Abstract
Aims
The present study was performed to investigate the efficacy and safety of anti-programmed death-1 (PD-1)/programmed death-ligand-1 (PD-L1) treatments in non-small cell lung cancer (NSCLC).
Methods
The potential articles were searched from Pubmed and Embase. The end points included overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and adverse events. p < .05 or I2 ≥ 50% meant obvious heterogeneity, and the random-effects model was adopted for pooled analysis, otherwise, the fixed-effects model was used. Subgroup analysis was performed based on the treatment line. Potential publication bias was tested with Begg’s funnel plot.
Results
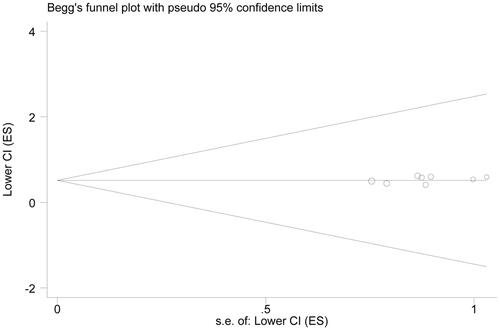
Eight eligible articles were included. OS rate of NSCLC patients was prolonged by anti-PD-1/PD-L1 treatments (HR = 0.685, 95%CI = 0.632–0.742), as well as PFS (HR = 0.821, 95%CI = 0.721–0.936). Meanwhile, anti-PD-1/PD-L1 treatments could greatly enhance the ORR (RR = 1.646, 95%CI = 1.382–1.961), with fewer adverse events (RR = 0.800, 95%CI = 0.688–0.931). Subgroup analysis indicated that anti-PD-1/PD-L1 treatments as second-line treatment could significantly improve the survival of patients without adverse events. The overall results were robust, without significant publication bias (p = .532).
Conclusion
Anti-PD-1/PD-L1 treatments show better clinical efficacy and higher safety than chemotherapy in NSCLC.
Introduction
Non-small cell lung cancer (NSCLC), accounting for 80–85% of lung cancer cases, is one of the main causes of cancer-related deaths [Citation1]. Platinum-based chemotherapy is the first-line treatment for metastatic NSCLC patients without anaplastic lymphoma kinse (ALK) or epidermal growth factor receptor (EGFR) mutations. It was reported that the response rate of this therapy ranged from 12% to 37%, median overall survival (OS) ranged from 8 to 13 months, and median progression-free survival (PFS) ranged from 4 to 7 months [Citation2]. Docetaxel is commonly applied in the second-line or third-line treatment of NSCLC patients. The objective response rate (ORR) of NSCLC patients for docetaxel, pemetrexed, and erlotinib ranged from 7% to 12%, median OS from 8 to 9 months, and median PFS from 2 to 3 months [Citation3,Citation4]. Due to the low response rates and dismal outcomes, novel and effective targeted therapies are urgently needed for patients with advanced NSCLC.
It is well known that the immune system plays an important role in cancer progression and treatments. T-lymphocytes-related immune response is regulated by the balance between activating signals and inhibitory checkpoints [Citation5–8]. Programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) are two main crucial immune checkpoints receptors, which can downregulate the functions of T-cell effector and contribute to the maintenance of toleration to tumour cells. The regulation process is commonly completed via binding their ligand B7 and is programmed by death-ligand-1 (PD-L1). The anti-PD-1, anti-PD-L1 and anti-CTLA4 antibodies may avoid this downregulation and enhance the antitumor actions of T-cells, thus leading to tumour cell death [Citation9–11].
Several antibodies against PD-L1 and PD-1 have been demonstrated with antineoplastic activity in NSCLC. These identified antibodies include atezolizumab (MPDL3280A, an IgG anti-PD-L1 antibody), nivolumab (a fully human IgG4 anti-PD-1 antibody), and pembrolizumab (lambrolizumab or MK-3475, a highly IgG4 monoclonal anti- PD-1 antibody) [Citation12]. This meta-analysis was performed to confirm the clinical efficacy and safety of anti-PD-1/PD-L1 in NSCLC. OS, PFS, ORR and adverse events based on the published investigations.
Materials and methods
Articles search
The potential relevant articles were obtained through the search on the databases of Pubmed and Embase. The search terms were as follows: “anti-PD-1” OR “anti-PD-L1”, “nivolumab” OR “BMS-936558” OR “ONO-4538”, “pembrolizumab” OR “MK-3475”, “atezolizumab” OR “MPDL3280A” AND “non-small cell lung cancer” OR “NSCLC”. Only the articles in English were considered. For the studies with the same population, the study with more comprehensive analysis was selected into the meta-analysis. The references of these articles were reviewed for potential publications.
Inclusion criteria
The articles must be evaluated according to the following criteria before the inclusion: (1) the study investigated the clinical efficacy of anti-PD-1/PD-L1 treatments, such as nivolumab, pembrolizumab or atezolizumab, on NSCLC patients; (2) comparison in clinical efficacy between anti-PD-1/PD-L1 treatment and chemotherapy was performed; (3) the end points included overall survival (OS), progression free survival (PFS) or objective response rate (ORR). Exclusion criteria were as follows: (1) letters, case reports and reviews; (2) studies without available data; (3) duplicate publications.
Data extraction
The key information in the included articles was extracted by two reviewers independently. The information included: name of first author, publication year, country, sample size in experiment and control group, regimens, hazards ratio (HR) and 95% confidence interval (CI) in the OS and PFS analysis, complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), and adverse events.
Statistical analysis
All the analysis was completed with Stata 12.0 software. HRs with 95%CIs were used to evaluate the influence of anti-PD-1/PD-L1 treatments on OS and PFS of NSCLC patients. Relative risks (RR) along with 95%CI represented the effects of anti-PD-1/PD-L1 treatments on ORR and adverse events. Between-study heterogeneity was analysed by I2 and p statistics. p values for heterogeneity (ph) < 0.05 or I2 ≥ 50% meant obvious heterogeneity. If significant heterogeneity presented, the random-effects model was adopted to pool data, otherwise, the fixed-effects model was used. Subgroup analysis was conducted based on the line of anti-PD-1/PD-L1 therapy. Sensitivity analysis was performed to test the robustness of overall results through deleting each single study in turn. Potential publication bias was tested with Begg’s funnel plot and Egger’s test. Quality evaluation of included articles was performed by Review Manager.
Results
Articles selection
A total of eight eligible articles (nine independent trails) were included in the present meta-analysis [Citation13–20] (). At the beginning of search, 214 relevant articles were identified. After the title and abstract were reviewed, 137 articles were excluded for review articles (n = 47), anti-PD-1/PD-L1 treatments and other diseases (n = 39) and single-arm investigations (n = 51). For the remaining 77 articles, 68 articles were removed from the analysis for unavailable data (n = 21), comparison between different doses of anti-PD-1/PD-L1 treatments (n = 32), case reports (n = 15) and repetitive study (n = 1). The detailed information of included articles was shown in . Among the nine independent studies, 8 was second-line therapy [Citation13–18,Citation20] and one was first-line therapy [Citation19]. The quality evaluation of the included articles was shown in . The results demonstrated that the eligible studies were with high quality, and the pooled analysis results were credible.
Figure 1. Flow chart about articles selection process. Eight eligible articles (nine independent studies) were included.
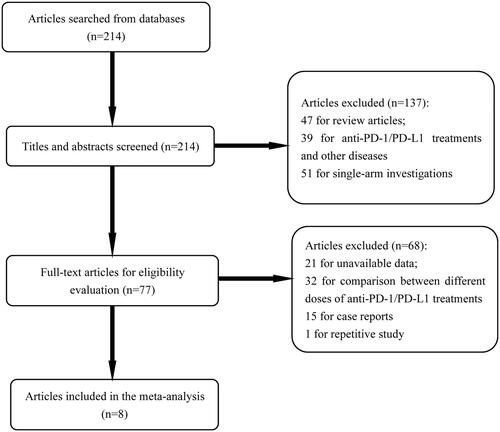
Figure 2. Quality evaluation of included articles. Analysis results demonstrated that the included studies were with high quality, and the conclusions obtained in this article were credible.
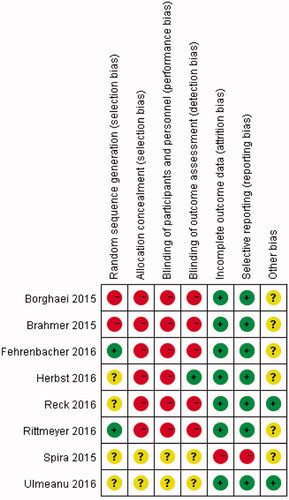
Table 1. Basic features of included articles.
The influences of anti-PD-1/PD-L1 treatments on OS
In the analysis of OS, no heterogeneity was observed (ph = .691) (, ). OS rate of the patients receiving anti-PD-1/PD-L1 treatments was much higher than those with chemotherapy (HR = 0.685, 95%CI = 0.632–0.742, p < .001). Based on the line of treatments, we performed the subgroup analysis. Then we found that patients receiving anti-PD-1/PD-L1 treatments had higher OS rate both in second-line therapy (HR = 0.689, 95%CI = 0.635–0.747, p < .001) and first-line therapy (HR = 0.600, 95%CI = 0.407–0.884, p = .010) than patients with chemotherapy.
Figure 3. OS analysis. OS rate of the patients receiving anti-PD-1/PD-L1 treatments was much higher than those with chemotherapy (HR = 0.685, 95%CI = 0.632–0.742). Both second-line and (HR = 0.689, 95%CI = 0.635–0.747, p < .001) and first-line therapy (HR = 0.600, 95%CI = 0.407–0.884, p = .010) of anti-PD-1/PD-L1 treatments were significantly improved the OS rate of NSCLC.
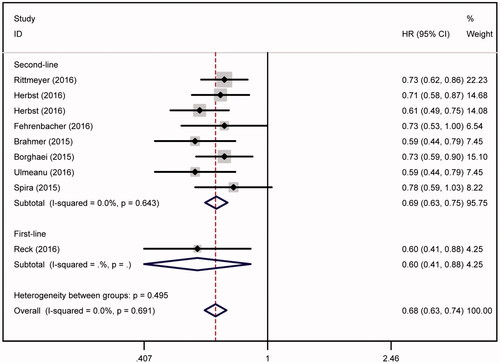
Table 2. Analysis results of clinical efficacy and safety of anti-PD-1/PD-L1 treatments in NSCLC.
The influences of anti-PD-1/PD-L1 treatments on PFS
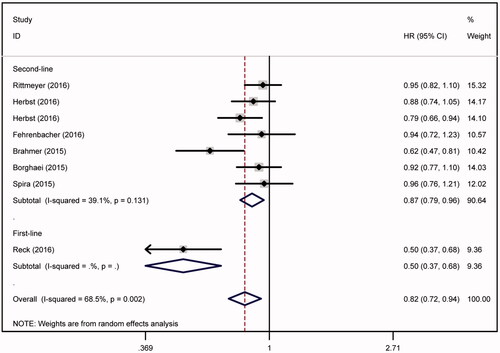
In the analysis of PFS, obvious heterogeneity was observed (ph = .002, ). Subgroup analysis indicated that the therapy line might lead to the existence of heterogeneity (). PFS rate of patients treated by anti-PD-1/PD-L1 regimen was much higher than those with chemotherapy (HR = 0.821, 95%CI = 0.721–0.936, p = .003). When compared with chemotherapy, anti-PD-1/PD-L1 regimen might elevate the PFS rate respectively in second-line (HR = 0.870, 95%CI = 0.791–0.958, p = .004) and first-line (HR = 0.500, 95%CI = 0.369–0.678, p < .001) therapy.
Figure 4. PFS analysis. PFS rate of patients treated by anti-PD-1/PD-L1 regimen was much higher than those with chemotherapy (HR = 0.821, 95%CI = 0.721–0.936). PFS rate was significantly elevated by anti-PD-1/PD-L1 regimen both in second-line (HR = 0.870, 95%CI = 0.791–0.958, p = .004) and first-line (HR = 0.500, 95%CI = 0.369–0.678, p < .001) of anti-PD-1/PD-L1 therapy.
The influences of anti-PD-1/PD-L1 treatments on ORR
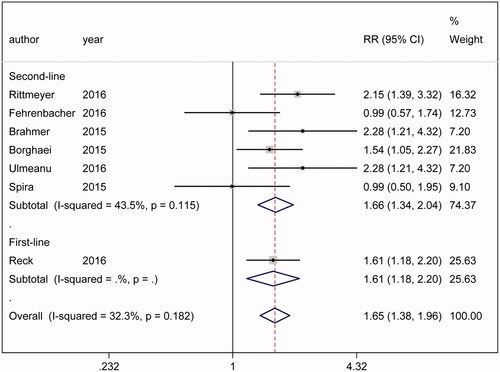
No significant heterogeneity was found in the analysis of ORR (ph = .182) (). The outcome indicated that anti-PD-1/PD-L1 treatments could greatly enhance the ORR of NSCLC patients compared with chemotherapy (RR = 1.646, 95%CI = 1.382–1.961, p < .001). Anti-PD-1/PD-L1 treatments might cause a higher ORR rate in second-line therapy (RR = 1.658, 95%CI = 1.345–2.045, p < .001) and first-line therapy (RR = 1.611, 95%CI = 1.180–2.199, p = .003), respectively, in comparison with chemotherapy.
Figure 5. ORR analysis. The outcome indicated that anti-PD-1/PD-L1 treatments could greatly enhance the ORR of NSCLC patients compared with chemotherapy (RR = 1.646, 95%CI = 1.382–1.961). Significantly higher ORR rate might caused by anti-PD-1/PD-L1 treatments both in second-line (RR = 1.658, 95%CI = 1.345–2.045, p < .001) and first-line (RR = 1.611, 95%CI= 1.180–2.199, p = .003) therapy.
The influences of anti-PD-1/PD-L1 treatments on adverse events
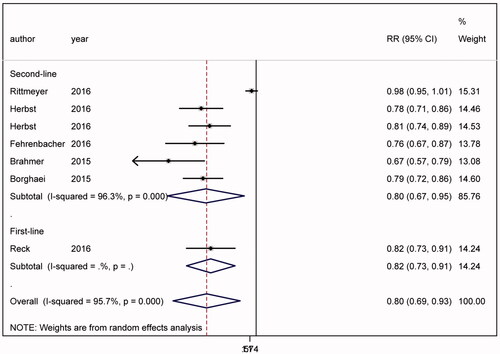
Significant heterogeneity was found in the analysis of adverse events (ph < .001) (). The outcome suggested that anti-PD-1/PD-L1 treatments cause less adverse events than chemotherapy (RR = 0.800, 95%CI = 0.688–0.931, p = .004). Both in second-line (RR = 0.797, 95%CI = 0.671–0.948, p = .010) and first-line (RR = 0.815, 95%CI = 0.731–0.909, p < .001) therapy subgroups, anti-PD-1/PD-L1 treatments significantly reduced the adverse events than chemotherapy.
Figure 6. Adverse events. The outcome suggested that anti-PD-1/PD-L1 treatments cause less adverse events than chemotherapy (RR = 0.797, 95%CI = 0.671–0.948). Anti-PD-1/PD-L1 treatments significantly reduced the adverse events in second-line (RR = 0.797, 95%CI = 0.671–0.948, p = .010) and first-line (RR = 0.815, 95%CI = 0.731–0.909, p < .001) therapy subgroups.
Sensitivity analysis
Sensitivity analysis was performed to evaluate the robustness of overall results. The results were demonstrated to be robust.
Publication bias analysis
Begg’s funnel plot was constructed to test potential publication bias. The outcome suggested no significant publication bias in the present meta-analysis (Begg’s test, p = .532; Egger’s test, p = .166; OS analysis) ().
Discussion
Platinum-based chemotherapy is the first-line treatment of advanced NSCLC patients without targetable mutations. It is characterised by toxicities, including non-haematological toxicities (nausea, vomiting, patigue and alopecia) and haematological adverse events. About 30% of patients show response to chemotherapy and half of patients expose to death within 1 year [Citation21–24]. Docetaxel is approved as the second-line treatment for advanced NSCLC patients for longer survival [Citation4,Citation25]. Novel agents, such as erlotinib and pemetrexed, have better side-effect profile than docetaxel, however, poorer effects on the OS of NSCLC patients [Citation3,Citation26]. No single-agent therapy has brought about better survival than that of docetaxel.
CTLA-4 and PD-1, antibodies that inhibit immune checkpoints, have been demonstrated to improve the outcomes of patients with many cancers [Citation9,Citation27–29]. PD-1 receptor is composed of ligands PD-L1 and PD-L2, which are produced by infiltrating immune cells and tumour cells [Citation30]. The interaction of PD-1 with PD-L1 and PD-L2 could inhibit the activation of T-cell and promote the escape of tumour cells from immune system [Citation7,Citation31,Citation32]. Nivolumab, a PD-1 inhibitor antibody, disturbs PD-1-mediated signalling and restores the antitumor function of immunity system [Citation11,Citation33,Citation34]. It has been reported that nivolumab could improve the OS of NSCLC patients compared with docetaxel [Citation17,Citation18]. About 15–20% of patients show response to nivolumab and the response could persist for months or years despite of the discontinuation of therapy [Citation17,Citation18]. Atezolizumab, engineered anti-PD-L1 antibody, can restore the antitumor activity of T-cell and enhance the priming of T-cell through blocking PD-L1-B7.1 and PD-L1-PD-1 interactions [Citation35–37]. In addition, blocking the binding of PD-L1-B7.1 with antigen-presenting cells and T cells contributes to improvements on immune responses, cytokine production and T-cell activation [Citation38,Citation39]. In the phase II, randomised POPLAR study, the OS of NSCLC patients was greatly improved by atezolizumab compared with docetaxel (12.6 months vs. 9.7 months) in the intention-to-treat population [Citation15]. Pembrolizumab is a highly selective IgG4 monoclonal PD-1 antibody. 2 mg/kg pembrolizumab every 3 weeks was approved in the USA for the metastatic NSCLC patients expressing PD-L1, who show disease progression during or after platinum-based chemotherapy. In KWYNOTE-001, NSCLC patients presenting high expression pattern of PD-L1 had a better therapeutic effect than those with negative expression [Citation40].
Although many studies have investigated the clinical application of anti-PD-1/PD-L1 antibody in NSCLC, no definitive conclusion was obtained on this issue. The present meta-analysis was to confirm the clinical efficacy and safety of anti-PD-1/PD-L1 treatments in NSCLC. The outcome indicated that anti-PD-1/PD-L1 treatments could improve OS, PFS, and ORR when compared with chemotherapy. The OS and PFS rates of patients receiving anti-PD-1/PD-L1 treatments were much higher than those with chemotherapy. Moreover, the patients receiving anti-PD-1/PD-L1 treatments showed better response rate. On the contrary, less adverse events were observed in patients with anti-PD-1/PD-L1 treatments. Subgroup analysis based on the therapy line demonstrated that anti-PD-1/PD-L1 treatments might increase the OS, PFS, ORR rates and reduce the adverse events in second-line therapy. However, only one of the included study investigated the effects of anti-PD-1/PD-L1 as a first-line treatment for NSCLC patients, thus the clinical efficacy and safety of first-line anti-PD-1/PD-L1 treatment for NSCLC needed further analysis. The similar results were also obtained in the previous pooled studies. The related meta-analysis with three included trials performed by Zhou and co-workers found that anti-PD-1/PD-L1 treatments significantly improved OS and reduced the occurrence of adverse events, but not PFS rate [Citation41]. The divergences might be attributed to the numbers of the included studies. In addition, the tumour features might also contribute to the difference. Further well-designed investigations were still required to address the issues.
Despite the relative accurate conclusion, there were several limitations in the analysis. First, individual effect of anti-PD-1/PD-L1 antibody was not analysed due to the limited resource. Growing evidences have demonstrated that the tumour proportion score (TPS) of PD-L1 expression could affect the therapeutic effects of anti-PD-1//PD-L1 for NSCLC patients [Citation14,Citation15]. Zhou et al. also demonstrated that both OS and PFS rates were distinctly improved in NSCLC patients with high PD-L1 expression level [Citation41]. Another meta-analysis conducted by Gandini and colleagues indicated that the ORR rate of anti-PD-1/PD-L1 treatments was higher in NSCLC patients with positive PD-L1 status than negative patients [Citation42]. Both of the related studies might reveal that NSCLC patients with positive expression of PD-L1 would benefit from the anti-PD-1/PD-L1 treatments. However, in this meta-analysis, the classification of PD-L1 TPS for every eligible study was different, we did not explore the influence of PD-L1 TPS for treatments among the study population. Second, almost studies were performed on Caucasian population rather than Asian population, therefore, the results might not apply to Asian population, and future investigations should be made in Asian countries. Third, there existed significant heterogeneity in PFS and adverse events analyses, which might result from the differences in regimen and population. Therefore, well-designed case-control trails were urgently needed to verify the results obtained in our analysis.
In conclusion, anti-PD-1/PD-L1 treatments (nivolumab, pembrolizumab and atezolizumab) greatly enhance the OS, PFS and ORR rates and lower the occurrence of adverse events for NSCLC patients. The present meta-analysis may guide the clinical application of nivolumab, pembrolizumab and atezolizumab in treatments of NSCLC.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13(Suppl 1):5–13.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–1597.
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. JCO. 2000;18(10):2095–2103.
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27(4):450–461.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723.
- Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. JCO. 2015;33(17):1974–1982.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Ulmeanu R, Antohe I, Anisie E, et al. Nivolumab for advanced non-small cell lung cancer: an evaluation of a phase III study. Expert Rev Anticancer Ther. 2016;16(2):165–167.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833.
- Spira AI, Park K, Mazieres J, et al. Effcacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol. 2015; 33 (suppl): abstr 8010. 2015 ASCO Annual Meeting.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550.
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. JCO. 2013;31(34):4349–4357.
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. JCO. 2009;27(8):1227–1234.
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. JCO. 2013;31(23):2895–2902.
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. JCO. 2000;18(12):2354–2362.
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813.
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319.
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98(6):751–755.
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–116.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. JCO. 2010;28(19):3167–3175.
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–856.
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567.
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562.
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587.
- Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113–1119.
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028.
- Zhou GW, Xiong Y, Chen S, et al. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: a meta-analysis of randomized clinical trials. Medicine. 2016;95(35):e4611.
- Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol/Hematol. 2016;100:88–98.