?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
At present, cancer is the first cause of death for humans, but early detection and treatment can help improve prognoses and reduce mortality. However, further development of carrier-assistant drug delivery systems (DDSs) is retarded by the aspects such as the low drug-carrying capacity, carrier-induced toxicity and immunogenicity, complex synthesis manipulation. The development of nanoscale drug delivery systems (NDDS) have been rapidly developed to address these issues. In this article, we used PLGA-PEG with good biocompatibility to encapsulate Fe3O4 nanoparticles (a magnetic resonance imaging contrast agent) and DOX (an antitumour drug) via the emulsion-solvent evaporation method, aimed at achieving a dual function of the early detection and the treatment of mammary cancer. The results showed that the Fe3O4/DOX/PLGA-PEG nanoparticles had a relatively uniform size, a high carrier rate of Fe3O4 and high encapsulation efficiency of DOX, and a relatively high activity of released DOX within 120 h. In addition, in vitro studies showed that the Fe3O4/DOX/PLGA-PEG nanoparticles were cytocompatibility in NIH 3T3 fibroblast cells culture study while had a special effect on destroying human breast cancer MCF-7 cells compared with pure DOX solution. In vitro studies revealed that the Fe3O4/DOX/PLGA-PEG enabled enhanced T2 contrast magnetic resonance. Overall, our multifunctional Fe3O4/DOX/PLGA-PEG nanoparticles, composed of biocompatible substances and therapeutic/imaging materials, have great potential for the early detection of cancer and accurate drug delivery via the dynamic monitoring using MRI.
Introduction
Chemotherapy, a predominant tactic for most cancer managements, showed certain anticancer effects in clinic and could prolong the survival period of cancer patients [Citation1,Citation2]. Unfortunately, several drawbacks of these available chemotherapeutic agents could not be ignored, such as insufficient water-solubility, rapid blood clearance, systemic side effects and non-specific delivery in physiological environments, which indirectly affect human health [Citation3,Citation4]. To overcome these above limitations, the booming nanoscale drug delivery systems (NDDS) have offered innovative ideas and approaches to fight malignant tumours, exhibiting high antitumour activity and reduced side effects in humans through controlled tumour-oriented drug release [Citation5,Citation6]. Numerous researches indicated that NDDS can reduce drug toxicity, prevent early drug release, protect drugs from rapid blood/renal clearance and achieve the preferential accumulation of drugs within solid tumours with enhanced permeability and retention (EPR) effect [Citation7–10]. In addition, precise tumour imaging has been recognized to be an important strategy for image-guided cancer treatment. Therefore, NDDS, which can simultaneously function for tumour treatment and imaging, are of great significance in the administration of antitumour drugs.
Doxorubicin (DOX), an anthracycline antibiotic, is widely used in chemotherapy of various types of cancers [Citation11,Citation12]. However, free DOX solution has high toxicity and adverse effects during treatment, which can affect all healthy tissues as well as tumours tissues. Recent studies have shown that the biomedical field has overcome the early problems of some clinical diseases in the field of drug carriers, and several drug carrier systems have been approved for the clinical treatment of anticancer and antifungal drug [Citation13,Citation14]. As the biomaterial approved by the Food and Drug Administration (FDA), poly (lactide-co-glycolides) (PLGA) can be degraded into non-toxic lactic acid in vivo, which was ranked as one the most popular biodegradable polymers due to its long-term clinical application, favourable degradation characteristics and sustained release ability [Citation15–17]. Besides, the hydrophilic block of poly(ethylene glycol) (PEG) could prolong the systemic circulation time and minimize opsonization which leads to the non-specific uptake in normal tissue [Citation18]. There are large number of previous studies that concerning PLGA-PEG micelles used for glioma treatments, which have been confirmed to have controllable particle size, good biocompatible, reduced systemic clearance and high capacity of drug loading [Citation19–21].
In recent years, MR contrast agents have been successfully encapsulated in polymer particles to prepare a new and simple strategy of drug distribution, such as gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) and Fe3O4 nanoparticle [Citation22–26]. Studies on the physical and chemical properties, distribution and degradation in animals confirmed the low biotoxicity of these MR contrast agents. They could be imaged on clinical magnetic resonance scanners to achieve highly efficient targeted therapy or detection of various diseases. However, some adverse effect limited the use of bare Fe3O4 nanoparticle for biomedical applications, such as instability under physiological conditions, formation of harmful free radical, and inappropriate surface binding of the ligand [Citation27]. Considering the adverse limitations of Fe3O4 nanoparticle, it is highly desirable to design the nanocarriers with stability capability.
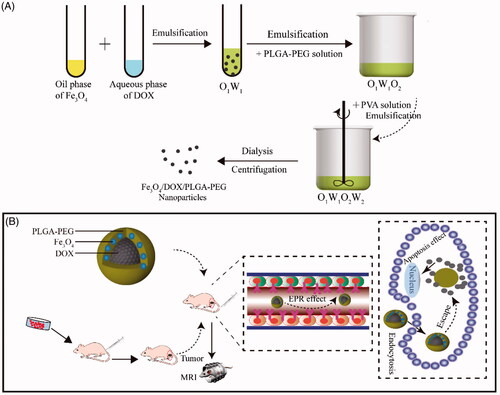
In this paper, we successfully designed and constructed Fe3O4/DOX/PLGA-PEG nanoparticles by the emulsion solvent evaporation method with a dual function of the early detection and the treatment of the tumours. As illustrated in Scheme 1(A), the as-designed Fe3O4/DOX/PLGA-PEG consist of two individual parts. First, PLGA-PEG with high biocompatibility and biodegradability were developed by the conjugation of COOH–PEG–NH2 to PLGA–COOH. Second, the antitumor drug doxorubicin (DOX) and Fe3O4 magnetic nanoparticles were encapsulated by PLGA-PEG matrix for endowing the nanocarriers with anticancer and T2-weighted magnetic resonance (MR) imaging capability. As illustrated in Scheme 1(B), such nanocarriers were based on PLGA-PEG in the shell, superparamagnetic Fe3O4 nanoparticles in the core, anticancer drug DOX encapsulated inside (designated as Fe3O4/DOX/PLGA-PEG nanocomposites). The synthesis and particle size of Fe3O4/DOX/PLGA-PEG were determined by different characterization techniques, including transmission electron microscope (TEM) and Fourier transform infra-red spectroscopy (FTIR). In addition, cancer treatment and MRI efficacies were examined in vitro using MCF-7 cell lines to demonstrate the potential of the Fe3O4/DOX/PLGA-PEG for the early detection and the treatment of the tumours.
Scheme 1. (A) Preparation of Fe3O4/DOX/PLGA-PEG nanoparticles via the emulsion solvent evaporation method. (B) A schematic illustration for Fe3O4/DOX/PLGA-PEG nanocarriers system and then Dox release from nanocarriers to kill tumour cells with a dual function of the early diagnosis and the treatment of the tumours.
Experimental section
Reagents and materials
Poly (D,L-lactide-co-glycolide) glycol)-carboxylic acid endcap (PLGA, Mw = 35 kDa), amino group of poly (ethylene glycol) amine and carboxylic acid endcap (NH2-PEG-COOH, Mw = 3500 Da), N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiiminde (EDC), Trimethylamine solution, Doxorubicin hydrochloride were purchased from Shanghai Aladdin Chemical Reagent Co., Ltd (Shanghai, China). Fe3O4 nanoparticles was obtained from Bai mage Biotechnology Co., Ltd (Wuxi, China) and N, N-Diisopropylethylamine was purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Cell culture reagents including DPBS (Dulbecco’s phosphate-buffered saline), DMEM (Dulbecco’s modified Eagle’s medium), foetal bovine serum (FBS), antibiotics (anti-anti) were obtained from Hyclone (UT, USA). Trypsin, cell counting Kit-8 was purchased from Dojindo Laboratories (Kumamoto, Japan). All other reagents used were at least of analytical grade.
Synthesis of PLGA-PEG copolymer
Carboxylate-functionalised copolymer PLGA-PEG was synthesized by conjugating COOH-PEG-NH2 to PLGA-COOH through a carbodiimide-mediated coupling reaction according to a previously-described method [Citation28]. Generally, the PLGA-COOH (1 g) was dissolved in methylene chloride (2 ml) to form PLGA-NHS with excess NHS (27 mg) in the presence of EDC (46 mg). PLGA–NHS was precipitated with ethyl ether, followed by dialysis to remove residual NHS and then lyophilised. N, N-Diisopropylethylamine and COOH-PEG-NH2 were added to chloroform dissolved with PLGA-NHS to form PLGA-PEG copolymer within 6 h. Finally, the excess co-polymer was precipitated with cold methanol for 12 h and washed out by same solvent and then lyophilized again to obtain pure PLGA-PEG.
Preparation of Fe3O4/DOX/PLGA-PEG nanoparticles
The emulsion solvent evaporation method (O1/W1/O2/W2) was used to prepare functionalized Fe3O4/DOX-loaded PLGA-PEG composite nanoparticles. First, Fe3O4 nanoparticles dispersed in chloroform mixed at a concentration of 10 mg/ml to form the primary organic phase (O1); then DOX-HCl dissolved in deionized water (5 mg/mL) to form the inner aqueous phase (W1), which was neutralized with trimethylamine, and immediately mixed O1 and W1 at W1/O1 volume ratio of 5:2. The emulsion was stirred continuously at room temperature for 1 h, then diluted two times with deionized water. Under the condition of ice bath, the particles were dispersed for 30 s. Then, three-volume secondary oil phase (PLGA-PEG solution dissolved in chloroform solution with concentration of 20 mg/mL) was mixed with the two-volume of W1/O1 emulsion to obtain the O1/W1/O2 dual emulsion, which was subsequently followed by short ultrasonication for 30 s. Finally, five volumes of O1/W1/O2 emulsion was added in eight volumes of W2 aqueous phase, containing 3% (w/v) polyvinyl alcohol (PVA) aqueous solution, followed by short ultrasonication for 30 s to form O1/W1/O2/W2 emulsion.
Material characterization
FTIR and 1H-NMR
The copolymer characterized by Fourier transform infra-red (FTIR) spectrometer (VERTEX 70, Bruker, Germany) at 500–4000 cm−1 for determining its functional groups. The copolymer was dissolved in CDCl3 and characterized by with nuclear magnetic resonance (1H-NMR) spectrometer (Bruker-500, Karlsruhe, Germany) operating at 400 MHz.
Particle size of Fe3O4/DOX/PLGA-PEG
The morphologies and structures of the materials were characterized by TEM (Technai G2S Twin) at 300 keV. The particle size distribution of nanoparticles was determined by laser particle size analyser (Zetasizer Nano ZS, Malvern, UK). The Fe3O4/DOX/PLGA-PEG composite nanoparticles were configured into aqueous solution of certain concentration and 1 ml solution was obtained.
Thermogravimetric analysis
The TG of polymer blends was measured by thermogravimetry analyser (TG209F3-ASC, NETZSCH, Germany). Typically, 4–6 mg samples was placed in a clean platinum disk and scanned at the heating rate of 10/min. The heating temperature ranged from 35 to 800 °C under nitrogen flow in 60 ml/min.
Carrier rate of the drug in the PLGA-PEG
The carrier rate of the drug in the PLGA-PEG was determined by UV-vis spectrophotometer (UV-2550, Shimadzu, Japan) to determine the amount of DOX loaded into the copolymer successfully. The carrier rate of drug was calculated using the following formula:
In this equation, Mt is the mass of the drug in the copolymer and Mo is the total mass of the copolymer.
In vitro drug release study
The in vitro release of DOX from nanoparticles was determined by UV-vis spectrophotometer. Briefly, the lyophilized nanoparticles was immersed in 100 ml PBS (pH 7.4) and incubated at 37 °C with stirring rate of 100 rpm. At predetermined time points, the sample was taken to analyse, and then, an equal amount of freshly prepared PBS was added. The DOX release was measured by UV-vis spectrophotometer at λmax of 483 nm.
MRI characterization
T2 MRI of the Fe3O4 nanoparticles
Fe3O4 nanoparticles were dispersed into aqueous solution, which was subsequently subjected by ultrasonication for 5 min, then a certain concentration gradient of polymer solution was prepared. Afterward, An MRI scan was performed using a 3 T MR scanner (Bruker-500, Rheinstetten, Germany). C Axial and sagittal images were recorded, and T2-weighted images were obtained using a gradient-echo sequence with time of repetition (TR) = 733 ms, time of echo (TE) = 5.4–35.1 ms, number of excitations (NEX) = 6, section thickness = 0.8 mm, flip angle = 45°, field of view (FOV) = 40 mm × 40 mm.
T2 MRI of the Fe3O4/DOX/PLGA-PEG nanoparticles
The cells were co-cultured with the material for 24 h, which were subsequently dispersed in a microcentrifuge tube and immobilised with 1% agarose for the following MRI analysis. Then, An MR scan was performed using a 3 T MR scanner. The T2-weighted sequence was performed using the previous parameters.
In vitro biocompatibility test
Cytotoxicity of PLGA-PEG
CCK-8 assay, as a common tool, was used to investigate the cytotoxicity of the developed PLGA-PEG Nano formulations in 3T3 cells. Generally, 3T3 cells were cultured at 37 °C under a humidified atmosphere containing 5% CO2. The 3T3 cells in the logarithmic phase of growth were seeded into 96-well plates with the cell density of 1 × 104 cells/well, and the outer-loop of the 96-well plates was filled with sterile PBS solution to keep the water in the inner hole. When an 80% confluence was reached, the cultured cells were treated with varying concentrations of PLGA-PEG solution based DMEM complete medium. The cells co-cultured in PLGA-PEG solution were used as the positive control, whereas the wells without cells and the material as the negative control. Three multiple wells were set as the parallel control at each concentration. Then the cells were incubated for 24 h at 37 °C, and the cell culture medium was replaced by 10 µL CCK-8 stock solution and 90 µL of fresh medium. After incubation 2 h at 37 °C, the absorbance intensity was measured at 570 nm using a microplate reader (Multiskan MK3, Thermo Fisher Scientific, USA) [Citation29]. Cell activity was calculated using the following formula:
In this equation, “As” is the sample group, “Ab” and “Ap” are the negative control and positive control group, respectively.
Haemolysis assay
Haemolysis test was used to evaluate the effect of the material on the dissolution of red blood cells (RBC). Typically, 1 ml of fresh blood from a healthy person (male, 26 years old) was centrifuged to obtain red blood cells (RBCs). The RBCs were purified and resuspended in PBS, the RBCs solution were finally obtained by diluting with PBS to a concentration of 16% (v:v). PLGA-PEG solutions (1 ml) in PBS with various concentration were added to the RBCs suspensions (50 μL). The same volume of RBCs incubated with PBS solution and ultrapure water was set as positive and negative control, respectively. After incubation for a period of time, the samples were centrifuged at 1000 rpm for 5 min. Last, the absorbance of the obtained supernatants was measured with a microplate reader at 540 nm.
Activated partial thromboplastin time (APTT) and prothrombin time (PT)
Fresh, anticoagulated whole blood was centrifuged at 1000 ×g for 10 min and the resulting supernatant (platelet-poor plasma) was collected. The platelet-poor plasma (360 μL) was mixed with the nanoparticles (40 μL in PBS). Normal saline-treated platelet-poor plasma was used as a negative control (n = 3). After adding the corresponding reagents, the APTT and PT of the samples were measured with an automatic coagulation analyser (STAR Evolution, Diagnostica Stago, Assiernes, France).
In vitro antitumour activity of nanoparticles
MCF-7 was chosen as a model cancer cell line. The antitumour activity of Fe3O4/DOX/PLGA-PEG to MCF-7 is similar to that of cytotoxicity of PLGA-PEG to 3T3. In addition, the cell culture conditions of MCF-7 are coincident with the 3T3 cells.
In vitro cellular uptake studies uptake experiment
A detailed uptake and internalisation studies of DOX in Fe3O4/DOX/PLGA-PEG were carried out by employing laser scanning microscopy for better understanding the uptake of nano formulations. The MCF-7 cells were seeded in confocal cell culture dish (WHB, China) at 2 × 105 cells/dish and incubated with different delivery formulations for 24 h. After incubation, the cells were washed thrice with cold PBS and fixed with 4% paraformaldehyde. Cell nuclei were stained with DAPI and imaged by confocal laser scanning microscopy (CLSM, Zeiss, Germany).
Statistical analysis
Data were expressed as the means ± standard deviation (SD) for at least thrice repeat. Statistical significance was tested using an unpaired, two-tailed Student's t-test. Value of * (p < .05), ** (p < .01), and *** (p < .001) were considered to be statistically significant.
Results and discussion
Synthesis and characterization of PLGA-PEG
The copolymer PLGA-PEG was newly designed and synthesized by directly encapsulating PLGA-COOH with NH2-PEG-COOH to produce PLGA-b-PEG-COOH with a fixed block length, and its molecular structure was confirmed by means of FT-IR, 1H NMR spectroscopy.
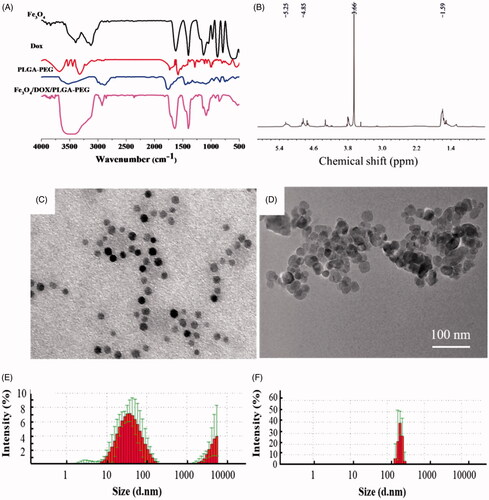
FITR spectra of Fe3O4, DOX, PLGA-PEG and Fe3O4/DOX/PLGA-PEG were presented in . The Fe3O4/DOX/PLGA-PEG preserved the characteristic peaks of each component can be seen in FTIR spectra of the pure PLGA–PEG. From pure PLGA-PEG, peak at 1660 cm−1 marked by a dotted line belonged to the stretching vibration of amide bonds [Citation30]. The FTIR spectrum of Fe3O4/DOX/PLGA-PEG showed peak at 565 cm−1 was characteristics to the absorption band of Fe–O; peak at 1458 cm−1 was assigned to the methyl absorption band; and peaks at 1758 cm−1 and 3500 cm−1 were related to the stretching vibration of carbonyl-induced absorption band (C=O) and hydroxyl group (O–H), respectively [Citation20,Citation31]. It can be noted that the characteristic peak of carbonyl-induced absorption band and hydroxyl group was obviously enhanced compared with the spectra of Fe3O4, DOX and PLGA-PEG, indicating that the Dox and Fe3O4 have been successfully coated in PLGA-PEG copolymer.
The 1H NMR spectra of PLGA-PEG was shown in . In the 1 H NMR spectrum, peak at δa 5.25 ppm was corresponding to δa (–CO–CH–(CH3)–); peak at 4.85 ppm was corresponded to δb (–CO–CH2–O–); peak at 3.7 ppm was assigned to δc (–OCH2CH2–O–); and peak at 1.6 ppm was attributed to δd (–CO–CH–(CH3)–). The apparent pKa of the copolymer was comparable to previously reported poly(L-histidine)-based copolymers [Citation28].
The particle size distribution
The particle morphology and size distribution of Fe3O4 and Fe3O4/DOX/PLGA-PEG was measured by TEM and laser nanocrystalline particle size analyser, respectively. The TEM image and size distribution histograms were shown in , and average hydrodynamic size and polydispersity index of each system were presented in Table S1. The experimental results showed that the particle size of Fe3O4 ranges from 10 nm to 100 nm with an average size of about 50 nm, and most of the particles are concentrated in 50 nm (). The size range of PLGA-PEG particles coated with DOX and Fe3O4 particles is 100–200 nm, and the distribution was uniform (). Therefore, the increase in the hydrodynamic size due to PLGA-PEG coating is 40–100 nm, which corresponds to monolayer coating of PLGA-PEG. Previous research reported that the nanotherapeutics with a sub-200 nm diameter had good extravasation and accumulation ability in solid tumour via the EPR effect [Citation32]. Our results showed that the particle size distributed in this range for EPR effect in tumour site, which was ideal particle size to cross the tumour barriers.
Thermogravimetric analysis
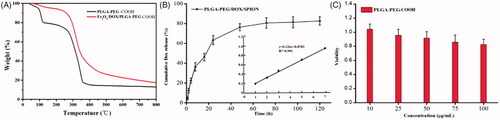
The amount of Fe3O4 in Fe3O4/DOX/PLGA-PEG composite particles was analysed by thermogravimetric analysis. As the weight loss curves presented in , the phase transition of PLGA-PEG copolymer from 300 °C to 350 °C belongs to polymer molecules, and the remaining mass after 800 °C belongs to Fe3O4 particles in Fe3O4/DOX/PLGA-PEG. These results showed that the Fe3O4 entrapment efficacy for Fe3O4/DOX/PLGA-PEG was around 7.36%, indicating that the Fe3O4 content of the NPs could be sufficient for the NPs to be applied as an imaging contrast agent in diagnostic applications. In addition, the entrapment efficiency of DOX was calculated to be 9.7% by UV-vis spectra. Therefore, PLGA-PEG has great potential as an antitumor drug carrier.
In vitro release study
The in vitro release of DOX from the Fe3O4/DOX/PLGA-PEG was measured by UV-Vis spectrum. As the results presented in , 62.35 ± 2.17% of DOX were released within 24 h. The initial burst release of DOX could be attributed to the portion of DOX that was not converted to the basic form of DOX, and therefore, it was poorly entrapped in PLGA-PEG matrix. Afterward, the DOX exhibited slow and constant release, nearly 20% of DOX were un-released in PLGA-PEG matrix even after 120 h. Such a stable slow-release ability can meet the needs of the drug carrier when utilising the EPR effect [Citation33]. It prevents premature release of DOX and thus reduces drug toxicity before the drug carrier passively targets the tumour site using the EPR effect.
In vitro biocompatibility evaluation
The effect of the PLGA-PEG carrier material on the proliferative activity of 3T3 cell was assessed by CCK-8 analysis. showed the cell viability of 3T3 cell after 24 h incubation with different concentrations of PLGA-PEG carrier material, which revealed no obvious toxicity. Although the decrease in the cell viability was correlated with increase in the concentration of materials, cell viability in the various concentration of carrier materials was not significantly different (p > .05). Moreover, the cell viability was still more than 80% even at the high concentration of 100 mg/mL of the RBC-like PLGA-PEG carrier materials, so the cytotoxicity of PLGA-PEG was classified as grade 0 and grade 1 according to RGR Score and Grade standard. These results showed that safe dose of carrier material could be applied to the treatment carrier without cytotoxicity.
Blood compatibility assay
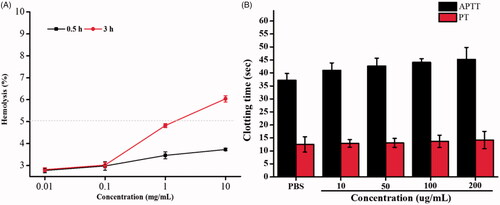
Haemolysis in vitro is regarded as an important and reliable measure for evaluating blood compatibility of materials. The results of haemolysis experiment of PLGA-PEG copolymer were presented in . Within a certain concentration range, the ruptures proportion of these erythrocytes were less than 5%. According to the American Society for Testing and Materials (ASTM F756), a material shall be classified as non-haemolytic (0–2% of haemolysis), slightly haemolytic (2–5% of haemolysis), and haemolytic (>5% of haemolysis) [Citation34]. Therefore, the PLGA-PEG copolymer within its normal concentration range may be classified as a slightly haemolytic material, indicating permissible haemolytic toxicity.
Figure 2. (A) Thermogravimetric chart of PLGA-PEG and Fe3O4/DOX/PLGA-PEG. (B) Cumulative DOX release profiles from Fe3O4/DOX/PLGA-PEG under 37 °C and the standard curve of DOX. (C) Cell viabilities of on 3T3 cells after incubation of various concentrations of PLGA-PEG nanocomposites for 24 h, as determined by the typical CCK-8 assay.
Prior to the nanomedical application of nanoparticles formulation, PT and APTT analysis must be performed. PT and APTT tests represent the external and intrinsic pathways of coagulation. The interaction between nanoparticles and coagulation factors was studied by detecting PT and APTT. The acceptable ranges of PT and APTT are 10.7–12.4 s and 28.7–40.8 s [Citation35]. As shown in , the coagulations time of four different concentrations PLGA-PEG copolymer was depicted, and PT and APTT evaluation were 43.2 s and 14.2 s in 200 μg/ml nanoparticles, respectively, illustrating the normal safe margin compared with the PBS control group which showed the PT and APTT value were 37.4 s and 13.2 s. respectively. The results showed that nanoparticles did not activate the coagulation pathway at the experimental concentration and was biocompatible with the coagulation pathway.
MRI characterization of the Fe3O4 nanoparticles
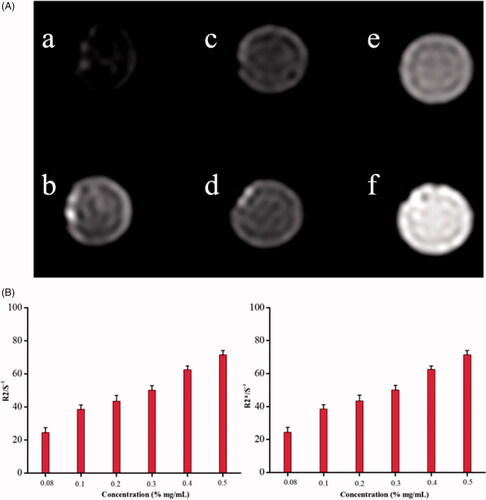
To assess the contrast enhancement of the Fe3O4 nanoparticles, six different concentrations of Fe3O4 were used: (A 0.5 mg/mL, B 0.4 mg/mL, C 0.3 mg/mL, D 0.2 mg/mL, E 0.1 mg/mL, F 0.08 mg/mL). As shown in , the different concentrations of Fe3O4 showed different degree of T2 signal and had good imaging effect to obtain clear T2WI images. In addition, the R2/R2 * value increased linearly with the increase in concentration of Fe3O4. The results confirmed that the current Fe3O4-nanoparticles could be expected to regard as a highly efficient contrast agent in vivo.
MRI characterization of the Fe3O4/DOX/PLGA-PEG nanoparticles
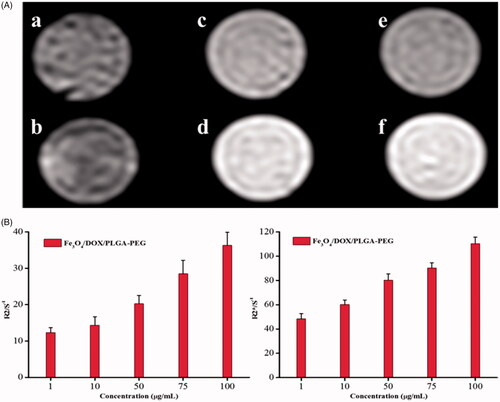
T2WI images and R2/R2* values were presented in , six different concentrations of Fe3O4/DOX/PLGA-PEG were used: (a 100 μg/mL, b 75 μg/mL, c 50 μg/mL, d 10 μg/mL, e 1 μg/mL, f control) all concentrations of Fe3O4/DOX/PLGA-PEG showed different levels of T2 signals, with good imaging effect and clear boundary (). In T2WI images, T2 signal decreased with the decline on the concentration of the composite particles, but still had good imaging effect with the concentration of 75 μg/mL (). That means when the concentration is lower than 75 μg/mL, the imaging effect may not be suitable for complex clinical imaging. Our results showed that 75 μg/mL was the minimum concentration of effective imaging of composite particles. Magnetic nanoparticles (Fe3O4) have been widely studied for the treatment of cancer because of their unique characteristics, such as the ability to produce heat in response to magnetic induction and magnetic resonance imaging (MRI) contrast. Yao et al wrapped Fe3O4 in PDA nanomaterials to monitor the change of the tumour and the results showed that the Fe3O4@PDA nanomaterials can achieve a precise positioning of the tumour and both qualitative detection and dynamic monitoring of the antitumor efficiency during in vitro and in vivo experiments [Citation36].
In vitro antitumour analysis of nanoparticles
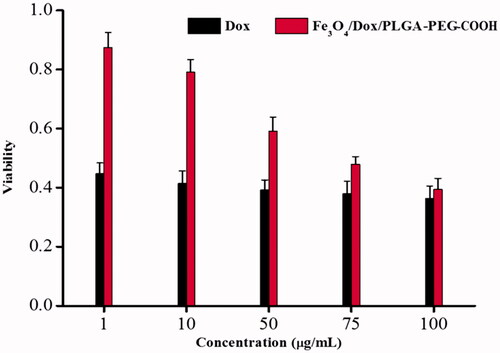
In this section, the MCF-7 cells were used to evaluate the antitumour effects of DOX and Fe3O4/DOX/PLGA-PEG complexes. shows the viabilities of MCF-7 cells after incubation with different complexes for 24 h. Pure DOX showed good antitumor effects in vitro, even at a very small concentration (1 μg/mL), and the cell viability was less than 50%. In addition, the antitumor effect of Fe3O4/DOX/PLGA-PEG complex had been found in a concentration-dependent manner. When the concentration was more than 75 μg/mL, cell viability was less than 50%. In general, the CCK8 study revealed that the Fe3O4/DOX/PLGA-PEG can maintain the integrity and activity of the DOX towards cancer cell, which made it a perfect candidate for the anticancer drug delivery. This novel dual functional nanocarriers can serve as a promising candidate for further studies on cancer therapy in vivo, due to its tumour treatment and MRI imaging-guidance, which will potentially provide the guidance and monitoring for the chemotherapy-based therapeutic process. Doxorubicin (DOX) is a first-line chemotherapeutic agent, which potently inhibits the proliferation of cancer cells [Citation37]. Tang et al. successfully designed a targeted and temperature-responsive theranostic nanoplatform (PFH/DOX@PLGA/Fe3O4-FA), which have been demonstrated to efficiently suppress the tumour growth based on the enhanced and synergistic chemotherapy in vitro and in vivo [Citation38].
Cellular uptake tests
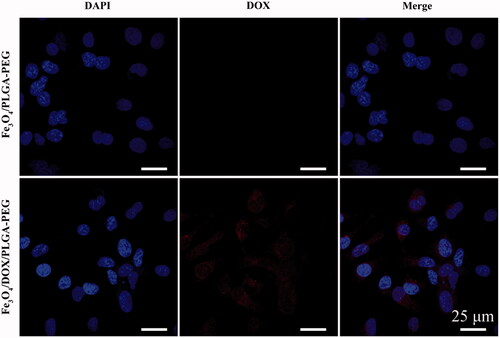
The confocal microscopy provided precise and progressive internalisation features of the nanoparticles in MCF-7 cancer cells. Hence, the internalisation of the nanoparticles by MCF-7 cancer cells was examined by confocal microscopy. After 24 h of incubation with the nanoparticles, nuclei were stained with DAPI and fluorescence images were acquired. Note that DOX inherently exhibits red fluorescence. As shown in , most cells appeared to take up DOX. Numerous red fluorescent dots were observed mostly in the nuclei, indicating good cellular uptake of the Fe3O4/DOX/PLGA-PEG. Thus, it can be clearly noted that DOX could be successfully internalised by tumour cells via endocytosis. The attachment of PEG to copolymers prolongs their blood circulation and due to the enhanced permeability and retention (EPR) effect, the polymer–drug conjugates are accumulated predominantly in solid tumours. Wei Hong et al. successfully synthesised pH-responsive copolymers based on PLGA-PEG-PLGA and poly(L-histidine) and achieved predominant antitumor effect in vitro [Citation39]. In additional, Longbao Feng et al. successfully developed a novel T7-conjugated redox-sensitive targeting amphiphilic molecule (PEG–PEI–PCL-SS-PCL–PEG) to co-deliver DOX and TRAIL, which showed a higher antitumor efficiency in vivo [Citation18].
Conclusions
In conclusion, the Fe3O4/DOX/PLGA-PEG nanoparticles were successfully constructed by the multiple emulsion solvent evaporation method (O1/W1/O2/W2). The nanoparticles prepared by the mentioned method manifested a relatively uniform size, a high co-encapsulation of Fe3O4 and DOX, excellent pattern of controlled drug release and sufficient magnetic properties. The magnetic properties of the nanoparticles promise their utility for both anti-cancer therapy and diagnostic objectives. The in vitro cellular uptake and cytotoxicity study of DOX-conjugates showed increased cellular uptake and cytotoxicity to MCF-7 cancer cells of PLGA-PEG-based nanoparticles. Moreover, at a concentration of less than 1 mg/mL, Fe3O4/DOX/PLGA-PEG hardly had any effect on RBC aggregation, morphology, RBC lysis or blood coagulation. In the near future, we believe this detection and treatment system are expected to serve as a potential dual function tool in the early detection of tumours and in the dynamic monitoring of drug distribution using MRI at the molecular level.
Supplementary_Data-0805.docx
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Discov EDJM, Rana S, Dixit S, et al. Anticancer effects of chemotherapy and nature products. J Med Discov. 2017;2:jmd17008.
- Jing YK, Wang L, Xia LJ, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97(1):264–269.
- Dong X, Yin W, Yu J, et al. Mesoporous bamboo charcoal nanoparticles as a new near-infrared responsive drug carrier for imaging-guided chemotherapy/photothermal synergistic therapy of tumor. Adv Healthcare Mater. 2016;5(13):1627–1637.
- Kouranos V, Dimopoulos G, Vassias A, et al. Chemotherapy-induced neutropenia in lung cancer patients: the role of antibiotic prophylaxis. Cancer Lett. 2011;313(1):9–14.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20.
- Dozono H, Yanazume S, Nakamura H, et al. HPMA copolymer-conjugated pirarubicin in multimodal treatment of a patient with stage IV prostate cancer and extensive lung and bone metastases. Targ Oncol. 2016;11(1):101.
- Kleynhans J, Grobler AF, Ebenhan T, et al. Radiopharmaceutical enhancement by drug delivery systems: a review. J Control Release. 2018;287:177–193.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6.
- Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79.
- Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151.
- Sun J, Sun G, Meng X, et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One. 2013;8(5):e64526.
- Kelishomi RB, Ejtemaeemehr S, Tavangar SM, et al. Morphine is protective against doxorubicin-induced cardiotoxicity in rat. Toxicology. 2008;243(1–2):96–104.
- Li J, Guo Y, Kuang Y, et al. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34(36):9142–9148.
- Tzeng SY, Green JJ. Therapeutic nanomedicine for brain cancer. Ther Deliv. 2013;4(6):687–704.
- Wang Y, Li P, Tran TD, et al. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials. 2016;6(2):26.
- Wang X, Cheng R, Cheng L, et al. Lipoyl ester terminated star PLGA as a simple and smart material for controlled drug delivery application. Biomacromolecules. 2018;19(4):1368.
- Bi Y, Liu L, Lu Y, et al. T7 peptide-functionalized PEG-PLGA micelles loading with carmustine for targeting therapy of glioma. ACS Appl Mater Interfaces. 2016;8(41):27465–27473.
- Feng L, Yan S, Zhu Q, et al. Targeted multifunctional redox-sensitive micelles co-delivery of DNA and doxorubicin for treatment of breast cancer. J Mater Chem B. 2018;6:3372–3386.
- Mosafer J, Teymouri M, Abnous K, et al. Study and evaluation of nucleolin-targeted delivery of magnetic PLGA-PEG nanospheres loaded with doxorubicin to C6 glioma cells compared with low nucleolin-expressing L929 cells. Mater Sci Eng C. 2017;72:123–133.
- Kumar R, Sahoo GC, Pandey K, et al. Development of PLGA-PEG encapsulated miltefosine based drug delivery system against visceral leishmaniasis. Mater Sci Eng C Mater Biol Appl. 2016;175:748–753.
- Yang AS, Liu W, Yang XL. Serum proteins opsonization and phagocytic uptake of peg-modified PLGA nanoparticles: effect of particle size. AMR. 2011;393–395:939–942.
- Ao M, Wang Z, Ran H, et al. Gd-DTPA-loaded PLGA microbubbles as both ultrasound contrast agent and MRI contrast agent--a feasibility research. J Biomed Mater Res. 2010;93:551–556.
- Zhou J, Guo D, Zhang Y, et al. Construction and evaluation of Fe3O4-based PLGA nanoparticles carrying rtPA used in the detection of thrombosis and in targeted thrombolysis. ACS Appl Mater Interfaces. 2014;6:5566.
- Huang Y, Boamah PO, Gong J, et al. Gd (III) complex conjugate of low-molecular-weight chitosan as a contrast agent for magnetic resonance/fluorescence dual-modal imaging. Carbohyd Polym. 2016;143:288–295.
- Lee PC, Lin CY, Peng CL, et al. Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater Sci. 2016;4:1742–1753.
- Jun-Qing S, Wang XJ, Zhu XL, et al. Multifunctional SPIO/DOX-loaded A54 homing peptide functionalized dextran-g-PLGA micelles for tumor therapy and MR imaging. Sci Rep. 2016;6.
- Li W, Zaloga J, Ding Y, et al. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci Rep. 2016;6:23140.
- Cheng J, Teply BA, Sherifi I, et al. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28(5):869–876.,
- Zhang D, Wu M, Zeng Y, et al. Chlorin e6 conjugated poly(dopamine) nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced cancer therapy. ACS Appl Mater Interfaces. 2015;7:8176–8187.
- Sun D, Ding J, Xiao C, et al. pH-responsive reversible PEGylation improves performance of antineoplastic agent. Adv Healthcare Mater. 2015;4(6):844–855.
- Arslanlar YT, Garcia-Guinea J, Kibar R, et al. Luminescence behavior and Raman characterization of jade from Turkey. Appl Radiat Isot. 2011;69:1299–1306.
- Du C, Qian J, Zhou L, et al. A biopolymer-drug conjugate nanotheranostics for multimodal imaging-guided synergistic cancer photothermal-chemotherapy. ACS Appl Mater Interfaces. 2017;9:31576.
- Basel MT, Shrestha TB, Troyer DL, et al. Protease-sensitive, polymer-caged liposomes: a method for making highly targeted liposomes using triggered release. ACS Nano. 2011;5(3):2162–2175.
- Standard practice for assessment of hemolytic properties of materials, ASTM, 2008, F756-08.
- Kim YA, Sun YK. Measurement of PT, aPTT, and fibrinogen by automatic coagulation analyzer, ACL9000. J Clin Pathol Qual Control. 2002;23:247–252.
- Yao Y, Zhao D, Li N, et al. Multifunctional Fe3O4@polydopamine@DNA-fueled molecular machine for magnetically targeted Intracellular Zn2+ imaging and fluorescence/MRI guided photodynamic-photothermal therapy. Anal Chem. 2019;91:7850-7857.
- Maayah ZH, Zhang T, Forrest ML, et al. DOX-Vit D, a novel doxorubicin delivery approach, inhibits human osteosarcoma cell proliferation by inducing apoptosis while inhibiting Akt and mTOR signaling pathways. Pharmaceutics. 2018;10(3):144.
- Tang H, Yuan G, Li P, et al. In vivo targeted, responsive and synergistic cancer nanotheranostics by MRI-guided synergistic HIFU ablation and chemotherapy. ACS Appl Mater Interfaces. 2018;10, acsami.8b01967.
- Wei H, Dawei C, Li J, et al. A mussel-derived one component adhesive coacervate. Acta Biomaterialia. 2014;10(4):1663–1670.