro-inflammatory cytokines, such as TNF-α and IL-6 [Citation5,Citation6] are essential to RA development owing to their role in synoviocyte activation. Regulating these pro-inflammatory cytokines may prevent disease progression and are candidate for RA therapies. TNF-α is a key inflammatory cytokine involved in the pathogenesis of RA and inhibition of TNF-α expression by antagonism or the use of therapeutic agents is an effective treatment for RA [Citation12]. IL-6 is present at high concentrations in the serum and synovial fluid of patients with RA and serves a key role in the pathogenesis of RA, including osteoporosis and an increased concentration of IL-6 in the joints around the body [Citation13,Citation14]. Chemokines play an important role in chronic synovitis and macrophage inhibitory protein (MIP-1α, CCL-3) and monocyte chemoattractant protein-1 (MCP-1, CCL-2) show abnormal expression in different stages of RA [Citation7,Citation15,Citation16].
The latest achievement obviously suggested that phosphatase and tension homolog deleted on chromosome 10 (PTEN) might contribute to the surviving and proliferation of FLSs in RA [Citation17,Citation18]. The PTEN, one of the most important tumor suppressors in mammals, is characterized as a ubiquitous modulator of cell growth, proliferation and inflammation, features mainly associated with its lipid phosphatase activity [Citation19,Citation20]. More importantly, PTEN has been known to have remarkable anti-inflammatory activities and anti-proliferation by blocking activation of the PI3-kinase/AKT pathway [Citation20]. What’s more, Wang et al. [Citation21] proposed that adenoviral vectors encoding human PTEN treatment significantly reduced ankle circumference, articular index scores and histology scores and also decreased levels of VEGF and IL-1β in collagen-induced arthritis (CIA). However, potential functions of PTEN in the activation of FLSs are still unknown. Hence, we predict that PTEN was significantly associated with RA, especially, expressions of pro-inflammatory cytokines and chemokines in activated FLSs.
To further elucidate the relationship between PTEN and cytokines and chemokines of FLSs in RA, in particular, we explored that PTEN may regulate the formation of pro-inflammatory cytokines and chemokines of FLSs and be closely associated with DNMT1 activation in RA pathogenesis.
Materials and methods
Materials and reagents
Bpv (PTEN) and 5-Aza-2′-deoxycytidine were obtained from Sigma Inc. (St. Louis, MO, USA). Complete Freund’s adjuvant was purchased from Chondrex, Inc. (WA, USA). Rabbit anti-PTEN and anti-TNF-α monoclonal antibody were obtained from Abcam (Cambridge, UK). Rabbit anti-DNMT1 and anti-Vimentin (Alexa Fluor 594 Conjugated) were acquired from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-IL-6 and anti-β-actin monoclonal antibody were purchased from Bioworld (Shanghai, China). Peroxidase-conjugated goat anti-rabbit IgG (H + L) was obtained from ZSGB-BIO (Beijing, China). PTEN, TNF-α, IL-6, IL-1β, CCL-2, CCL-3, VCAM-1, and VEGF-α and β-actin primers were obtained from Sangon Biotech (Shanghai) Co., Ltd (Shanghai, China).
Adjuvant-induced arthritis (AIA) of rat model
Adult Sprague-Dawley (SD) rats (80–120 g, female) were subcutaneously injected with complete Freund's adjuvant (0.1 ml/100g body weight) for 24 days in the left hind paw to induce AIA model [Citation8,Citation22]. Meanwhile, normal control rats were injected with normal saline. SD rats were purchased from the Experimental Animal Center of Anhui Medical University. Efforts were made to reduce the number of animals used and their suffering in all animal experiments. The animals and all experiments were performed using protocols approved by the institutions’ subcommittees on animal care of Anhui Medical University (approval number: 20160253).
Histopathology
The synovial tissues from rats AIA knee joint were dipped in 4% paraformaldehyde for 24 h and then embedded in paraffin wax. Immunohistochemistry and Hematoxylin and eosin (H&E) staining were performed according to standard protocols [Citation8,Citation22]. Also, pathological changes were observed and photographed under the Olympus BX-51 microscope(Tokyo, Japan).
FLSs cultured
FLSs were harvested from the synovium of AIA and control rats knee joint by tissue explants cultivation method [Citation8]. Also, FLSs were maintained in high-glucose DMEM medium supplemented with 20% (v/v) FBS (Cyagen Biosciences Inc, Guangzhou, China) and penicillin-streptomycin (Beyotime, Shanghai, China). Cells were cultured at 37 °C and atmosphere of 5% CO2. The FLSs were used for 3–8 generation in the experiments.
Immunofluorescence staining
FLSs with a density of 1–2 × 105 cells/ml were cultured in 6-well plates for 24 h. After fixing with methanol, both rabbit anti-PTEN and anti-Vimentin were followed by immunofluorescence staining overnight at 4 °C. Also, the cells were incubated with Alexa Fluor 488-Conjugated Goat anti-rabbit IgG (H + L) (ZSGB-BIO, Beijing, China) for 1 h. After washing with PBS, counterstaining was implemented with 4′, 6-diamidino-2-phenylindole (DAPI; Beyotime, China) for 5 min and then the cells were observed and photographed under Olympus BX-51 microscope.
Small interfering RNA transfection after the cells were cultured with a density of 1–2 × 106 cells/ml in 6-well plates overnight, according to the manufacturer’s instructions [Citation8]. FLSs were transfected with Lipofectamine 2000 (Invitrogen, CA, USA) and100 nM of small interfering RNA (RNAi) (GenePharma, Shanghai, China). The PTEN-RNAi (rat) oligonucleotide sequences were as follows: 5′-CCGAUACUUCUCUCCAAAUTT-3′ for the sense strand and 5′-AUUUGGAGAGAAGUAUCGGTT-3′ for the antisense strand. At the same time, the negative scrambled RNAi was used in study. After transfection for 6 h, the FLSs were cultured with DMEM supplemented with 20% FBS for 48 h.
Plasmid construction
Plasmid construction of PTEN-GV141 was obtained from Genechem (Shanghai, China). We acknowledge the principal investigators sincerely. FLSs were transfected with GV141-PTEN to induce the ectopic expression of PTEN and empty GV141 vector (GV141) as a control. Cells transfection was performed with the Lipofectamine TM2000 according to the manufacturer’s manuals [Citation8].
Quantitative real-time PCR (q-PCR)
After extracting from cells and tissues by TRIZol reagent (Invitrogen, USA), the total RNA were reverse transcribed into cDNA using iScript™ cDNA kit (Bio-Rad, USA) [Citation8]. Also, according to the manufacture’s instruction, the SYBRGreen q-PCR Master Mix (Vazyme Biotech, Nanjing, China) was used to prepare reaction mixture for q-PCR. The mRNA expression of PTEN, TNF-α, IL-6, IL-1β, CCL-2, CCL-3, VCAM-1, VEGF-α and β-actin were detected by the CFX Connect Real-Time PCR Detection System (Bio-Rad, USA). The primers used were listed as following: PTEN (forward: 5′-TCCTCCAACTCAGGACCCAC-3′; reverse: 5′-TCACCACACACAGGCAATGG-3′), TNF-α (forward: 5′-ACTCCCAGAAAAGCAAGCAA-3′; reverse: 5′-CAGTTCCACATCTCGGATCA-3′), IL-6 (forward: 5′-GAGCCCACCAGGAACGAAAGTC-3′; reverse: 5′-TGTTGTGGGTGGTATCCTCTGTGAA-3′), IL-1β (forward: 5′-TGACCCATGTGAGCTGAAAG-3′; reverse: 5′-AGGGATTTTGTCGTTGCTTG-3′), CCL-2 (forward: 5′-TAGCATCCACGTGCTGTCTC-3′; reverse: 5′-TGCTGCTGGTGATTCTCTTG-3′), Ccl-3 (forward: 5′-ACTGCCTGCTGCTTCTCCTA-3′; reverse: 5′-CGGTTTCTCTTGGTCAGGAA-3′), VCAM-1 (forward: 5′-GTCAGCGAAGGAAACTGGAG-3′; reverse: 5′-ACCGTGCAGTTGACAGTGAC-3′), VEGF-α (forward: 5′-CGACAGAAGGGGAGCAGAAAG-3′; reverse: 5′-GCACTCCAGGGCTTCATCATT-3′) and β-actin (forward: 5′-CCCATCTATGAGGGTTACGC-3′; reverse: 5′-TTTAATGTCACGCACGATTTC-3′). Relative mRNA expression of interesting genes was measured by normalization to the β-actin, and all samples were analyzed three times.
Western blotting The FLSs and tissues with bpv, PTEN-RNAi or PTEN-GV141 were lysed with RIRA Lysis Buffer (Beyotime, China) and collected. After protein denaturation with heat, it was fractionated by electrophoresis through SDS-PAGE and transferred to PVDF membranes (Millipore, Massachusetts, USA). After closing with defatted milk for 3 h, the blots were incubated with primary antibodies for more than 12 h. Rabbit antibodies PTEN, TNF-α and IL-6 were used at a dilution of 1:500 and β-actin was used at a dilution of 1:1000. After blots were washed four times in TBS/Tween-20 and incubated for 1 h in peroxidase-conjugated goat anti-rabbit IgG (H + L)at 1:10000 dilutions. After washing four times, the protein blots were excited with the ImmobilonTM Western Chemiluminescent HRP Substrate (Millipore, USA).
Statistical analysis
All data are collated as means ± SD and analyzed by SPSS16.0 software(Armonk, New York, USA). Statistical significances were determined by one-way ANOVA with the post-hoc Dunnett's test. In all cases, values of p < .05 were considered to be statistically significant.
Results
The expression of PTEN was down-regulated in AIA FLSs
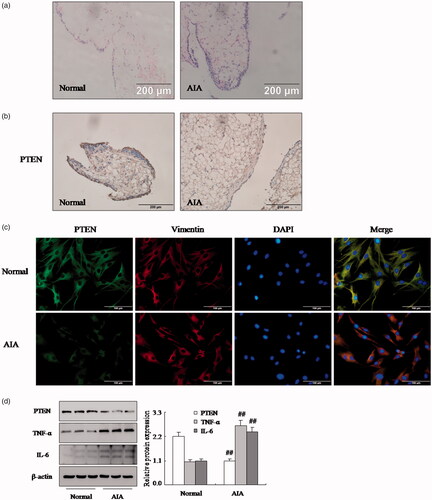
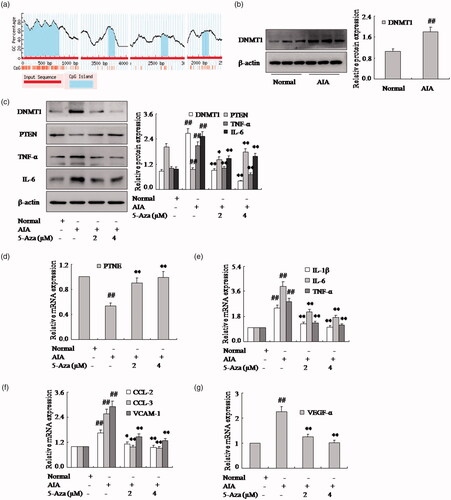
The AIA model, which was established by complete Freund’s adjuvant, is investigating the role of PTEN and one of RA model. Also, the synovial tissues from rat knee were confirmed by HE analysis () that the increased remarkable inflammatory cells infiltrations in AIA model. At the same time, the immunohistochemical showed (as shown in ) that the PTEN protein expression was down-regulated significantly in AIA synovial compared with normal synovial. Moreover, we also isolated FLSs from synovial tissue and performed the expression of Vimentin by immunofluorescence staining in FLSs. indicated that the cells were FLSs which were derived from synovial tissues. What’s more, the protein expression of PTEN was lower than normal FLSs in AIA by immunofluorescence staining (. Similarly, Western blotting analysis also proved that the levels PTEN protein () was reduced observably in AIA FLSs. More significantly, the protein expression of TNF-α and IL-6 have improved apparently in AIA FLSs compared with normal, as shown in . Consequently, these results suggested that the expression of PTEN was observably reduced in AIA FLSs.
Figure 1. The expression of PTEN was down-regulated in RA FLSs. (a) Representative H&E staining of AIA and normal synovial tissues in rat (original magnification, ×20). (b) The expression of PTEN in AIA and normal synovial tissue was analyzed by IHC staining analysis in rats. (c) The expression of PTEN and Vimentin were analyzed by double immunofluorescence staining analysis in rats AIA and normal FLSs. (d) The protein levels of PTEN, TNF-α and IL-6 were analyzed by Western blotting in AIA and normal FLSs. All values were expressed as mean ± SD. ##p < .01 vs. normal group.
Inhibition of PTEN expression with bpv increases pro-inflammatory cytokines and chemokines of FLSs
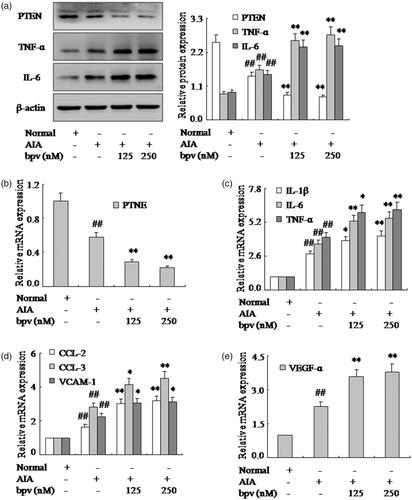
To identify the significance of PTEN in the RA FLSs, we have measured the effect of PTEN on the pro-inflammatory cytokines and chemokines of FLSs by treated with PTEN inhibitor bpv in AIA. The results of western blotting and q-PCR showed the expression of PTEN were down-regulated markedly in varying degrees with bpv in AIA FLSs as shown in . More significantly, Western blotting and q-PCR have verified the expression of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were up-regulated markedly by PTEN inhibitor bpv in AIA FLSs (. In addition, the mRNA expression of chemokines CCL-2 and CCL-3 were also strengthened observably by bpv in AIA FLSs (). Remarkably, evidence was q-PCR analysis (), whisch showed that the VCAM-1 and VEGF-α mRNA were up-regulated apparently. Thus, these researches had proved that the effect of PTEN inhibitor bpv increased markedly pro-inflammatory cytokines and chemokines of FLSs in AIA rats.
Figure 2. Inhibition of PTEN expression with bpv increases pro-inflammatory cytokines and chemokines of FLSs. (a) The protein levels of PTEN, TNF-α and IL-6 were analyzed by Western blotting in FLSs with bpv in AIA. (b) The mRNA levels of PTEN were analyzed by q-PCR assays with bpv. (c) The q-PCR assays analyzed IL-1β, IL-6 and TNF-α mRNA in FLSs with bpv. (d) After FLSs were incubated with bpv, the mRNA levels of CCL-2, CCL-3 and VCAM-1 were analyzed by q-PCR assays in AIA. (e) FLSs were treated with bpv, the mRNA level of VEGF-α was analyzed by q-PCR assays in AIA. All values were expressed as mean ± SD. ##p < .01 vs. normal group. *p < .05, **p < .01 vs. AIA group.
Inhibition of PTEN expression with PTEN-RNAi promotes pro-inflammatory cytokines and chemokines of FLSs
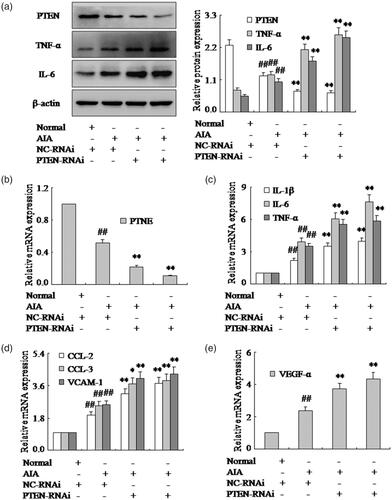
In order to provide additional evidence that PTEN is involved in the pro-inflammatory cytokines of RA FLSs, siRNA specific for rat PTEN was used to knock down gene expression in AIA FLSs. FLSs treated with NC-RNAi or PTEN-RNAi was exposed to 48 h following the transfection with 100 nM PTEN-RNAi, the level of PTEN mRNA and protein were reduced remarkably compared with the cells transfected with NC-RNAi (). As expected, the expression of TNF-α, IL-6 and IL-1β mRNA and protein were up-regulated by PTEN siRNA in AIA FLSs as shown in . Furthermore, CCL-2 and CCL-3 mRNA expression were also promoted observably by PTEN-RNAi in AIA FLSs (). Similarly, the VCAM-1 and VEGF-α mRNA were up-regulated apparently by PTEN-RNAi in AIA FLSs (). Altogether, our findings suggested that inhibited PTEN expression could increase pro-inflammatory cytokines and chemokines of FLSs in AIA rats and PTEN may contribute to the progression of RA.
Figure 3. Inhibition of PTEN expression with PTEN-RNAi promotes pro-inflammatory cytokines and chemokines of FLSs. (a) The protein levels of PTEN, TNF-α and IL-6 were analyzed by Western blotting in FLSs with PTEN-RNAi in AIA. (b) The mRNA levels of PTEN were analyzed by q-PCR assays with PTEN-RNAi. (c) The q-PCR assays analyzed IL-1β, IL-6 and TNF-α mRNA in FLSs with PTEN-RNAi. (d) After FLSs were incubated with PTEN-RNAi, the mRNA levels of CCL-2, CCL-3 and VCAM-1 were analyzed by q-PCR assays in AIA. (e) FLSs were treated with PTEN-RNAi, the mRNA level of VEGF-α was analyzed by q-PCR assays in AIA. All values were expressed as mean ± SD. ##p < .01 vs. normal group. *p < .05, **p < .01 vs. AIA group.
Overexpression vector of PTEN expression with PTEN-GV141 inhibits pro-inflammatory cytokines and chemokines of FLSs
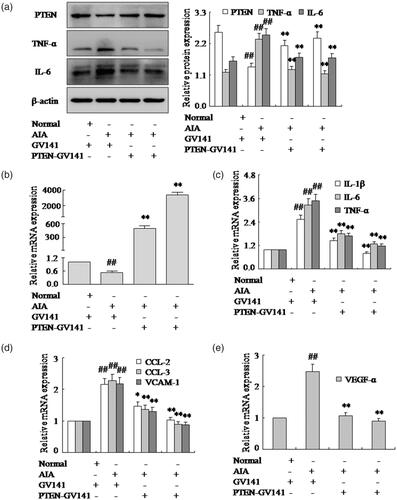
This is so clear that inhibited PTEN expression could increase pro-inflammatory cytokines and chemokines of FLSs in AIA. Based on the above research, we proposed over-expression of PTEN may inhibit pro-inflammatory cytokines and chemokines of FLSs. To further determine the underlying mechanism of PTEN during pro-inflammatory cytokines and chemokines of FLSs, overexpression vector with PTEN-GV141 was used for over-expression of PTEN. Western blotting and q-PCR () showed the expression of PTEN was up-regulated observably with PTEN-GV141 in AIA FLSs. In addition, after treating with PTEN-GV141, expressions of TNF-α, IL-6 and IL-1β mRNA and protein were down-regulated remarkably in AIA FLSs (). Meanwhile, chemokines CCL-2 and CCL-3 mRNA expression were also down-regulated observably by PTEN-GV141, as shown in . What is more, over-expression of PTEN by PTEN-GV141 resulted in decreasing the VCAM-1 and VEGF-α mRNA by q-PCR in AIA FLSs (). These evidences proved that the treatment of FLSs with PTEN-GV141 had a profound inhibitory effect on pro-inflammatory cytokines and chemokines of FLSs. It may be involved that PTEN plays a pivotal role with the pro-inflammatory cytokines and chemokines of FLSs in the pathogenesis of RA.
Figure 4. Over expression vector of PTEN expression with PTEN-GV141 inhibits pro-inflammatory cytokines and chemokines of FLSs. (a) The protein levels of PTEN, TNF-α and IL-6 were analyzed by Western blotting in FLSs with PTEN-GV141 in AIA. (b) The mRNA levels of PTEN were analyzed by q-PCR assays with PTEN-GV141. (c) The q-PCR assays analyzed IL-1β, IL-6 and TNF-α mRNA in FLSs with PTEN-GV141. (d) After FLSs were incubated with PTEN-GV141, the mRNA levels of CCL-2, CCL-3 and VCAM-1 were analyzed by q-PCR assays in AIA. (e) FLSs were treated with PTEN-GV141, the mRNA level of VEGF-α was analyzed by q-PCR assays in AIA. All values were expressed as mean ± SD. ##p < .01 vs. normal group. *p < .05, **p < .01 vs. AIA group.
DNA methylation regulates PTEN expression
It is evident that the PTEN negatively regulates the expression of pro-inflammatory cytokines and chemokines of FLSs in RA. To clarify the reason for the down-expression of PTEN in AIA FLSs, we have measured whether DNA methylation mediates the PTEN gene expression. First, we projected near the transcript first exon and first exon upstream found four CPG island with CG point rich, and it becomes obvious that the decrease of PTEN gene expression may be related to CpG island methylation (). Moreover, we found the expression of DNMT1 protein was up-expression in AIA FLSs compared with normal rat, as shown in . What is more, after with methylation inhibitor 5-Aza in FLSs, the PTEN expression was over-expression obviously in AIA, as shown in . As same as expected, the expression of TNF-α, IL-6 and IL-1β mRNA and protein were reduced in FLSs (). Meanwhile, chemokines CCL-2 and CCL-3 mRNA expression were also down-regulated observably by 5-Aza (). Moreover, the VCAM-1 and VEGF-α mRNA were also decreased of FLSs with 5-Aza in AIA (). Altogether, with methylation, inhibitor 5-Aza of FLSs could suppress the expression of pro-inflammatory cytokines and chemokines and be involved with methylation.
Figure 5. DNA methylation regulates PTEN expression. (a) We projected near the transcript first exon and first exon upstream found four CPG island with CG point rich form rat PTEN gene. (b) The protein level of DNMT1 was analyzed by Western blotting in AIA and normal FLSs. (c) The protein levels of PTEN, TNF-α and IL-6 were analyzed by Western blotting in FLSs with 5-Aza in AIA. (d) The mRNA levels of PTEN were analyzed by q-PCR assays with 5-Aza. (e) The q-PCR assays analyzed IL-1β, IL-6 and TNF-α mRNA in FLSs with 5-Aza. (f) After FLSs were incubated with 5-Aza, the mRNA levels of CCL-2, CCL-3 and VCAM-1 were analyzed by q-PCR assays in AIA. (g) FLSs were treated with 5-Aza, the mRNA level of VEGF-α was analyzed by q-PCR assays in AIA. All values were expressed as mean ± SD. ##p < .01 vs. normal group. *p < .05, **p < .01 vs. AIA group.
PTEN may modulate FLSs pro-inflammatory cytokines and chemokines and be closely associated with AKT signaling pathway
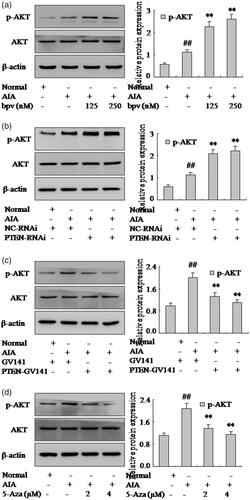
It has proved PTEN is closely associated with AKT signaling pathway in pathophysiological mechanisms. To explore whether PTEN regulates pro-inflammatory cytokines and chemokines through AKT signaling pathway in FLSs, the expression of p-AKT, one of the PI3K/AKT pathway activation marker, was examined in rat AIA. First, the expression of p-AKT was increased obviously by Western blotting analysis in AIA FLSs compare with normal. Moreover, bpv or PTEN-RNAi () could enhance significantly the expression of p-AKT in AIA FLSs. In particular, the expression of p-AKT was suppressed by PTEN-GV141 (as shown in ) in AIA FLSs. It showed that PTEN negatively regulated the AKT signaling pathway in AIA FLSs. In addition, showed that expressions of p-AKT were also suppressed remarkably in AIA FLSs treating with 5-Aza. Altogether, all the above results indicated that PTEN could modulate FLSs pro-inflammatory cytokines and chemokines and be closely associated with AKT signaling pathway.
Figure 6. PTEN may modulate FLSs pro-inflammatory cytokines and chemokines and be closely associated with AKT signaling pathway. (a) The protein level of p-AKT was analyzed by Western blotting in FLSs with bpv. (b) The protein level of p-AKT was analyzed by Western blotting in FLSs with PTEN-RNAi. (c) The protein level of p-AKT was analyzed by Western blotting in FLSs with PTEN-GV141. (d) The protein level of p-AKT was analyzed by Western blotting in FLSs with over expression vector 5-Aza. All values were expressed as mean ± SD. #p < .05, ##p < .01 vs. normal group. **p < .01 vs. AIA group.
Discussions
The results of this study show that: (1) the expression of PTEN was down-regulated substantially in RA FLSs; (2) PTEN inhibitor 5-Aza or PTEN-RNAi could increase observably FLSs pro-inflammatory cytokines and chemokines in AIA; (3) overexpression vector with PTEN-GV141 could suppress substantially pro-inflammatory of cytokines and chemokines FLSs in AIA; (4) DNA methylation regulates PTEN expression; (5) PTEN may modulate FLSs pro-inflammatory cytokines and chemokines and be closely associated with AKT signaling pathway.
The AIA model, injection Completes Freund’s adjuvant, has been widely used in the mechanism research of RA [Citation8,Citation22]. The rats AIA model has similar characteristics with RA in histology and immunology and it is one of the models to evaluate the treatment and research of RA [Citation23]. Therefore, we chose AIA model to affirm the role of PTEN in the RA FLSs. The exact pathogenesis of RA remains to be fully delineated, but an important feature involves communication between a number of different inflammatory cells, notably macrophages and T lymphocytes, and cells resident in the joint [Citation2,Citation24]. These cells communicate via a network of proteins known as cytokines, some of which exert pro-inflammatory actions and others that provide anti-inflammatory or immunoregulatory effects [Citation25,Citation26]. In normal physiology, these cytokines are maintained in balance; however, in RA, the balance shifts in favour of the pro-inflammatory cytokines [Citation27]. Recent research has proved FLSs play a pivotal role in RA pathogenesis through aggressive migration and matrix invasion and certain pro-inflammatory cytokines may affect synoviocyte invasion [Citation2,Citation28,Citation29]. However, there are no drugs that target FLSs and the mechanism that regulates FLS activation remains unclear. Therefore, how to target the inhibition pro-inflammatory cytokines and chemokines of FLSs has positive significance for RA treatment. PTEN is a novel tumour suppressor which exhibits tyrosine phosphatase activity as well as homology to the cytoskeletal proteins tensin and auxilin [Citation17,Citation18]. In this paper, we agree with Pap et al. [Citation30] that PTEN is observably reduced by immunohistochemical and Western blotting in AIA FLSs compared with normal. Moreover, we also have measured inhibition of PTEN expression with bpv or PTEN-RNAi could active the expression of IL-1β, IL-6 and TNF-α in AIA FLSs. The most important pro-inflammatory cytokines are IL-1β, IL-6 and TNF-α in RA [Citation25]. These cytokines produce an overlapping spectrum of biological actions, but it is likely that they act in concert with each other and other cytokines to produce many of their path physiological effects [Citation10,Citation31]. What is more, over-expression of PTEN with PTEN-GV141 could down-regulate expression of IL-1β, IL-6 and TNF-α mRNA and protein in AIA FLSs. It is well established that pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α which are deeply involved in the pathogenesis of RA, they are key cytokine in driving inflammation due to influencing various cells in the synovium such as synoviocytes, immune cells and osteoclasts. Biologic agents against these cytokines have been developed to treat human auto-inflammatory diseases, with agents against TNF-α and IL-6 proving particularly useful in RA [Citation12,Citation13]. Hence, PTEN has a positive effect on the treatment of RA by suppressing the production of pro-inflammatory cytokines.
Along with FLSs activation and migration that is stimulated by several chemokines including CCL2 and CCL3 and these chemokines are abundantly expressed in the RA synovium, these chemokines may facilitate fibroblast recruitment to the synovium and subsequently induce activation of FLSs [Citation32]. Interestingly, we also have measured inhibition of PTEN expression with bpv or PTEN-RNAi that could active the expression of CCL2 and CCL3 in AIA FLSs. Chemokines regulate chemotaxis in nearby responsive cells and are implicated in chronic inflammatory diseases, such as RA, atherosclerosis and adipose inflammation [Citation33]. Furthermore, overexpression of PTEN with PTEN-GV141 could down-regulate expression of CCL2 and CCL3 mRNA in AIA FLSs. Rump et al. [Citation34] proved the chemokines CCL7, CCL14, CCL16 and CCL22 occurred in RA synovial fluid and endothelial cells and have a highly significant correlation with the level of anti-CCP. In addition, with bpv or PTEN-RNAi of FLSs, the mRNA expression of VCAM-1 and VEGF-α were improved evidently. It has been proved that the serum VCAM-1 levels increased in RA, which could possibly be associated with the autoimmune and inflammatory reactions found in RA [Citation35]. The VEGF-α is the most potent proangiogenic factor in RA angiogenesis and promotes the formation of invasive pannus, a thickened layer of synovial tissue that erodes cartilage and bone. More importantly, over-expression of PTEN with PTEN-GV141 reduced expression of VCAM-1 and VEGF-α mRNA. Consequently, PTEN also negatively regulates the expression of chemokines, VCAM-1 and VEGF-α of FLSs in AIA rats.
DNA methylation is a kind of epigenetic modification which plays a critical role in regulating gene expression and potentially contributes to immune dysregulation [Citation36]. Several studies have demonstrated that DNA methylation is closely associated with RA [Citation37]. For example, Nakano et al. [Citation37] found that differentially methylated genes could contribute to the pathogenesis of RA. We also proved DNMT1 was overexpression in AIA FLSs. In particular, with methylation inhibitor 5-Aza in FLSs, the PTEN mRNA and protein expression was overexpression obviously in AIA. On the other hand, the pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), chemokines (CCL-2 and CCL-3), VCAM-1 and VEGF-α expression all were reduced in FLSs with 5-Aza. To summarize, DNA methylation may regulate PTEN expression in the pathogenesis of AIA.
This is so clear that PTEN mediates cell activation by negatively regulating the PI3K/AKT signaling pathway [Citation20]. The loss of PTEN can result in the activation of AKT kinases, which play key roles in cell growth, proliferation and invasion [Citation38]. Our results also demonstrate this viewpoint that inhibition PTEN by bpv or PTEN-RNAi, p-AKT expression was enhanced significantly in AIA FLSs. In particular, PTEN-GV141 suppressed AKT signaling, the expression of p-AKT was substantially decreased in AIA FLSs. Altogether, these dates suggested that PTEN mediated pro-inflammatory cytokines and chemokines of FLSs might regulate activation of PI3K/AKT signaling pathway.
In summary, our findings in the present study suggested that PTEN might play a pivotal role during pro-inflammatory cytokines and chemokines of FLSs through activation of AKT signaling pathway. In addition, PTEN expression may be regulated by DNA methylation in the pathogenesis of AIA. It is one more competent and qualified opinion that the overexpression of PTEN suppresses pro-inflammatory cytokines and chemokines of FLSs in AIA, indicating the potential of PTEN as a therapeutic target for RA. Further research is needed to clarify if PTEN could be used as a diagnostic marker and prognostic indicators of RA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85(3):307–310.
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223(1):252–270.
- Elshabrawy HA, Chen Z, Volin MV, et al. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18(4):433–448.
- Kim EK, Kwon JE, Lee SY, et al. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017;8(1):e2565.
- Zhu LJ, Yang TC, Wu Q, et al. Tumor necrosis factor receptor-associated factor (TRAF) 6 inhibition mitigates the pro-inflammatory roles and proliferation of rheumatoid arthritis fibroblast-like synoviocytes. Cytokine. 2017;93:26–33.
- Mu N, Gu J, Huang T, et al. A novel NF-kappaB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep. 2016;6(1):20059.
- Sato H, Muraoka S, Kusunoki N, et al. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):263.
- Li XF, Sun YY, Bao J, et al. Functional role of PPAR-gamma on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Sci Rep. 2017;7(1):12671.
- Bastiaansen-Jenniskens YM, Wei W, Feijt C, et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2alpha. Arthritis Rheum. 2013;65(8):2070–2080.
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14(1):397–440.
- Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2004;43(suppl_3):iii2–iii9.
- Dulic S, Vasarhelyi Z, Sava F, et al. T-cell subsets in rheumatoid arthritis patients on long-term anti-TNF or IL-6 receptor blocker therapy. Mediators Inflamm. 2017;2017:1.
- Diaz-Torne C, Ortiz MDA, Moya P, et al. The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin Arthritis Rheum. 2017;47(6):757–764.
- Johansson L, Arlestig L, Kokkonen H, et al. An increased concentration of receptor activator of nuclear factor kappa-B ligand pre-dates the onset of rheumatoid arthritis. Rheumatology (Oxford). 2017;56(12):2190–2196.
- Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69(12):2283–2291.
- Orr C, Najm A, Biniecka M, et al. Synovial immunophenotype and anti-citrullinated peptide antibodies in rheumatoid arthritis patients: relationship to treatment response and radiologic prognosis. Arthritis Rheumatol. 2017;69(11):2114–2123.
- Chen D, Liu D, He M, et al. Rheumatoid arthritis fibroblast-like synoviocyte suppression mediated by PTEN involves survivin gene silencing. Sci Rep. 2017;7(1):367.
- Kim HS, Jang SW, Lee W, et al. PTEN drives Th17 cell differentiation by preventing IL-2 production. J Exp Med. 2017;214(11):3381–3398.
- Chen L, Guo D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol Immunol. 2017;14(7):581–589.
- Zhang W, Du Z, Zhu J, et al. Sprouty2 suppresses the inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes through regulating the Raf/ERK and PTEN/AKT signals. Mol Immunol. 2015;67(2):532–539.
- Wang CR, Shiau AL, Chen SY, et al. Amelioration of collagen-induced arthritis in rats by adenovirus-mediated PTEN gene transfer. Arthritis Rheum. 2008;58(6):1650–1656.
- Li XF, Shen WW, Sun YY, et al. MicroRNA-20a negatively regulates expression of NLRP3-inflammasome by targeting TXNIP in adjuvant-induced arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2016;83(6):695–700.
- Verhoeven F, Totoson P, Marie C, et al. Diclofenac but not celecoxib improves endothelial function in rheumatoid arthritis: a study in adjuvant-induced arthritis. Atherosclerosis. 2017;266:136–144.
- Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–485.
- Ridgley LA, Anderson AE, Pratt AG. What are the dominant cytokines in early rheumatoid arthritis? Curr Opin Rheumatol. 2017;30(2):207–214.
- Cutolo M, Capellino S, Montagna P, et al. Anti-inflammatory effects of leflunomide in combination with methotrexate on co-culture of T lymphocytes and synovial macrophages from rheumatoid arthritis patients. Ann Rheum Dis. 2006;65(6):728–735.
- McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol. 2017;35 Suppl 107(5):94–101.
- Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, et al. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52(7):1999–2002.
- Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233(1):256–266.
- Pap T, Franz JK, Hummel KM, et al. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2(1):59–64.
- Ferraccioli G, Bracci-Laudiero L, Alivernini S, et al. Interleukin-1beta and interleukin-6 in arthritis animal models: roles in the early phase of transition from acute to chronic inflammation and relevance for human rheumatoid arthritis. Mol Med. 2010;16(11–12):552–557.
- Sucur A, Jajic Z, Artukovic M, et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res Ther. 2017;19(1):142.
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602.
- Rump L, Mattey DL, Kehoe O, et al. An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium. Cytokine. 2017;97:133–140.
- Wang L, Ding Y, Guo X, et al. Role and mechanism of vascular cell adhesion molecule-1 in the development of rheumatoid arthritis. Exp Ther Med. 2015;10(3):1229–1233.
- Zheng Y, Josefowicz S, Chaudhry A, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812.
- Nakano K, Whitaker JW, Boyle DL, et al. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2013;72(1):110–117.
- Huang, Li T, Li X, et al. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 2014;8(6):1930–1942.
