 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this study was to investigate the mechanism underlying miR-21-associated apoptosis in HB. In this study, HB and adjacent tissues were collected from patients with HB. RT-PCR, FISH, western blot, apoptosis assay, migration, invasion and wound healing assays, caspase activity assay, luciferase reporter assays, and xenografts mouse model were used to determine the effects of miR-21 on HB cell apoptosis. The results revealed that miR-21 was up-regulated in both HB cell and tissue and was associated with progression of HB. MiR-21 inhibitor enhanced the apoptosis level in HB cells. MiR-21 inhibitor showed reduced abilities of migration and invasion. ASPP2 was a target gene of miR-21. Inhibition of ASPP2 increased abilities of migration and invasion in HB cells. Furthermore, miR-21 inhibitor caused increased activity p-38 signaling. In a xenografts mouse model, miR-21 inhibitor could significantly suppress tumor growth in nude mice along with enhanced expressions of ASPP2 and p38. Taken together, the results suggest that upregulation of miR-21 is related to HB progression and miR-21-associated apoptosis in HB is mediated through ASPP2/p38 signaling pathway in vitro and in vivo. This study provides novel insight into the effects of miR-21 on HB apoptosis and clue to develop new therapies.
Keywords:
Introduction
Hepatoblastoma (HB) is the most common malignant liver cancer that mainly occurs in early childhood [Citation1]. Growing studies have been demonstrated that HB is closely related to several environmental risks, for example, smoking, premature birth and low-birth weight [Citation2]. In the past few decades, the diagnosis, treatment, and outcomes of children with HB have been substantially improved [Citation3]. However, there is still much to be improved in the outcome of HB patients due to 53% of 3-year survival [Citation4].
With regards to anti-cancer therapy, it is essential to elucidate various types of tumor cell death mechanisms, including necrosis, autophagy, mitotic catastrophe, and apoptosis [Citation5]. Of which, apoptosis is thought an important factor for cancer therapy and applying pro-apoptosis mechanisms of tumor cell have been profoundly the treatment efficacy in clinical practices [Citation4]. MicroRNAs (miRNAs), 19–25 nucleotides in length, are a group of non-coding RNAs that plays an important role in gene expression regulation and thus involves in the various biological process [Citation6]. It has been demonstrated miR-21 inhibits apoptosis in various cancer types [Citation7], indicating miR-21 is an important regulator in the apoptosis process of the tumor cell.
Apoptosis stimulated protein of p53-2 (ASPP2), a binding protein of p53, promote the damage-induced apoptotic role of p53 by activating the promoters of several proapoptotic genes, for example, p53-inducible gene 3 (PIG3) and Bax [Citation8], suggesting ASPP2 functions as a stimulatory protein of p53. Also, downregulated expression of ASPP2 is observed to be related to poor prognosis and metastasis in many cancers [Citation9]. Furthermore, ASPP2 has been identified as one of the target genes of miR-21 and also mediates the effect of miR-21 on glioma cells apoptosis [Citation10]. Collectively, miR-21/ASPP2 pathway may be an important mechanism for the apoptotic regulation of tumor cell.
However, there are very few studies focusing on the association between miR-21 and HB apoptosis. Therefore, the objectives in this study were to investigate the effect of miR-21 on HB apoptosis in vitro and in vivo. This study would provide a novel clue for understating the mechanism of HB and the development of new treatment.
Materials and methods
Patients
Before surgery, all patients did not receive any anti-cancer treatment, such as oral chemotherapy, radiotherapy, and biological immunotherapy. All samples were confirmed by pathologic examination. Ethics approval from the Ethics Committee of Linyi people’s hospital was obtained. Written, informed consents were obtained from the parents of patients with HB before the study. In this study, samples were collected between June 2015 and December 2016 from 10 patients (including HB and adjacent tissue samples) in the Linyi people's hospital, Linyi, China. Ten patients included six males and four females aged 0.5–12 years (median age: 5.3 years). The stage and risk stratification of HB were assessed by the PRETEXT system [Citation11]. The overall survival (OS) rate of patients with HB with high or low miR-21 expression was predicted by OncoLnc database [Citation12].
Cell culture
HB and control cells were isolated from HB and correspondingly adjacent tissues, respectively, according to the protocol [Citation13]. After isolation, HB and control cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, NY, USA), and 100 U/mL penicillin (Invitrogen, CA, USA). The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2.
Real-time PCR (RT-PCR)
Total RNA was extracted from HB and control cells following the manufacturer’s instructions for the RNeasy Mini kit (Qiagen, Hilden, Germany). The protocol of stem-loop reverse transcription of mature miR-21 was described in [Citation14]. Stem-loop primers were used as [Citation15]: miR-21 (5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGCC-3′) and U48 (5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGGTCAG-3′). The primers for amplifying mature miR-21 were as following: miR-21:5′-CGCGCCAACACCAGTCGATG-3′ and 5′-GTGCAGGGTCCGAGGT-3′ and U48: 5′-CGACGAGTGATGATGAC-3′and 5′-GTGCAGGGTCCGAGGT-3′). Real-time PCR reactions were performed on a Lightcycler (Roche, Mannheim, Germany). RT-PCR data were analyzed using with the comparative threshold cycle relative-quantification method, and the mRNA expression was normalized to that of U48 (housekeeping miRNA) as a reference control.
Western blot
Total protein was extracted by using the cell lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Protein extracts were isolated by 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis and electrically then transferred to polyvinylidene difluoride membranes that were blocked with 5% nonfat dry milk in PBS-0.05% Tween 20 for 1 h. Then, the primary antibodies for Blc-2, Bax, ASPP2, c-Jun-N-terminal kinase (JNK), p-JNK, p38, p-p38, extracellular signal-regulated kinase (ERK), p-ERK and β-actin (Santa Cruz Biotechnology, CA, USA) were employed overnight at 4 °C. The membranes were then incubated with their correspondingly secondary antibodies for 1 h at 37 °C. The results were visualized by enhanced chemiluminescence assays (Thermo Fisher Scientific, Rockford, IL, USA).
Fluorescence in situ hybridization (FISH)
FISH assay was applied to detect miR-21 expression in HB and control tissue sections following the manufacturer’s instructions for the mirVana™ miRNA Probe Construction Kit (Thermo Fisher Scientific, MA, USA). The images were obtained by confocal laser scanning microscopy (Leica, Solms, Germany).
Cell transfection
MiR-21 inhibitor, corresponding negative control, and ASPP2-siRNA were obtained from GenePharma Co., Ltd (Shanghai, China). The transfection procedure was performed according to the manufacturer’s instructions of Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Waltham, MA, USA). Twenty-four hours post-transfection, treated cells were used to subsequent experiments.
Flow cytometry assay
HB cells transfected with miR-21 inhibitor were collected and subjected to the flow cytometry analysis according to the protocol reported in [Citation16]. The results were analyzed using ModFit software and FACSCaliber (BD Bioscience, San Jose, CA, USA). Each treatment group comprised of 3–5 replicates
Invasion, migration and wound healing assay
HB cells transfected with miR-21 inhibitor were subjected to the invasion assay (BD Biosciences, NJ, USA), Transwell assay (migration) (BD Biosciences, NJ, USA) and wound healing assay. The detailed protocol was followed previous report [Citation17]. Each treatment group comprised of 3–5 replicates.
Caspase activity assay
Caspase-3, 8, 9 activities were evaluated by the caspase activity assay kit (Promega, Fitchburg, WI, USA). HB cells transfected with miR-21 inhibitor were treated with 10 µL of lysis buffer and then incubated with 5 µL of chromogenic substrate for 20 min at 37 °C. The results were evaluated by using a multi-scan plate reader (Fisher, MA, USA). Each treatment group comprised of 3–5 replicates.
Luciferase reporter assays
TargetScan (www.targetscan.org), MiRanda (www.microrna.org), and RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/) were used to online predict target genes of miR-21. ASPP2 was identified to be a potential binding site of miR-21. The luciferase reporter assay (Promega, WI, USA) was applied to identify whether ASPP2 3′-UTR is the binding site of miR-21. The detailed protocol was identical to the previous method [Citation18].
Animal experiments
All animal experiments and care were performed according to the Animal Care and Treatment Administration of the National Ministry of Health and the requirement of the Ethics Committee in Linyi people's hospital. Eight male BALB/c athymic nude mice (body weight: 18.01 ± 1.86 g, 4–6 weeks old) were purchased from the Animal Center Laboratory at Peking University (Beijing, China). All mice were individually housed at pathogen-free conditions (20–26 °C with 40–70% relative humidity and a 12:12 h light-dark cycle). Water and food were given ad libitum. HB cell line HUH-6 was purchased from Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). To establish xenograft model, HUH6 cells (3.0 × 106) were suspended in 100 μl phosphate-buffered saline and subcutaneously injected into the flank of each mouse. Eight days post-injection, the injected mice were randomly divided into two groups (n = 4 each). Cholesterol-conjugated 2-O-methyl-modified miR-21 inhibitor or control (hsa-miR-NC) (GenePharma Co., Ltd, Shanghai, China) was injected into the tumor every four days for seven times (1 nmol in 20 μl phosphate-buffered saline) [Citation19]. The tumor volume was calculated with the formula Volume = (Length × Width2) × 0.5.
Statistical analysis
Data were expressed as means ± SEM and were analyzed by SPSS 19.0 software (SPSS Inc, Chicago, USA). Mean differences between groups were analyzed using the Tukey’s test. In this study, p < .05 was regarded to be statistically significant.
Results
MiR-21 expression increased in HB
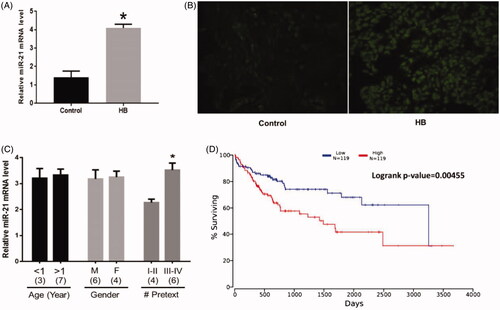
RT-PCR and FISH results revealed that miR-21 expression was increased in HB cells () (p = .023) and HB tissues () (p = .010) compared with the control group, respectively. Also, miR-21 expression was higher in patients who were classified in group III and IV compared with those in group I and II according to the PRETEXT system [Citation11] () (p = .032). However, miR-21 expression had no correlation with age (p = .739) and gender (p = .804). Furthermore, the OS analysis conducted in the OncoLnc database revealed that patients with high levels of miR-21 had shorter survival time than those with low levels of miR-21 expression (p = .005) ().
Figure 1. Increased miR-21 expression in HB. (A) miR-21 expression was upregulated in HB cells. (B) miR-21 expression was upregulated in tissues. (C) The correlations between miR-21 expression and patient information. Numbers in parentheses denote the number of samples. (D) The overall survival (OS) rate of patients with HB with high or low miR-21 expression. (*) denotes difference from control (p < .05). Values are means ± SEM. Three samples at least in each treatment group were available for the analysis.
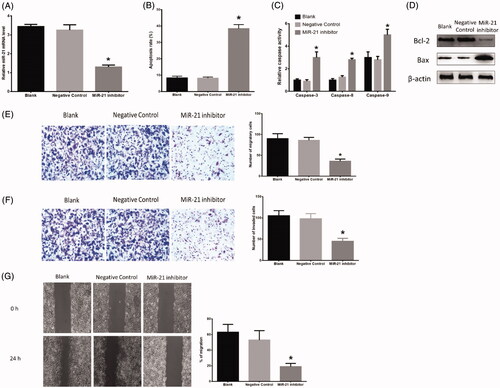
Effects of the miR-21 inhibitor on HB apoptosis
Through transfection with the miR-21 inhibitor, HB cells expressed less miR-21 than those in the blank (p = .021) (). No difference was found between the blank and negative group. Flow cytometry assay revealed that HB cells transfected with the miR-21 inhibitor displayed higher apoptosis level than those treated with blank (p = .013) (). The miR-21 inhibitor decreased Blc-2 (p = .032) while increased Bax (p = .021) protein expression compared with those treated with blank (. Also, HB cells treated with the miR-21 inhibitor showed higher activity of caspase-3 (p = .009), caspase-8 (p = .021) and caspase-9 (p = .027) compared with those in blank-treated group (. Furthermore, HB cells transfected with the miR-21 inhibitor showed the reduced ability of migration in Transwell assay (p = .038) () and wound healing assay (p = .021) (), respectively, compared with blank-treated cells. The miR-21 inhibitor treatment also caused decreased ability of invasion in invasion assay compared with blank treatment (p = .031) (. In all experiments, there was no difference between the blank and negative group.
Figure 2. Effects of miR-21 inhibitor on HB apoptosis. (A) miR-21 expression in HB cells transfected with negative control or miR-21 inhibitor. (B) Inhibition of miR-21 increased HB apoptosis. (C) Inhibition of miR-21 affected protein expressions of Bcl-2 and Bax. (D) Inhibition of miR-21 increased protein expressions of caspase-3, 8, 9. (E) Inhibition of miR-21 decreased HB cell migration ability in Transwell assay. (F) Inhibition of miR-21 decreased HB cell invasion ability in invasion assay. (G) Inhibition of miR-21 decreased HB cell migration ability in wound healing assay. (*) denotes difference from control (p < .05). Values are means ± SEM. Three samples at least in each treatment group were available for the analysis.
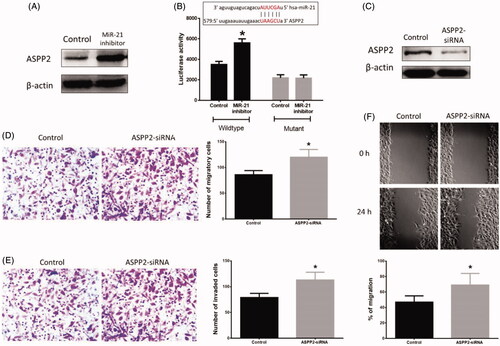
Effects of inhibition of ASPP2 on HB apoptosis
To further investigate the effect of miR-21 in HB apoptosis, we first identified the target gene of miR-21. ASPP2 expression was increased in HB cells treated with the miR-21 inhibitor (p = .025) compared with those in the control group (. After transfection with luciferase reporter vector containing the wild type or mutant 3′UTR of ASPP2, the results revealed that the miR-21 inhibitor treatment increased the luciferase activity in wide type transfected HB cells (p = .033), but not in mutant-treated cells (. Also, HB cells treated with ASPP2-siRNA showed less ASPP2 protein expression ( (p=.027). Furthermore, HB cells transfected with ASPP2-siRNA displayed enhanced ability of migration in Transwell assay (p = .041) () and wound healing assay (p = .030) (), respectively, compared with the control group. In invasion assay, ASPP2-siRNA-treated HB cells had a higher ability of invasion (p = .037) () than those in the control group.
Figure 3. Effects of inhibition of ASPP2 on HB cells. (A) Inhibition of miR-21 increased protein expression of ASPP2. (B) The predictive binding sequence of miR-21 in the 3′UTR of ASPP2 and relative luciferase activity. (C) ASPP2 siRNA treatment decreased protein expression of ASPP2 in HB cells. (D) Inhibition of ASPP2 increased HB cell migration ability in Transwell assay. (E) Inhibition of ASPP2 increased HB cell invasion ability in invasion assay. (F) Inhibition of ASPP2 increased HB cell migration ability in wound healing assay. (*) denotes difference from control (p < .05). Values are means ± SEM. Three samples at least in each treatment group were available for the analysis.
Effects of MAPKs family on HB apoptosis
To further investigate the potential signaling pathways mediating miR-21-involved apoptosis in HB cells, some key proteins in MAPKs family were detected in HB cells treated transfected with miR-21 inhibitor. The results suggested that the miR-21 inhibitor treatment increased the protein expressions of p-ERK (p = .010), ERK (p = .027), p-p38 (p = .039), and p38 (p = .013), but did not alter protein levels of p-JNK (p = .077) and JNK (p = .082) (. Moreover, selective p38 MAPK inhibitor SB203580 reduced apoptosis level induced by the miR-21 inhibitor treatment (p = .030), but such effect was not found in ERK kinase inhibitor PD98059 (p = .069) compared with those only treated with the miR-21 inhibitor (), indicating p38 signaling may mediate effects of miR-21 inhibitor on apoptosis in HB cells.
Figure 4. (A) Effects of miR-21 inhibitor on HB apoptosis in vivo. (B) Inhibition of miR-21 affected protein expressions of MAPKs family in vitro. (C) Effects of MAPKs inhibitor on HB apoptosis. (D) Tumor growth curve. (E,F) Representative tumors from mice treated with has-miR-NC or miR-21 inhibitor. (G) Inhibition of miR-21 affected protein expressions of MAPKs family in vivo. (*) denotes difference from control (p < .05). Values are means ± SEM. Three samples at least in each treatment group were available for the analysis.
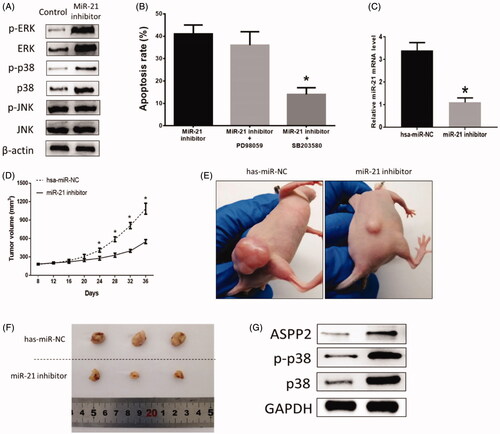
Downregulation of MiR-21 inhibits tumor growth in hepatoblastoma xenografts model
Hepatoblastoma HUH6 cell line was subcutaneously injected into the flanks of athymic mice to establish hepatoblastoma xenografts mouse model. Intratumor injection of miR-21inhibitor was performed every four days for seven times. The miR-21 expression was evaluated by RT-PCR in tumor tissues on day 12 to verify the efficiency of miR-21inhibitor in xenografts mice model. The results suggested that miR-21 was downregulated by miR-21inhibitor in tumor tissues (p = .021) (. In addition, miR-21inhibitor significantly suppressed tumor growth (). The tumor growth curve indicated that the tumor size became to be significant on day 24 between two groups (p = .032) (. Furthermore, miR-21inhibitor caused increased expressions of ASPP2 (p = .035), p-p38 (p = .019), and p38 (p = .008) (), which is consistent with results from vitro experiment. These results indicated that inhibition of miR-21 could promote HB cell apoptosis through ASPP2/p38 signaling pathway.
Discussion
Numerous previous studies reveal that the miR-21-involved oncogenic processes are closely associated with enhanced invasion and metastasis, increased proliferation rate, as well as inhibited apoptotic level [Citation20,Citation21]. For HB, the elevated miR-21 expression level is observed in both plasma and exomes samples in patients with HB [Citation22], and the expression profile of miR-21 may be a good potential predictor for prognosis [Citation23]. In this study, miR-21 expression level also was increased in both HB cell and tissue level compared with normal adjacent tissues. Furthermore, our study first reports miR-21 expression was higher in the patients with higher difficulty of the expected surgical treatment (III & IV) compared with patients who were classified into group I & II [Citation11]. However, miR-21 expression was not observed to be related to other factors, such as age and gender, in this study. Furthermore, HB patients with high expression of miR-21 were associated with shorter survival time than those with low miR-21 level. Thus, these results together suggest that miR-21 expression level may be a potential signature of HB progression and indicator for therapeutic strategy.
Since miR-21 plays important roles in various cancers [Citation24], it thus has been a key factor in studying tumor mechanism and exploring the potential therapy. Apoptosis is a series of programmed cellular events in aging or damaged cells [Citation25]. In various tumor cells, however, the disrupted apoptotic process results in cell survival and proliferation along with DNA mutation or damage [Citation24]. It has been demonstrated that miR-21 is extensively involved in apoptosis via suppressing cellular apoptosis [Citation25]. In this study, inhibition of miR-21 caused increased apoptosis level and altered apoptosis-associated protein expressions, such as caspase-3/8/9, Blc-2, and Bax. Moreover, inhibited the effect of miR-21 was found to lead to decreased HB cell migration and invasion. In our animal study, administration of miR-21 inhibitor to HUH6-xenografts mouse significantly downregulated tumor growth, which agrees with the observations from in vitro studies. Zhou et al. reported that the inhibition of miR-21 promotes cell apoptosis in lung cancer in both in vitro and in vivo [Citation19]. Also, Knockdown of miR-21 exerts inhibitory roles in tumor growth in breast cancer [Citation26]. Thus, these results imply that miR-21 exert essential roles in apoptosis of HB cells and miR-21 may be a novel therapeutic target for HB intervention.
Next, to investigate the functions of miR-21 in HB cell, the downstream gene of miR-21 ASPP2 was identified via luciferase reporter assay. Since multiple target genes of miR-21 have been reported to be involved in apoptosis [Citation27], thus inhibition of ASPP2 via siRNA transfection in HB cells was applied to further identify the roles of ASPP2 in miR-21-mediated HB apoptosis. Our results revealed that the inhibition of ASPP2 enhanced HB cell migration and invasion and ASPP2 was increased in the growth-inhibited tumor in vivo. Collectively, ASPP2 is not only the target gene of miR-21 but also is a major mediator in miR-21-involved apoptosis in HB. ASPP2 plays critical roles in tumorigenesis and stimulates the transactivation function of p53 on the promoters of proapoptotic genes via binding to the DNA binding domains of p53, in turn, enhances the proapoptotic effect of p53 [Citation8]. Thus, the functions of ASPP2 in HB apoptosis provide clues to further explore the signaling pathway of miR-21-involved HB apoptosis.
The mitogen-activated protein kinases (MAPKs), belonging to the serine/threonine kinase family, play essential roles to regulate cell proliferation and apoptosis [Citation28]. Due to the functional interaction between MAPKs and p53 in cell cycle regulation, thus three primary signaling pathways including p38 kinase, ERK, and JNK were detected in HB cells with inhibition of miR-21 [Citation29]. The results revealed that inhibition of miR-21 up-regulated protein expression of both p38 and ERK, but not JNK. Meanwhile, selective p38 MAPK inhibitor decreased apoptosis level caused by inhibition of miR-21, but no such effect was not found in ERK kinase inhibitor. Also, p38 was also activated in vivo study. These results collectively suggest that miR-21-involved apoptosis in HB is mediated via p38, but not ERK and JNK signaling pathways. P38 has been reported to be associated with cell cycle and apoptosis, in particular, p38 inhibits cell growth by suppressing cyclin D and engaging the p16/Rb and p19ARF/p53 antitumor signaling pathway [Citation30]. In this study, the results together indicate that the activated p38 MAPK pathway induced by miR-21 may eventually affect apoptosis via altering the cell cycle.
In conclusion, the results suggested that miR-21 expression is associated with HB progression and that miR-21-involved apoptosis in HB is mediated through ASPP2/p38 signaling pathway in vitro and in vivo. This study provided novel evidence for understanding the mechanisms underlying the roles of miR-21 in HB apoptosis and clue to develop new therapeutic strategies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- von Schweinitz D. Hepatoblastoma: recent developments in research and treatment. In Seminars in pediatric surgery, vol. 21, no. 1, p. 21-30. WB Saunders, 2012.
- Birch JM. Epidemiology of pediatric liver tumors, In Pediatric Liver Tumors. Berlin, Heidelberg: Springer; 2011. p. 15-26.
- Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer. 2012;59:818–821.
- Lieber J, Kirchner B, Eicher C, et al. Inhibition of Bcl‐2 and Bcl‐X enhances chemotherapy sensitivity in hepatoblastoma cells. Pediatr Blood Cancer. 2010;55:1089–1095.
- Marin J, Romero M, Martinez-Becerra P, et al. Overview of the molecular bases of resistance to chemotherapy in liver and gastrointestinal tumours. CMM. 2009;9(9):1108–1129.
- Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163.
- Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci. 2009;106(29):12085–12090.
- Samuels-Lev Y, O'Connor DJ, Bergamaschi D, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol cell. 2001;8(4):781–794.
- Lossos IS, Natkunam Y, Levy R, et al. Apoptosis stimulating protein of p53 (ASPP2) expression differs in diffuse large B-cell and follicular center lymphoma: correlation with clinical outcome. Leuk lymphoma. 2002;43:2309–2317.
- Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68(19):8164–8172.
- Roebuck DJ, Aronson D, Clapuyt P, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37(2):123–132.
- Anaya J. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci. 2016;2:e67.
- Cui Y, Lu P, Song G, et al. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37.
- Doberstein K, Steinmeyer N, Hartmetz A-K, et al. MicroRNA-145 targets the metalloprotease ADAM17 and is suppressed in renal cell carcinoma patients. Neoplasia. 2013;15(2):218.
- Doberstein K, Bretz NP, Schirmer U, et al. miR-21-3p is a positive regulator of L1CAM in several human carcinomas. Cancer Lett. 2014;354(2):455–466.
- Zhang FZ, Ho DH-H, Wong RH-F. Triptolide, a HSP90 middle domain inhibitor, induces apoptosis in triple manner. Oncotarget. 2018;9(32):22301.
- Pulito C, Mori F, Sacconi A, et al. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017;3(1):17022.
- Liu W, Xia P, Feng J, et al. MicroRNA-132 upregulation promotes matrix degradation in intervertebral disc degeneration. Exp Cell Res. 2017;359(1):39–49.
- He X, Chang Y, Meng F, et al. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31(28):3357.
- Wang P, Zou F, Zhang X, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009;69(20):8157–8165.
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033.
- Liu W, Chen S, Liu B. Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study. Pediatr Surg Int. 2016;32(11):1059–1065.
- Gyugos M, Lendvai G, Kenessey I, et al. MicroRNA expression might predict prognosis of epithelial hepatoblastoma. Virchows Arch. 2014;464(4):419–427.
- Liu M-F, Jiang S, Lu Z, et al. Physiological and pathological functions of mammalian microRNAs; 2010; 427–446.
- Koff JL, Ramachandiran S, Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. IJMS. 2015;16(2):2942–2955.
- Yan LX, Wu QN, Zhang Y, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast cancer Res. 2011;13(1):R2.
- Buscaglia LEB, Li Y. Apoptosis and the target genes of microRNA-21. Chinese J Cancer. 2011;30(6):371.
- Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: from an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25(3):120–123.
- Tseng S-H, Wang C-H, Lin S-M, et al. Activation of c-Jun N-terminal kinase 1 and caspase 3 in the tamoxifen-induced apoptosis of rat glioma cells. J Cancer Res Clin Oncol. 2004;130(5):285–293.
- Manke IA, Nguyen A, Lim D, et al. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol cell. 2005;17(1):37–48.
