?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bladder cancer is a common malignant tumour with high recurrence rate. Cytokeratin 19 fragments (Cyfra21-1) in urine has been regarded as a promising biomarker for the prognosis and diagnosis of bladder cancer due to the relevance of its high urinary level to the bladder cancer patients. However, currently detection methods of Cyfra21-1 have their limits, such as complicated steps, limited sensitivity or unsatisfying specificity. In this study, we developed a novel time-resolved fluoroimmuno test strip by using europium chelate microparticle (Eu-CM). Detection was performed in simple steps by carrying drops of sample into the well of the test strip, waiting for 15 min and inserting the strip into a fluorescence strip reader for quantitation. The standard curve equation of the test strip was y = 0.0177x + 0.01 (R2 = .9993). In the analysis of human urine samples (n = 115), it demonstrated a good performance (accuracy: CV < 10%, AUC: 0.989). With the cut-off value of 81 ng/mL, the sensitivity and specificity for bladder cancer were 92.86 and 100%, respectively. In comparison to ELISA and electrochemiluminescence methods, the Eu-CM based time-resolved fluoroimmuno test strip provided a rapid, sensitive and reliable method for monitoring bladder cancer. It may be applied as a non-invasive approach for in point-of-care for bladder cancer detection.
Introduction
Bladder cancer (BC) is one of the most common cancers with an estimated 165,000 deaths per year in the world [Citation1]. Although the diagnosis and treatment techniques of bladder cancer have improved greatly in recent years, the probabilities of recurrence and progression is still high after local treatment, the 5-year survival rate and recurrence rate of high-grade BC can be approximately 5 and 80%, respectively [Citation2–4], and the 5-year progression rate is about 45% [Citation5]. Therefore, regular re-examination is required after surgery. Currently, cystoscopy is the gold standard for BC detection and surveillance, which requires invasive sampling to pathological examination of suspicious tissue, but the sensitivity to diagnose carcinoma in situ is low and patients often bear a high economic burden [Citation6]. Urine cytology is widely used as a non-invasive test but with unsatisfying sensitivity. To explore non-invasive urine-based detection methods, numbers of urinary biomarkers have been developed in laboratories and clinics [Citation7–9].
Cytokeratin 19 fragments (Cyfra21-1) is a soluble fragment of keratin CK19. Serum Cyfra21-1 is an effective diagnostic biomarker for cancers such as lung cancer and oral/oropharyngeal squamous cell carcinoma [Citation10–12], and previous studies also confirmed that the diagnostic specificity and sensitivity of urinary Cyfra21-1 for bladder cancer were about 60–90% and 40–92%, respectively [Citation8,Citation13–16]. Immunoassay-based techniques such as electro chemiluminescent immunoassay, enzyme-linked immunosorbent assay (ELISA) and immunochromatographic assay were most often used for urinary Cyfra21-1 detection [Citation17–19]. These detection methods suffer from some problems. The reaction system of chemiluminescence method is unstable, with complexity of the procedures and high instrument cost. The ELISA needs to pre-treat urine to extract protein so that the analysis time is relatively long. It is not suitable for rapid detection. Membrane-based lateral flow Immunochromatographic assay, often referred to as a point-of-care test, offers several advantages, such as rapidity, simplicity, selectivity and low cost. The colloidal gold is the most common type, but it can only be qualitative or semi-quantitative detection and cannot be used for trace analysis, it also occurs large false-negative rate because of the haematuria interference [Citation20,Citation21]. Therefore, there an increasing demand for improvement of immunochromatographic methods and to design new ones to achieve quantitative detection with great convenience, ideal sensitivity and specificity.
Several types of materials such as colloidal gold, coloured latex particles, carbon and selenium nanoparticles, chemiluminescent and fluorescent nano. particles can be used as label for immunochromatographic assay. Among these, fluorescent labels offer high sensitivity and time-resolved fluorescent immunoassay by using lanthanide elements such as europium (Eu) and samarium (Sm) in which fluorofores possess a longer fluorescence lifetime, narrow emission spectra, large stokes shifts and outstanding photostabilities [Citation22–25]. The quantitative range is directly related to the number of specific proteins involved in the reaction system, and the performance index is much higher than other rapid detection techniques [Citation26–28]. Polystyrene nanoparticles containing thousands of lanthanide chelates have developed as attractive labels with their remarkable signal amplification potential, which contributes to high sensitivity and accuracy.
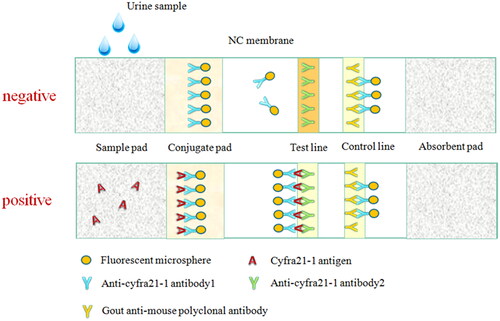
In this paper, we developed a novel time-resolved fluoro-immunochromatographic test strip by using europium chelate microparticle (Eu-CM) as a label, which was based on a conventional sandwich immunoassay. When the sample containing Cyfra21-1 was added to the sample pad, the analytes migrated to the conjugate pad and combined with Eu-CM labelled one Anti-Cyfra21-1 coated on the NC membrane and formed Eu-CM-labelled antibody-antigen-antibody sandwich complexes. Then, the labelled microparticles were immobilized at the test line (T line), and the excess fluorescent microsphere complexes continue moving along the membrane and were captured in the internal control line (C line) by the other antibody, such as goat anti-mouse IgG. And then, the surplus complexes migrated into the absorption pad. The whole reaction might take about 15 min. Finally, a fluorescence strip reader was employed to analyze the test strip by calculating the fluorescence intensities of the T line and C line. On the basis of the above, the concentration of Cyfra21-1 in the samples would be directly measured with the ratio of T/C. If only the T line or no line was observed by the reader, the strip was considered invalid.
The results for the test strip were based on the T/C ratio, the performance of the Eu-CM based time-resolved fluoroimmuno test strip was evaluated in terms of linearity, minimum detection limit, precision, sensitivity and specificity. The developed test strip was also compared with a commercially available ELISA kit for analysis of 26 urine samples of healthy people. The data illustrated that the new time-resolved fluoro-immunochromatographic test strip had the following advantages: (1) Convenience and rapidity, (2) higher sensitivity, (3) wide quantitative detection range. It showed great potential further development for rapid point-of-care screening and clinical as well as domestic monitoring of bladder cancer.
Materials and methods
Specimen sources
A collection of 115 urine samples was used in this study, which were from Shenzhen Luohu district people’s hospital and Tumour Hospital Affiliated to Zhongshan University, Guangzhou from March to October 2018. The tumour group: included 42 hospitalized patients with bladder cancer and 22 patients who were re-examined after bladder cancer surgery (59 males and 4 females, age: 34–75 years-old, average age: 61.9 years-old). The control group: included 30 people with healthy physical examination results, 11 patients with other urinary neoplasms (renal carcinoma, prostatic carcinoma, ureteral tumours), 10 patients with benign diseases of the urinary system (urinary calculi, urinary tract infection).Urine samples were collected and stored in a sterile centrifuge tube at 2–8 °C and then detected within 2 h.
Main reagents
Anti-Cyfra21-1 antibodies 1, anti-Cyfra21-1 antibodies 2, Cyfra21-1 antigen, goat-anti-mouse IgG, N-hydroxy-succinimide (NHS), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 2-Aminoethanol, MES monohydrate, boric acid, sodium tetraborate were purchased from Shenzhen Baisiheng Technology co., Ltd. (Shenzhen, China). Nitrocellulose filter membrane (NC membrane), glass cellulose membrane (conjugate pad), absorbent pad, PVC baseplate were purchased from Shanghai Jinbiao biotechnology co., Ltd. (Shanghai, China). Europium chelate coated nanosphere (PS-COOH) were purchased from Bangs Laboratories, Inc.
Main instruments
HM3035 three-dimension platform dispenser and HGS201 strip cutting machine was purchased from Shanghai Jinbiao biotechnology co., Ltd. WH1000 fluorescence immunoassay instrument was purchased from Shenzhen WWHS Biotech co., Ltd. (Shenzhen, China).
Preparation of fluorescent microsphere markers
Anti-Cyfra21-1 antibodies 1 was conjugated to the europium chelate nanosphere by the EDC/NHS coupling reaction. The first wash: pipette 500 μL fluorescent microspheres, add 1 mL 0.1 M MES and fully mix using an ultrasonic cleaning instrument, centrifugation at 13,000 rpm for 15 min, abandon the supernatant, resuspend the precipitate with 1 mL 0.1 M MES and mix well for 2 min with ultrasonic cleaning instrument, centrifugation at 13,000 rpm for 15 min, abandon the supernatant, repeat this step thrice, resuspend the precipitate with 1 mL 0.1 M MES after washing for the last time, to ensure that the microspheres are monodispersed. Activation: add 20 mg/mL EDC 100 μL and 20 mg/mL NHS 300 μL to fluorescent microsphere suspension, centrifugation at 13,000 rpm for 15 min, abandon the supernatant, resuspend with 1 mL coupling solution (0.2 M boric acid, 0.1 M sodium tetraborate). Coupling: Ultrasound mixing the coupling fluid which contains fluorescent microspheres, performed at 13,000 rpm for 15 min, abandon the supernatant, add 1 mL coupling solution and resuspend it. Repeat this step thrice. After the last resuspension, add 7.5 μL anti-Cyfra21-1 antibodies 1, mix it for 3 h with a rotary mixer under minimum speed. Blocking: After the coupling, add 30 μL ethanolamine (10%) and 800 μL blocking solution (PBS, 0.5%BSA, 0.4% tween-20) to fluorescent microsphere suspension, mix it for 2.5 h with the rotary mixer under minimum speed. Then, centrifugate it for 15 min at 13,000 rpm, abandon the supernatant. Final cleaning: resuspend it with 1 mL blocking solution, repeat the cleaning step thrice with blocking solution. The precipitates were dissolved in 500 μL of blocking solution.
Preparation of each part of test strips
Conjugate pad: Glass cellulose membrane was selected as bonding cushion solid material, near-infrared fluorescent nanosphere-labelled anti-Cyfra21-1 antibodies 1 were sprayed and dried in a strong convection air oven at 37 °C for 10 h. Sample pad: Glass cellulose membrane was selected, sample pad treatment solution (Tris, BSA, twin-20) was sprayed and dried in the oven for use. NC membrane: Anti-Cyfra21-1 antibodies 2 (Test line: line T) and goat-anti-mouse IgG (Control line: line C) were sprayed on the NC membrane with a platform dispenser and dried in the oven for use.
Test strips assembling
In the environment with humidity less than 35%, as shown in , the NC membrane, conjugate pad, sample pad, and absorbent paper are pasted successively on the PVC viscous bottom plate, strips of 0.38 cm width were put into the plastic shell and then stored at room temperature in sealed aluminium foil bags.
Test strips optimization
Determine the selection of labelled antibody
Anti-Cyfra21-1 antibodies 1 and anti-Cyfra21-1 antibodies 2 were labelled on fluorescence nanosphere respectively, and one of the two antibodies was used as the test line (line T) to spray on the NC membrane to detect the properties of the test strips.
Determine the antibody concentration of the test line
0.8 mg/mL, 1.0 mg/mL and 1.2 mg/mL anti-Cyfra21-1 antibodies 2 were respectively used as the test line to spray on the NC membrane to detect the properties of the test strips.
Performance evaluation of the test strips
Establishment of the standard curve
Cyfra21-1 antigen was evaluated by Roche electrochemical luminescence method to detect Cyfra21-1 antigen. The valued Cyfra21-1 standard was diluted with different concentrations (0, 1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 ng/mL), and the prepared strips were used for sequential determination. The measurements were repeated 5 times per concentration, and the standard curve was drawn according to the results. The equation used for calculation was of the form y = a + bx, and the correlation coefficient R2 value should be ≥.995.
Minimum detection limit
The sample diluent was taken as a blank sample and detection was repeated 20 times. The mean and standard deviation of the detection results were calculated. The detection limit and sensitivity are the corresponding concentration obtained from the standard curve with the mean ± double standard deviation.
Precision
The test paper was randomly selected and the concentration of 25 and 50 ng/mL were tested 10 times each to calculate the interior CV. Interior CV < 10% is required.
Sensitivity and specificity
The measurement data were expressed as ± S, and SPSS22.0 software was used for data analysis. The rank sum test was used to compare the two groups of data, and p < .05 had statistical significance. The sensitivity, specificity, false-positive rate and false-negative rate of Cyfra21-1 detection for bladder cancer recurrence monitoring were calculated by ROC curve.
Results
This test strips combines the fluorescence microsphere labelling technology with the immunochromatography technology, and adopts the principle of double antibody sandwich-type to detect the concentration of Cyfra21-1 in urine. The structure diagram of the strip is shown in . When the sample was dripped to the hole of the test strip, under the effect of chromatography, formatted of antibody–antigen complex. Then along the NC membrane chromatography to line T, the complex stayed and fixed on the line T, the antibodies marked by fluorescence nanosphere that did not participate in the reaction were further chromatographed to line C and immuno-binding reaction was conducted with the goat-anti-mouse IgG fixed at line C, reaction for 15 min, and then the measurement results were read in the ultraviolet signal reader. The concentration of Cyfra21-1 in the sample was proportional to the ratio of the optical signal intensity of line T and C (T/C). The concentration of Cyfra21-1 in the sample can be obtained by calculating the standard curve.
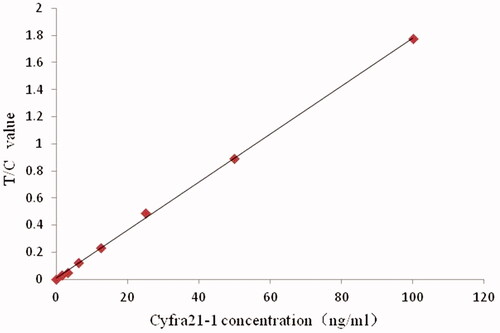
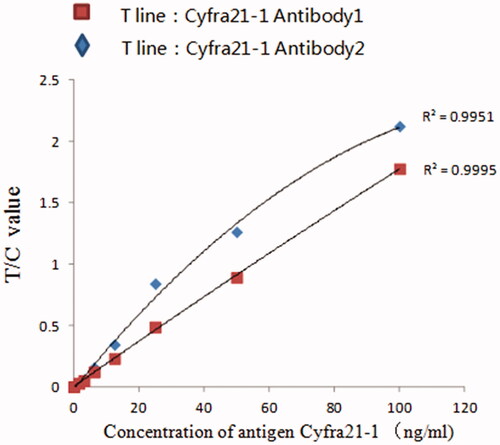
Figure 2. Standard curve of Cyfra21-1 quantitatively detected by fluorescence microspheres immunochromatography with different antibody markers.
Optimization of process conditions
Selection of microsphere labelled antibody
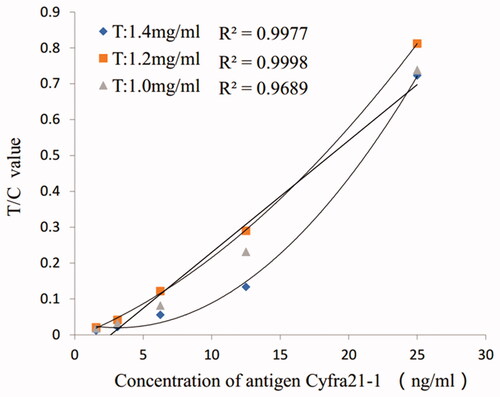
As shown in , compared with anti-Cyfra21-1 antibodies 2, anti-Cyfra21-1 antibodies 1 labelled on fluorescence microspheres have better linearity.
Detection of antibody concentration in line T
As seen from , when the antibody concentration of line T was 1.2 mg/mL, the detection signal value was the strongest. Considering the performance and cost of the test paper, it was appropriate to select an antibody concentration of 1.2 mg/mL.
Determination of the properties of the test strips
Detection of linearity
With the sample concentration as the abscissa and the ratio of the test line to the control line (T/C) as the ordinate, the Cyfra21-1 standard curve prepared is shown in , y = 0.0177x + 0.01. The logistic curve (four parameters) was used for fitting, and the fitting degree R2 was .9993, n = 7. The T/C value has a good correlation with the standard product in the range of 0–100 ng/mL.
Sensitivity
The mean value of the blank sample, detected 20 times, was 0.0075 ng/mL, and the standard deviation was 0.00088 ng/mL. The sensitivity was 0.071 ng/mL by bringing the blank mean plus twice the standard deviation into the standard curve formula. The results showed that the fluorescence nanosphere immunochromatographic test strips of Cyfra21-1 had high sensitivity and fully met the detection requirements.
Precision
As shown in , the intra CV of each test concentration was less than 10%.
Table 1. Precision analysis of test strips.
Clinical samples test
To demonstrate the clinical application of the immunochromatographic test strip of Cyfra21-1, a total of 115 urine samples, included those from 42 bladder cancer hospitalized patients, 22 patients who re-examined after surgery (the results of microscopic examination were negative), 30 healthy people identified by physical examination, 10 patients with benign diseases (ureteral calculi, renal calculi, urinary tract infection), and 11 patients with other urinary tumours (ureteral tumour, kidney cancer, prostate cancer), were analyzed. In order to investigate the normal reference range of Cyfra21-1 in urine, the minimum value and the standard value, as well as the mean value of healthy people were 3.38, 5.38 and 4.23 ng/mL, respectively.
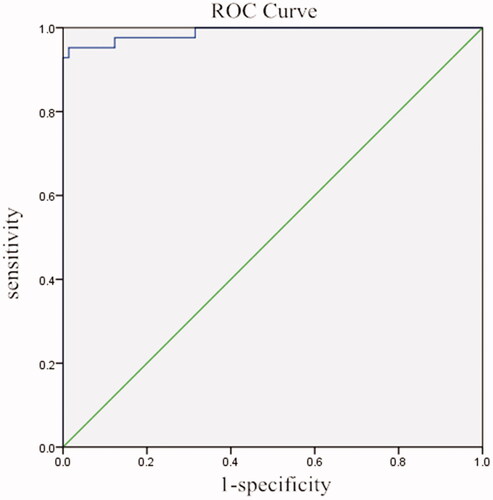
As shown in , there were significant differences between the urinary Cyfra21-1 level of bladder cancer patients and those of non-bladder cancer people (p < .01; p < .01; p < .05; p < .01). The ROC curve is shown in , the area under the curve was 0.989, p = .08, which is of statistical significance, indicating that the result of the test strips has predictive value for bladder cancer. By calculating the Youden index, it was found that when 81 ng/mL was the cut-off value, the sensitivity, specificity, false-positive rate, and false-negative rate were 95.24, 100, 0 and 7.14% respectively.
Table 2. Clinical samples test results.
For further investment, the results from the bladder cancer patients with low grade were compared with those from the patients with high grade. As shown in , the average level of urinary Cyfra21-1 from high grade and low-grade patients were 246.56 ± 86.65 ng/mL and 104.27 ± 63.07 ng/mL, respectively. It suggested that the test strip showed an advantage in indicating high grade type of bladder cancer.
Table 3. Clinical samples test results.
Conclusions
Urine is the most ideal non-invasive test sample. In this study, we developed a novel immunochromatographic test strip by using europium chelate coated fluorescent nanosphere, and applied it for quantitative detection of bladder cancer biomarker Cyfra21-1in the urine. Compared with the performance of traditional rapid detection technology, this method has the advantages of higher sensitivity, wider quantitative detection range. The sensitivity of Cyfra21-1 detection by this method can reach 1.164 ng/mL, and the performance index is much higher than the other rapid detection technologies. Quantitative detection of Cyfra21-1 in urine can be used for early detection and recurrence monitoring of bladder cancer. However, in the present study, the urine sample of bladder cancer patient was limited, more clinical samples need to be tested and more data needed to be accumulated to determine the sensitivity and specificity, other verified specific biomarkers for bladder cancer could be employed to reduce the false-negative rate in the future. The test strip is time-saving and labour-saving, and suitable for a wide range of clinical applications, so as to reduce the frequency of postoperative review of bladder cancer patients and provide clinicians with a reference for the early diagnosis of bladder cancer.
Ethical approval
All procedures performed in the study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclosure statement
There are neither ethical nor financial conflicts of interest involved in the manuscript. The manuscript contains only original unpublished work and is not being submitted for publication elsewhere.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
- Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8(1):1129.
- Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–119.
- Lodewijk I, Duenas M, Rubio C, et al. Liquid biopsy biomarkers in bladder cancer: a current need for patient diagnosis and monitoring. Int J Mol Sci. 2018;19(9):2514.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466.
- Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461.
- Agarwal PK, Black PC, Kamat AM. Considerations on the use of diagnostic markers in management of patients with bladder cancer. World J Urol. 2008;26(1):39–44.
- D’Costa JJ, Goldsmith JC, Wilson JS, et al. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016;2:301–317.
- Chen LM, Chang M, Dai Y, et al. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1804–1812.
- Zhang L, Liu D, Li L, et al. The important role of circulating Cyfra21-1 in metastasis diagnosis and prognostic value compared with carcinoembryonic antigen and neuron-specific enolase in lung cancer patients. BMC Cancer. 2017;17(1):96.
- Zhao T, Jin Y, Mao G, et al. Cyfra21-1 is an early predictor of chemotherapeutic effectiveness in advanced nonsmall cell lung cancer: an observational study. Medicine (Baltimore). 2016;95(52):e5748.
- Yuan C, Yang K, Tang H, et al. Diagnostic values of serum tumor markers Cyfra21-1, SCCAg, ferritin, CEA, CA19-9, and AFP in oral/oropharyngeal squamous cell carcinoma. Onco Targets Ther. 2016;9:3381–3386.
- Yuan-Lan Huang JC, Yan W, Zang D, et al. Diagnostic accuracy of cytokeratin-19 fragment (Cyfra21–1) for bladder cancer: a systematic review and meta-analysis. Tumor Biol. 2015;36(5):3137–3145.
- Leiblich A. Recent developments in the search for urinary biomarkers in bladder cancer. Curr Urol Rep. 2017;18(12):100.
- Darwiche F, Parekh DJ, Gonzalgo ML. Biomarkers for non-muscle invasive bladder cancer: current tests and future promise. Indian J Urol. 2015;31(4):273–282.
- Miyake M, Morizawa Y, Hori S, et al. Diagnostic and prognostic role of urinary collagens in primary human bladder cancer. Cancer Sci. 2017;108(11):2221–2228.
- Chen J, Tao F, Zhang B, et al. Elevated squamous cell carcinoma antigen, cytokeratin 19 fragment, and carcinoembryonic antigen levels in diabetic nephropathy. Int J Endocrinol. 2017;2017:1.
- Liu Yan-Hui L-L, Xiao-Hou WU, Xiao-Zhong CAI, et al. Research of the strip of immunocolloidal-gold with monoclonal antiCK20 antibody. Immunol J. 2007;23(1):101–106.
- Wang HY, Hsieh CH, Wen CN, et al. Cancers screening in an asymptomatic population by using multiple tumour markers. PLoS One. 2016;11(6):e0158285.
- Hou W, Wang S, Wang X, et al. Development of colloidal gold immunochromatographic strips for detection of Riemerella anatipestifer. PLoS One. 2015;10(3):e0122952.
- Niu Y, Wang D, Cui L, et al. Monoclonal antibody-based colloid gold immunochromatographic strip for the rapid detection of Tomato zonate spot tospovirus. Virol J. 2018;15(1):15.
- Chun-Yan Zhang J-X, Li W-J, Ou L-J, et al. [Preparation and fluorescence sensing properties of monodisperse bonded polystyrene-containing microspheres]. Chem J Chin Univ. 2019;40:153–159. Chinese
- Cordina NM, Sayyadi N, Parker LM, et al. Reduced background autofluorescence for cell imaging using nanodiamonds and lanthanide chelates. Sci Rep. 2018;8(1):4521.
- Liang RL, Deng QT, Chen ZH, et al. Europium (III) chelate microparticle-based lateral flow immunoassay strips for rapid and quantitative detection of antibody to hepatitis B core antigen. Sci Rep. 2017;7(1):14093.
- Lai XH, Liang RL, Liu TC, et al. A fluorescence immunochromatographic assay using europium (III) chelate microparticles for rapid, quantitative and sensitive detection of creatine kinase MB. J Fluoresc. 2016;26(3):987–996.
- Gahlaut N, Miller LW. Time-resolved microscopy for imaging lanthanide luminescence in living cells. Cytometry. 2010;77(12):1113–1125.
- Yang M, Liang Y, Gui Q, et al. Multifunctional luminescent nanomaterials from NaLa(MoO4)2: Eu3+/Tb3+ with tunable decay lifetimes, emission colors, and enhanced cell viability. Sci Rep. 2015;5(1):11844.
- Shen Y, Xu S, He D. A novel europium chelate coated nanosphere for time-resolved fluorescence immunoassay. PLoS One. 2015;10(6):e0129689.