Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of the immature myeloid cells that are derived from the myeloid progenitors with immunosuppressive functions. MDSCs are accumulated in the inflammatory sites during some autoimmune disorders, such as rheumatoid arthritis (RA) and can be an important factor in the pathogenesis of these diseases. Some research has shown the anti-inflammatory role of MDSCs during the RA progression and supports the hypothesis that MDSCs can be a potential treatment option for autoimmunity with their immunosuppressive activity. In contrast, some papers have reported the opposite effects of MDSCs, and support the hypothesis that MDSCs have a pro-inflammatory role in autoimmune disease. MDSCs functions in RA have not been fully understood, and some controversies, as well as many unanswered questions, remain. Although the two well-known subgroups of MDSCs, M-MDSC, and PMN-MDSC, seem to have different suppressive functions and regulate the immune system responses in a different manner; some studies have shown these cells are converted to each other and even to other cells under different pathological conditions. This review summarises some of the latest papers with respect to the MDSCs functions and discusses the relationship between MDSCs and inflammation in the context of rheumatoid arthritis.
Graphical Abstract
Introduction
Myeloid-derived suppressor cells (MDSCs) were originally described as a heterogeneous population of immature cells that are derived from the myeloid progenitors with an immune-suppressive function. MDSCs can inhibit immune responses through different mechanisms, including the suppression of T cells and the induction of regulatory T (Treg) cell populations [Citation1,Citation2]. Over the course of past decades, researchers have mainly focussed on the role of these cells in the field of cancer researches [Citation3–5]. Considering the immunosuppressive properties of MDSCs, it is not surprising that new studies focus on the potential suppressive role of these cells in autoimmune diseases like rheumatoid arthritis (RA).
Rheumatoid arthritis is a systemic autoimmune disorder characterised by a hyperplastic synovial membrane that is capable of destroying adjacent articular cartilage and bone [Citation6]. Although the pathogenesis of RA has not been well understood; it is obvious that helper T (Th) 1 and Th17 cells have a critical role in the pathogenesis of the disease [Citation7]. In the case of RA, Biologic agent treatments targeting pro-inflammatory cytokines, such as interleukin (IL)-6 (tocilizumab) and tumour necrosis factor (TNF)-α (adalimumab), have shown many beneficial effects compared to conventional disease-modifying anti-rheumatic drugs (DMARDs) [Citation8–10]. However, there is still a problem, biological agents are not effective in some RA patients, just like DMARDs [Citation11]. These findings suggest the need to develop new therapies for treatment.
Based on studies on the role and function of MDSC cells in cancer and autoimmune diseases, it has been shown that MDSCs can suppress the immune system through various mechanisms. Hence, these cells have the potential to be a probable therapeutic tool for RA patients. However, the role of MDSCs in RA is still controversial [Citation12]. The purpose of this study is to review the results of several studies on the role of MDSCs in rheumatoid arthritis, as well as the causes of differences in the results of these findings.
Types and phenotypic features of MDSCs
Studies in the field of rheumatoid arthritis have shown the presence of two major subtypes of MDSCs, 1) monocytic MDSCs (M-MDSCs) which are mononuclear, and granulocytic 2) polymorphonuclear MDSCs (PMN-MDSCs) [Citation13]. As will be discussed later in this article, there are still doubts about their exact role, but almost all studies in rheumatoid arthritis show an increase in these two populations (or one of them) at the onset or during the progression of the disease. Human studies (especially for cancer) also indicate the presence of a third population of MDSCs, called the early-stage of MDSCs, which are not complete immune suppressive cells [Citation14]; however, the presence of these cells in rheumatoid arthritis has not yet been reported. Recently, a new subtype of MDSCs with the name Eo-MDSCs was identified in the mice infected with bacteria, which is known as CD11b+Ly6CmedLy6G−Siglec-F+ cells [Citation15]. The existence of such a population of MDSCs in humans has not yet been proven.
M-MDSC and PMN-MDSC phenotypes are almost identical to those of inflammatory monocytes and neutrophils, and approximately the same markers are used to identify them. This problem can make it difficult to distinguish these cells from each other, as well as to judge the function of these cells. Therefore, cell surface markers are not a reliable indicator for the differentiation of these cells, and the recognition of transcription factors (C/EBP β, RB, STAT3 and etc.) and immune-regulatory molecules (Arg-1, NO, ROS and etc.) should be considered in their diagnosis. The phenotypes reported in these studies were shown in .
Phenotypic features of MDSCs in mice
In mice, M-MDSCs are defined as Ly6Chi CD11b+Ly6G– F4/80med/hi cells with low side scatter, while PMN-MDSCs can be defined as Ly6CloCD11b+Ly6G+ F4/80low/−cells with high side scatter in flow cytometry plots [Citation16]. This phenotype is typical for neutrophils, but in some experimental models, PMN-MDSCs can also express markers that do not normally exist on neutrophils. For example, PMN-MDSCs from mice express CD224 and CD115, whereas neutrophils do not express these molecules [Citation17]. In addition, M-MDSCs can be separated from inflammatory monocyte on the basis of expression of F4/80. Because inflammatory monocyte is F4/80low/− in mice [Citation18].
Phenotypic features of MDSCs in human
In human, M-MDSCs are characterised by CD14 expression, and PMN-MDSCs by expression of CD15 and CD66b. Both cells do not express lymphocytes and NK cell lineage markers but express the general myeloid maker, namely CD33. Classic monocytes, inflammatory monocytes (CD16low/-CD14hi) and M-MDSCs can be separated on the basis of expression of the Major histocompatibility complex (MHC) class II molecules. M-MDSCs have a CD33+CD11b+CD14+CD15– human leukocyte antigen (HLA)-DR–/low phenotype, whereas classic monocytes and inflammatory monocytes are HLA-DR+ [Citation19].
PMN-MDSCs and neutrophils share a similar phenotype as CD14–CD11b+CD33+CD15+ (or CD66b+). However, different densities of these marker’s expressions allow for the distinction between these cells. A recent study shows that PMN-MDSCs and neutrophils have a different expression of some cell surface markers, such as the lectin-type oxidised LDL receptor 1 (LOX-1) and reduced expression of the lysosome-associated protein LAMP-2 [Citation15].
PMN-MDSC under endoplasmic reticulum (ER) stress, express LOX-1, that it can be a good marker for diagnosing of human neutrophils from the PMN-MDSCs [Citation20]. In addition, early-stage MDSCs are HLA-DR–CD33+Lin– (Lin: CD15, CD14, CD3, CD56, and CD19). Therefore, these cells can be distinguished from the other two types of MDSCs with lack of CD14, CD15 and etc. [Citation21].
MDSCs accumulation, activation, and survival
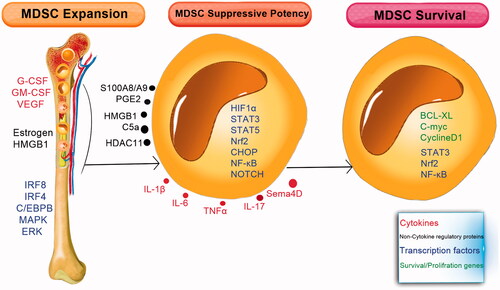
MDSCs are generated in the bone marrow from the myeloid progenitor cells and then traffic through the circulatory system or migrate into the tissues (). Multiple factors involved in the expansion and activation of MDSCs. These factors are divided into three main groups () [Citation22]. The first group induces the expansion of MDSCs through stimulation of myelopoiesis and inhibits the differentiation of mature myeloid cells [Citation23]. The second group directly activates the MDSCs, and the third group of factors is involved in the survival of MDSCs [Citation24].
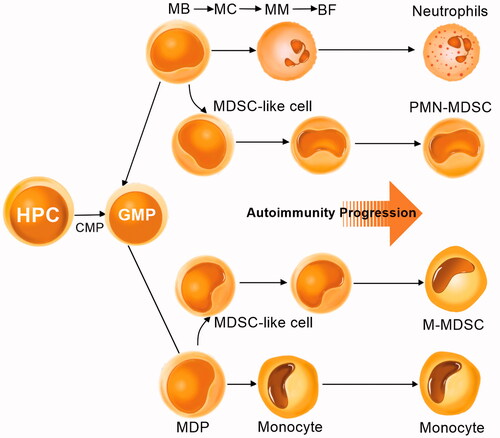
Figure 2. MDSCs differentiation and expansion. Neutrophils and monocytes are differentiated in bone marrow from common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and haematopoietic progenitor cells (HPCs). Then neutrophils are differentiated from myeloblasts (MBs), myelocytes (MCs), metamyelocytes (MMs), and band forms (BFs), while monocytes are differentiated from monocyte/macrophage and dendritic cell precursors (MDPs). Under inflammatory conditions, the immature myeloid cells can convert into MDSCs, called MDSC-like cells. These cells have some features of MDSCs but do not have complete immunosuppressive activity. In patients with autoimmune disorders, MDSCs, monocytes, and neutrophils coexist, and MDSCs can be increased during autoimmune progression.
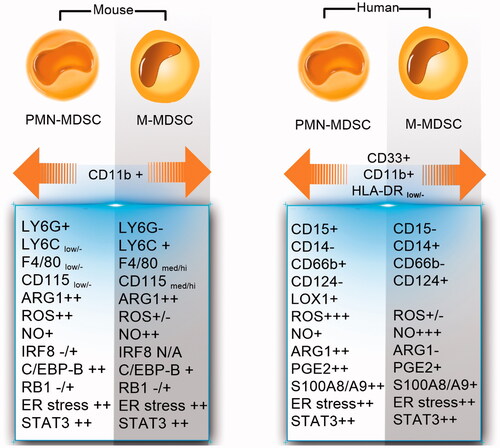
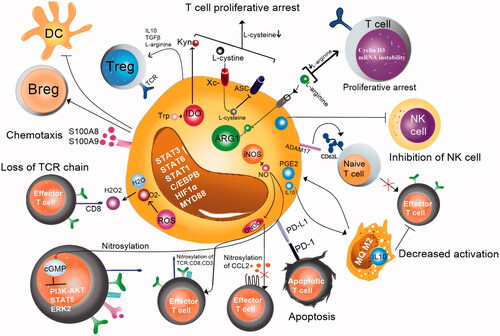
Figure 3. Cytokines, non-cytokine regulatory proteins, and transcription factors control the expansion, suppressive potency, and survival of MDSCs. Different cytokine and non-cytokine factors which regulate myelopoiesis, promote the expansion of MDSCs in the bone marrow. Within the inflammatory condition, different molecules are produced by immune cells (e.g. DCs, macrophages, lymphocytes, and fibroblasts) and increase the suppressive potency of MDSC by activating the transcription factors and different mediators in these cells. Survival of MDSCs is also mediated by the same factors that induce the expansion of MDSCs plus factors that limit apoptosis.
Accumulation and differentiation of MDSCs
The factors that induce proliferation of MDSCs include: macrophage colony-stimulating factor (M-CSF), stem-cell factor (SCF), vascular endothelial growth factor (VEGF), granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage CSF (GM-CSF), IL-1β, IL-6, IL-17, TNF-α, high mobility group box protein 1 (HMGB1), oestrogens, and polyunsaturated fatty acids [Citation24–27]. Moreover, the transcriptional factors/regulators, such as signal transducer activator of transcription (STAT) 3, interferon regulatory factor (IRF) 8, STAT5, NOTCH, and CCAAT/enhancer-binding protein beta isoform b (C/EBP-β) have a critical role in this process [Citation28]. Other factors involved in this process include adenosine receptor A2b, retinoblastoma protein 1 (RB1), cytoplasmic receptor NLRP3, and calprotectin (S100A9, S100A8) [Citation27,Citation29].
Upregulation of STAT3 is a hallmark for MDSCs [Citation30,Citation31]. Janus kinase (JAK) protein family members and STAT3 have a major role in the proliferation, differentiation, and cell survival [Citation32]. STAT3 activation can increase the proliferation and survival of myeloid progenitor cells through the upregulation of B-cell lymphoma XL (BCL-XL), cyclin D1, and C-MYC [Citation33,Citation34]. Recent studies revealed that STAT3 also has a role in MDSC expansion by inducing the S100 calcium-binding protein A8 (S100A8) and S100A9 [Citation35,Citation36]. The S100A8 and S100A9 are members of the S100 calcium-binding proteins family that have been suggested to play a major role in the inflammation [Citation37]. S100A8/A9 are regulated by STAT3 and nuclear factor NFκB, and their overexpression results in the expansion of MDSCs [Citation36].
IRF4 and IRF8 are other important transcription factors in the MDSC expansion. In contrast to STAT3, which promotes MDSC expansion, IRF8 blocks the expansion of MDSC, especially PMN-MDSCs. IRF8 inhibits the STAT3 activation and thereby, decreases the production of reactive oxygen species (ROS). Studies have shown that the IRF8-deficient mice have high levels of PMN-MDSCs [Citation38,Citation39], and overexpression of IRF8 in the myeloid lineage reduces the MDSC expansion [Citation40,Citation41].
Furthermore, Myeloid differentiation primary-response gene 88 (MyD88), an adaptor for Toll-like receptors (TLRs), is another factor which can promote the expansion of MDSCs. [Citation42,Citation43]. In addition, the C/EBP-β transcription factor, which presents oftentimes in the inflammatory conditions, including infections, autoimmunity, obesity, and stress, increases the accumulation of MDSCs. C/EBP-β preferentially regulates M-MDSC, because mice deficient for this transcription factor have reduced levels of M-MDSC [Citation44]. In addition, elements of the ER stress pathway also contribute to MDSC accumulation. The ER stress response is the conserved mechanism used by cells for protection from oxidative stress, dysregulated proliferation, hypoxia, acidic extracellular PH, and nutrient deprivation [Citation45].
PGE2 drives the differentiation of MDSC from the human haematopoietic stem cells [Citation46,Citation47], and the p38MAPK/ERK pathway is involved in the M-MDSC induction [Citation48,Citation49]. HMGB1 is another factor that stimulates the differentiation of MDSCs from the bone marrow progenitor cells [Citation50].
Nowadays, immune checkpoint blockade is one of the hottest topics in immunology, and even the 2018 Nobel Prize was given to James P. Allison and Tasuku Honjo for their research on immune checkpoint blockade [Citation51]. The inhibition by PD-1 is one of the mechanisms that MDSCs use to suppress the immune system. In addition, in a new study in 2019, it has been shown that PD-1 is also necessary for the development of MDSCs in the tumour microenvironments [Citation52]. This study showed that PD-1-positive MDSCs had increased proliferation and suppressive functions compared to PD-1-negative MDSCs. Moreover, according to a recent study in 2019, LPS stimulated MDSCs (especially PMN-MDSC) express a high level of CD180, a TLR-like protein. In addition, anti-CD180 treatment in mice diminished the expansion of MDSCs through prevention of STAT3 phosphorylation and reduced the immune suppressive function of these cells. As a result, it seems that CD180 also has a major role in the expansion and the immune suppressive function of MDSCs [Citation53].
Activation and suppressive potency of MDSCs
There are many factors that affect the activation and suppressive potency of MDSCs, which, as discussed in the previous section, are also effective in the accumulation of MDSCs. Phosphorylation of STAT3 can increase the expression of gp47 and gp91 (two subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase) and, thereby, induce the suppressive potency of MDSC [Citation54]. NADPH oxidase generates superoxide anion by reducing oxygen. When superoxide reacts with nitric oxide (NO), peroxynitrite is produced. Peroxynitrite adds nitrite to tyrosine residues in T cell receptor (TCR) and T cell co-receptors, leading to change in TCR chains and inhibition the T cell activation [Citation55,Citation56]. S100A8/A9 also increases the MDSC suppressive activity and serves as a chemoattractant for MDSC. This heterodimer acts by binding to the N-glycan motif of the receptor for advanced glycation end products (RAGE) [Citation57]. Interestingly, TNF-α also drives the suppressive activity of MDSCs by increasing the S100A8/A9 levels that signal through RAGE [Citation57].
Although ROS has toxic effects on many cells; MDSCs are almost resistant to both the internal and external ROS which they are released during their activation. NF erythroid 2–related factor 2 (Nrf2) is a transcription factor that is responsible for this tolerance [Citation29]. Moreover, C/EBP homologous protein (CHOP), an indicator of ER stress, can increase the MDSC suppressive potency by upregulating arginase1 (Arg1) and inducible NO synthase (iNOS) [Citation45,Citation58]. The ER stress response also regulates the activation of MDSCs and favours MDSC apoptosis through TNF-related apoptosis-induced ligand receptor 2 (TRAIL-R2) and caspase-8 activation [Citation59].
HMGB1 can increase the production of IL-10 from MDSC, enhances MDSC cross-talk with macrophages, and promotes the MDSC-mediated downregulation of the L-selectin expression on the naive T cells. Histone deacetylase 11 (HDAC11) is another factor that regulates MDSC suppressive potency through IL-10 production, and HDAC11-knockout mice have higher levels and more suppressive MDSC, suggesting that HDAC11 is a negative regulator for the development of MDSC [Citation60]. Furthermore, IL-4Rα (CD124) and its signalling pathway through STAT6, a transcription factor for both IL-4 and IL-13 signalling, have an important role in the MDSCs activation. IL-13 and IL-4 can upregulate the activity of Arg1, which increases the suppressive function of MDSCs [Citation61,Citation62]. In addition, IFN-γ-mediated signalling (by STAT1) is involved in the upregulation of Arg1 and iNOS expression in MDSCs [Citation63]. PGE2 also drives the suppressive potency of MDSC by increasing the expression of Arg1 [Citation64]. IL-1β, IL-6, and IL-17 also can induce suppressive potency of these cells, in addition to the role involved in the expansion of MDSCs [Citation65,Citation66]. Moreover, the complement system can also be effective in MDSC activity. for example, complement component C5a can drive accumulation and activation of MDSCs [Citation67].
Survival of MDSCs
As mentioned above, genes or transcription factors like STAT3, Nrf2, NFκB, BCL-XL, cyclin D1, and C-MYC can play a role in MDSC survival in addition to the role that they have in expansion or activation of MDSCs. STAT3 activation can increases the proliferation and survival of myeloid progenitor cells through the upregulation of B-cell lymphoma XL (BCL-XL), cyclin D1, and C-MYC [Citation33,Citation34]. Moreover, HMGB1 regulates MDSC survival by rendering the autophagic pathways in the cell [Citation68].
The immunosuppressive activity of MDSCs
MDSCs can suppress the immune responses via different mechanisms, including the production of Arg1, nitration of the TCR or chemokines and activation of inducible NO synthase (iNOS). In addition, MDSCs can inhibit T cell and macrophage activity by downregulating the production of the type 1 cytokines, such as IL-12 and upregulating the production of ROS, prostaglandin (PG) E2 (through cyclooxygenase 2) and a number of anti-inflammatory cytokines [Citation27,Citation29]. Arginine (Arg), a non-essential amino acid, plays a major role in different biological processes, including the immune responses [Citation69]. Arg1 and cationic amino acid transporter (CAT2B), which are expressed by MDSCs, can lead to depletion of Arg from the environment and, in turn, T cell dysfunction. Low Arg levels induce loss of the CD3ζ chain, inhibition of the T cell proliferation (by cyclin D3 mRNA instability) and diminish of cytokine production [Citation70]. MDSCs also use other mechanisms to inhibit the innate and adaptive immunity. They sequester cysteine that is an essential amino acid for the T cell activation and function. T cells do not have cystathionine which is necessary for the conversion of intracellular methionine to cysteine. Moreover, they do not have the xCT chain of the xc − transporter. Therefore, T cell needs the extracellular cysteine produced by other cells. Cysteine is imported by T cells through the alanine-serine-cysteine (ASC) neutral amino acid transporter [Citation71].
Production of NO by the enzymatic activity of iNOS can lead to formation peroxynitrite (ONOO−) formation by the cooperative activity of ROS and NO. Peroxynitrite causes nitration/nitrosylation of tyrosine residues in the TCR and T cell co-receptor, leading to CD3ζ chain dissociation from the TCR molecule and, ultimately, TCR signalling disruption [Citation56]. In addition, MDSCs can suppress the T cell proliferation with the help of indoleamine 2, 3-dioxygenase (IDO), a tryptophan (Trp) degrading enzyme, which catalyses the oxidative degradation of Trp. Low Trp concentration in combination with high kynurenine (downstream metabolite) decreases the expression of the TCR ζ-chain, leading to the T cell anergy [Citation72]. Moreover, MDSCs can inhibit the dendritic cells (DCs) activation and antigen presentation of DCs to T cells, and finally suppress the immune response [Citation73].
CD8+ and CD4+ T cells are suppressed by downregulation of L-selectin by MDSCs [Citation74]. L-selectin is essential for the naive T cell extravasation from blood circulation into the secondary lymph nodes [Citation75]. MDSCs express the disintegrin and metalloproteinase ADAM17 that is responsible for L-selectin cleavage [Citation74]. MDSCs can also induce and recruit Treg and Breg cells and mediate apoptosis by interactions of its programmed cell death ligand 1 (PD-L1) with PD-1 on natural killer (NK) cells or T cells [Citation76,Citation77]. Furthermore, they can suppress the cytotoxicity of NK cells via the production of transforming growth factor (TGF)-β [Citation78]. The immunosuppressive activity of MDSCs was shown in .
MDSCs in autoimmune diseases
The immunosuppressive roles of MDSCs in tumours have been well defined, but their roles in autoimmune diseases have not been well established yet. It was shown that MDSCs were accumulated in experimental models of several autoimmune diseases, such as multiple sclerosis, namely experimental autoimmune encephalitis (EAE) [Citation79–82], inflammatory bowel disease (IBD) [Citation83], experimental autoimmune uveoretinitis (EAU) [Citation84], alopecia areata (AA) [Citation85], ankylosing spondylitis (AS) [Citation86] and type 1 diabetes (T1D) [Citation87]. Accumulation of MDSCs has also been reported in inflammatory conditions, e.g. autoimmune hepatitis [Citation88,Citation89] and experimental autoimmune arthritis in mice [Citation90]. This suggests the potential suppressing role for these cells in T cell responses, but the exact function of MDSCs remains controversial. Various studies show the positive and negative effects of MDSCs in autoimmune diseases, but the key question is why these cells, which act as an immune system inhibitor cannot limit the incidence of autoimmune diseases.
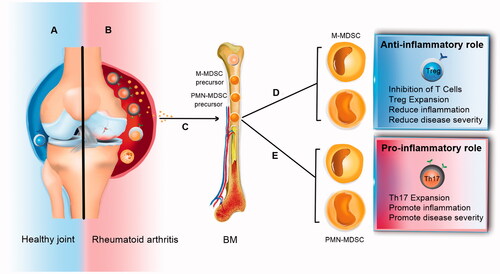
In the EAE model, different studies have shown both anti- and pro-inflammatory roles of MDSCs [Citation79,Citation81]. In several investigations, MDSCs have been found to accumulate dramatically in the spleen of mice and show a suppressive role [Citation79,Citation82]. It has been shown that MDSCs restore myelin and decrease the disease severity by inhibiting Th1 and Th17 cells after transferring to mice [Citation79]. Paradoxically, it has been reported that these cells contribute to the pathogenesis of EAE, and sustain the differentiation and accumulation of Th17 cells [Citation81]. The function and frequency of MDSCs in autoimmune diseases in different stages and organs such as the peripheral blood, bone marrow, spleen and synovial fluid are described in detail in .
Table 1. The role and frequency of MDSCs in different stages and organs in autoimmune diseases.
MDSCs in rheumatoid arthritis
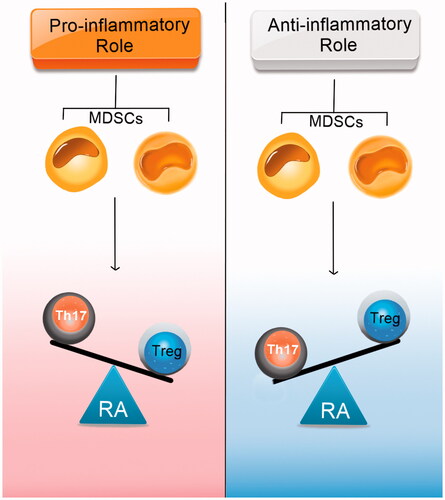
Similar to EAE, in mouse collagen-induced arthritis (CIA) model (an animal model of RA), some recent papers suggest MDSCs play an immunosuppressive role [Citation91]. Nevertheless, some studies have shown that MDSCs can also have a pro-inflammatory role and promote the RA severity in mice by sustaining Th17 cell differentiation () [Citation92]. In this section, we discuss some of the most recent articles that reviewed MDSC roles in rheumatoid arthritis.
Figure 5. Two current hypotheses about MDSC roles in rheumatoid arthritis. Some researchers suggest that MDSCs can induce Th17 cells and inhibit Treg cells. On the other hand, some data show the opposite effects of MDSCs on Th17/Treg cell balance. This inconsistency can be due to the different expansion patterns of Th17 cell and Treg cell populations and the plasticity between Th17 and Treg cells.
Evidence for pro-inflammatory effects of MDSCs
Obviously, MDSCs and Th17 cells play a major role in autoimmune diseases, but the relationship between these two immune cells remains controversial. A recent study in the CIA model (DBA/1J strain) showed MDSCs were increased in the spleen of mice, and adoptive allogenic transfer of MDSCs (isolated from spleen and BM of CIA mice) promoted disease progression and Th17 cell differentiation [Citation92]. In this study, the anti-Gr-1 treatment in CIA mice decreased the Th17 cells in the draining lymph nodes (DLN), IL-1β, and IL-17A in serum. Furthermore, MDSCs expressed a higher level of IL-1β and induced Th17 cell differentiation. This study showed that the frequency of HLA-DR−/lowCD14+ MDSCs was increased in the peripheral blood of newly diagnosed RA patients compared to the control group. The percentage of MDSCs was higher in RA patients compared to Osteoarthritis (OA) patients. In addition, the percentage of HLA-DR−/low CD14+ MDSCs was correlated with the expansion of Th17 cells and with the disease progression, suggesting the participation of MDSCs in the early stage of RA. These results support the hypothesis that MDSCs may play a pro-inflammatory role in the pathogenesis of RA.
A recent study shows that the number of MDSCs in the lymphoid tissues and inflamed paws of arthritic mice (C57BL/6 strain) are increased, and there is a positive correlation between Th17 cells and MDSCs with RA severity [Citation93]. This study showed that mice with a high frequency of MDSCs had higher levels of pro-inflammatory cytokines, such as IL-1β and TNF-α. MDSCs from arthritic mice did not show suppression of T cells activity over the disease progression. Also, the syngeneic transfer of MDSCs (isolated from the spleen of CIA mice) promoted RA severity in arthritic mice, while their deletion decreased disease severity, accompanied by a reduction of Th17/IL-17A expansion and secretion, respectively. Data from this study also showed that circulating MDSCs were increased in patients with high disease severity compared to the healthy control group or patients with low disease severity. Based on the study discussed above, the positive correlation between IL-17A level and frequency of MDSCs (in synovial fluid of RA patients) and also the ability of human MDSCs to induce Th17 differentiation support the hypothesis that MDSCs may play a pro-inflammatory role in autoimmune arthritis.
Evidence for anti-inflammatory effects of MDSCs
Some studies have shown that MDSCs can play an immunosuppressive role in the experimental model of autoimmune arthritis. A recent study in 2018 showed that in vitro treatment with MDSCs induced forkhead box P3 (FOXP3), the main Treg cells transcription factor, in CD4+ T cells and decreased IL-17 in mice (DBA/1J strain) [Citation91]. In addition, treatment of mice with MDSCs (syngeneic transfer of MDSCs isolated from the spleen of CIA mice) significantly reduced the severity of disease and the frequency of Th17 and Th1 cells but increased the frequency of Treg cells. Moreover, the data from this study showed that the adoptive transfer using MDSCs lacking IL-10 (isolated from the spleen of CIA induced IL-10 KO mice) did not suppress inflammation in mice. As a result, these data demonstrate that MDSCs can expand the FOXP3+ T cell population probably through IL-10 manner.
The study of Fujii et al. indicated that PMN-MDSCs in the spleen of CIA mice (DBA/1J strain) suppressed the pro-inflammatory cytokine production and CD4+ T cell proliferation in response to non-specific stimulation [Citation90]. This study indicated that the adoptive (syngeneic) transfer of PMN-MDSCs (isolated from the spleen of CIA mice) ameliorates the disease symptoms, and elimination of MDSCs promoted an inflammatory condition. Co-culture of CD4+ T cells with PMN-MDSCs suppressed Th17 cell differentiation, and also the adoptive transfer of PMN-MDSCs to CIA mice decreased Th17 cells in the DLNs. Similar to the research of Park et al. [Citation91], this study also supports the hypothesis that MDSCs suppress the Th17 cell differentiation and play an anti-inflammatory role in CIA [Citation90].
Kurko et al. have also illustrated that the synovial fluid in the joints of mice with proteoglycan-induced arthritis (PGIA) contains a population of PMN-MDSCs. The data from this study indicated that MDSCs potently suppressed the DC maturation and T cell proliferation and might have the potential to limit the expansion of autoreactive T cells [Citation94].
As discussed above, not only studies that support the pro-inflammatory role of MDSCs but also studies that support the anti-inflammatory role of MDSCs have shown that MDSCs can be elevated in RA and CIA model. Some cytokines are responsible for MDSCs accumulation in RA. Investigations show that the level of TNF-α which has a major role in the development of RA and CIA is increased [Citation95]. TNF-α also induces MDSC proliferation in the experimental models of autoimmune disease [Citation96]. Similar to TNF-α, the level of GM-CSF which is a key cytokine in the development of MDSCs [Citation23], also increased in the experimental model of RA [Citation97], and GM-CSF, IL-6 and IL-17 are another pro-inflammatory cytokines that participate in the pathogenesis of RA [Citation98] and can promote the MDSC accumulation and its suppressive potency either [Citation66].
Contrary to similar results about the accumulation of MDSCs in rheumatoid arthritis in several studies, there are many contradictions in the function of these cells. Several factors can be responsible for these contradictions in rheumatoid arthritis studies, including unreliable diagnostic cell markers or different conditions and genetics of the models and patients under study. Of course, these contradictions are not limited to rheumatoid arthritis researches, and there are contradictions in MDSCs function, even in cancer and other pathological conditions. For example, a study in the malignant tumours demonstrated that the suppressive effect of MDSCs might be due to M-MDSCs rather than PMN-MDSCs [Citation44] (similar to the study in patients with ankylosing spondylitis [Citation86]). While PMN-MDSCs are isolated from tumour-bearing mice by Youn et al., data indicate M-MDSCs are precursors of PMN-MDSCs [Citation17]. In fact, based on articles written in the context of MDSCs, this would appear that the suppressive responses of these cells are very plastic and are completely influenced by the microenvironment of the diseases. That is why microenvironmental changes should be considered in all studies in this area in order to avoid unrealistic conclusions.
Conclusion and future perspectives
As discussed in this review, currently there are two different hypotheses related to MDSCs function in rheumatoid arthritis, an inflammatory and anti-inflammatory theory (). By reviewing these two categories of articles, it can be said that most of the papers supporting inflammatory theory believe that MDSCs can increase Th17 cell population, while others focus on anti-inflammatory theory and believe that MDSCs can increase the population of Tregs. Moreover, it seems that the two well-known subgroups of MDSCs, namely M-MDSC and PMN-MDSC, have different suppressive functions and regulate the immune response in different manners. For example, recent studies in cancers and autoimmune disorders have shown that PMN-MDSCs are much less effective than M-MDSC in the apoptosis and inhibition of the T cell proliferation. But the interesting point is that some other studies show that MDSCs subtypes can be converted to each other and even into other cell populations, such as PMN-MDSCs to neutrophils, or M-MDSCs to inflammatory dendritic cells based on micro-environmental conditions [Citation44,Citation86]. Similarly, Th17 and Treg cell populations can be converted to each other under different microenvironmental states [Citation99]. So it can be said that microenvironment can affect the development and function of MDSCs. In addition, the local state may be equally critical in the amplification of these responses during autoimmune disorders, as seen in infections related studies [Citation15].
Another reason for controversy results is the unreliability of surface markers for detecting MDSCs cells. As mentioned in the previous sections, these cells have similar phenotypes to their precursors or neutrophils and monocytes. Therefore, along with surface markers, measurement of transcription factors and immune-regulatory molecules are necessary for the precise identification of MDSCs subtypes. Moreover, the use of different MDSCs (as effector cells) to T cells (as target cells) ratio in cell-culture based studies may lead to this controversy in results. Data from new studies suggest that the in vitro regulatory functions of MDSCs are completely dependent on the frequency of these cells that are used as effector cells.
In conclusion, increasing the population of MDSCs in rheumatoid arthritis, regardless to their anti- or pro-inflammatory roles, and the effect of microenvironment on the plasticity of MDSCs, it is possible to manipulate microenvironment influences as a therapeutic approach for the treatment of rheumatoid arthritis. Thus, further studies should be accomplished in both humans and experimental models to clarify the effect of various microenvironmental conditions on MDSC functions, in order to find the appropriate factors for enhancing the anti-inflammatory properties of MDSC cells in rheumatoid arthritis.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
| Abbreviations | ||
| ADAM17 | = | ADAM metallopeptidase domain 17 |
| Arg | = | arginine |
| ARG1 | = | arginase1 |
| ASC | = | alanine-serine-cysteine |
| Bcl-xL | = | B-cell lymphoma-extra-large |
| C/EBPs | = | CCAAT-enhancer-binding proteins |
| CAT2B | = | cationic amino acid transporter |
| CHOP | = | C/EBP homologous protein |
| CIA | = | collagen-induced arthritis |
| Cox-2 | = | cyclooxygenase 2 |
| DLN | = | draining lymph nodes |
| DMARDs | = | disease-modifying anti-rheumatic |
| EAE | = | experimental autoimmune encephalitis |
| FOXP3 | = | forkhead box P3 |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| HDAC11 | = | histone deacetylase 11 |
| HMGB1 | = | high mobility group box protein 1 |
| IDO | = | indole amine 2,3-dioxygenase |
| iNOS | = | inducible NO synthase |
| IRF4 | = | IFN-inducible regulatory factor 4 |
| IRF8 | = | IFN-inducible regulatory factor 8 |
| LOX-1 | = | lectin-type oxidised LDL receptor 1 |
| MDSC | = | myeloid-derived suppressor cell |
| M-MDSC | = | monocytic MDSC |
| NF-κB | = | nuclear factor kappa B |
| Nrf2 | = | NF erythroid 2–related factor 2 |
| PGE2 | = | prostaglandin E2 |
| PMN-MDSC | = | polymorphonuclear MDSC |
| RA | = | rheumatoid arthritis |
| RB1 | = | retinoblastoma 1 |
| ROS | = | reactive oxygen species |
| STAT3 | = | signal transducer activator of transcription 3 |
| TCR | = | T cell receptor |
| TGF-β | = | transforming growth factor beta |
| TNF-α | = | tumour necrosis factor α |
| Trp | = | tryptophan |
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Kong YY, Fuchsberger M, Xiang SD, et al. Myeloid derived suppressor cells and their role in diseases. CMC. 2013;20(11):1437–1444.
- Nagaraj S, Youn J-I, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191(1):17–23.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253.
- Solito S, Marigo I, Pinton L, et al. Myeloid‐derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci. 2014;1319(1):47–65.
- Messmer MN, Netherby CS, Banik D, et al. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64(1):1–13.
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85(3):307–389.
- Gizinski AM, Fox DA. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol. 2014;26(2):204–210.
- Lipsky PE, van der Heijde DM, St. Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343(22):1594–1602.
- Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis. J Rheumatol. 2010;38(1):10–20.
- Feldmann M, Maini RN. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245.
- Ramos-Casals M, Diaz-Lagares C, Cuadrado M-J, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9(3):188–193.
- Wang Y, Tian J, Wang S. The potential therapeutic role of myeloid-derived suppressor cells in autoimmune arthritis. Semin Arthritis Rheum. 2016;45(4):490–495.
- Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood. 2008;111(8):4233–4244.
- Dumitru CA, Moses K, Trellakis S, et al. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61(8):1155–1167.
- Peñaloza HF, Alvarez D, Muñoz‐Durango N, et al. The role of myeloid‐derived suppressor cells in chronic infectious diseases and the current methodology available for their study. J Leukoc Biol. 2019;105(5):857–872.
- Damuzzo V, Pinton L, Desantis G, et al. Complexity and challenges in defining myeloid‐derived suppressor cells. Cytometry. 2014;88(2):n/a– 91.
- Youn JI, Collazo M, Shalova IN, et al. Characterization of the nature of granulocytic myeloid‐derived suppressor cells in tumor‐bearing mice. J Leukoc Biol. 2012;91(1):167–181.
- Yang J, Zhang L, Yu C, et al. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1.
- Mandruzzato S, Brandau S, Britten CM, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65(2):161–169.
- Condamine T, Dominguez GA, Youn J-I, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2):aaf8943.
- Bronte V, Brandau S, Chen S-H, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7(1):12150.
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25.
- Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid‐derived suppressor cell subsets is determined by GM‐CSF. Eur J Immunol. 2009;40(1):22–35.
- Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid‐derived suppressor cells. J Leukoc Biol. 2015;98(6):913–922.
- Mantovani A, Sica A, Allavena P, et al. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330.
- Ribechini E, Hutchinson JA, Hergovits S, et al. Novel GM-CSF signals via IFN-γR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Adv. 2017;1(14):947–960.
- Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–119.
- Cheng P, Kumar V, Liu H, et al. Effects of notch signaling on regulation of myeloid cell differentiation in cancer. Cancer Res. 2014;74(1):141–152.
- Ostrand-Rosenberg S, Fenselau C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 2018;200(2):422–431.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162.
- Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172(1):464–474.
- Rébé C, Végran F, Berger H, et al. STAT3 activation: a key factor in tumor immunoescape. Jak-stat. 2013;2(1):e23010.
- Nefedova Y, Nagaraj S, Rosenbauer A, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65(20):9525–9535.
- Xin H, Zhang C, Herrmann A, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69(6):2506.
- Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249.
- Sinha P, Okoro C, Foell D, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–4675.
- Gebhardt C, Németh J, Angel P, et al. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72(11):1622–1631.
- Stewart TJ, Greeneltch KM, Reid JE, et al. Interferon regulatory factor‐8 modulates the development of tumour‐induced CD11b + Gr‐1+ myeloid cells. J Cell Mol Med. 2009;13(9b):3939–3950.
- Waight JD, Netherby C, Hensen ML, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–4478.
- Netherby CS, Abrams SI. Mechanisms overseeing myeloid-derived suppressor cell production in neoplastic disease. Cancer Immunol Immunother. 2017;66(8):989–996.
- Netherby CS, Messmer MN, Burkard-Mandel L, et al. The granulocyte progenitor stage is a key target of IRF8-mediated regulation of myeloid-derived suppressor cell production. J Immunol. 2017;198(10):4129–4139.
- Liu Y, Xiang X, Zhuang X, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–2499.
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, et al. MyD88-dependent expansion of an immature GR-1+ CD11b + population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474.
- Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity. 2010;32(6):790–802.
- Thevenot PT, Sierra RA, Raber PL, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41(3):389–401.
- Sinha P, Clements VK, Fulton AM, et al. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–4513.
- Talmadge JE, Hood KC, Zobel LC, et al. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int immunopharmacol. 2007;7(2):140–151.
- Mao Y, Poschke I, Wennerberg E, de Coaña YP, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2–dependent mechanisms. Cancer Res. 2013;73(13):3877.
- Mao Y, Sarhan D, Steven A, et al. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20(15):4096.
- Li J, Sun J, Rong R, et al. HMGB1 promotes myeloid-derived suppressor cells and renal cell carcinoma immune escape. Oncotarget. 2017;8(38):63290.
- Kaiser J, Couzin-Frankel J. Cancer immunotherapy sweeps Nobel for medicine. Science. 2018;362(6410):13.
- Nam S, Lee A, Lim J, et al. Analysis of the expression and regulation of PD-1 protein on the surface of myeloid-derived suppressor cells (MDSCs). Biomol Ther. 2019;27(1):63.
- Dong G, Yao X, Yan F, et al. Ligation of CD180 contributes to endotoxic shock by regulating the accumulation and immunosuppressive activity of myeloid-derived suppressor cells through STAT3. Biochim Biophys Acta Mol Basis Dis. 2019;1865(3):535–546.
- Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–5701.
- Lu T, Ramakrishnan R, Altiok S, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121(10):4015–4029.
- Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828.
- Sade-Feldman M, Kanterman J, Ish-Shalom E, et al. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38(3):541–554.
- Lee B-R, Chang S-Y, Hong E-H, et al. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget. 2014;5(23):12331.
- Condamine T, Kumar V, Ramachandran IR, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R–mediated apoptosis. J Clin Invest. 2014;124(6):2626–2639.
- Sahakian E, Powers JJ, Chen J, et al. Histone deacetylase 11: a novel epigenetic regulator of myeloid derived suppressor cell expansion and function. Mol Immunol. 2015;63(2):579–585.
- Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419.
- Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSC) inhibit graft-versus-host (GVHD) disease via an arginase-1 dependent mechanism that is upregulated by IL-13. Blood. 2010;116(25):5738–5747.
- Greifenberg V, Ribechini E, Rößner S, et al. Myeloid‐derived suppressor cell activation by combined LPS and IFN‐γ treatment impairs DC development. Eur J Immunol. 2009;39(10):2865–2876.
- Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939.
- Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer cell. 2008;14(5):408–419.
- He D, Li H, Yusuf N, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281. ji_0902574.
- Markiewski MM, DeAngelis RA, Benencia F, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225.
- Parker KH, Sinha P, Horn LA, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74(20):5723.
- Sikalidis AK. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015;21(1):9–17.
- Wu H, Zhen Y, Ma Z, et al. Arginase-1–dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med. 2016;8(331):331ra40–331ra40.
- Srivastava MK, Sinha P, Clements VK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2009;70(1):68–77.
- Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–3797.
- Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–807.
- Hanson EM, Clements VK, Sinha P, et al. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183(2):937.
- Arbonés ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1(4):247–260.
- Serafini P, Mgebroff S, Noonan K, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449.
- Lindau D, Gielen P, Kroesen M, et al. The immunosuppressive tumour network: myeloid‐derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–115.
- Li H, Han Y, Guo Q, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β1. J Immunol. 2009;182(1):240–249.
- Ioannou M, Alissafi T, Lazaridis I, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2011;188(3):1136–1146.
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C + myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113(14):3190–3197.
- Yi H, Guo C, Yu X, et al. Mouse CD11b + Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189(9):4295–4304.
- Zhu B, Bando Y, Xiao S, et al. CD11b + Ly-6Chi suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179(8):5228–5237.
- Haile LA, Von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135(3):871.e5–881.e5.
- Kerr EC, Raveney BJ, Copland DA, Dick AD, et al. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31(4):354–361.
- Marhaba R, Vitacolonna M, Hildebrand D, et al. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179(8):5071–5081.
- Liu Y-F, Zhuang K-h, Chen B, et al. Expansion and activation of monocytic-myeloid-derived suppressor cell via STAT3/arginase-I signaling in patients with ankylosing spondylitis. Arthritis Res Ther. 2018;20(1):168.
- Yin B, Ma G, Yen C-Y, et al. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185(10):5828–5834.
- Cripps JG, Wang J, Maria A, et al. Type 1 T helper cells induce the accumulation of myeloid‐derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52(4):1350–1359.
- Zuo D, Yu X, Guo C, et al. Scavenger receptor a restrains T‐cell activation and protects against concanavalin A‐induced hepatic injury. Hepatology. 2013;57(1):228–238.
- Fujii W, Ashihara E, Hirai H, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191(3):1073–1083.
- Park M-J, Lee S-H, Kim E-K, et al. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. 2018;8(1):3753.
- Zhang H, Wang S, Huang Y, et al. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin Immunol. 2015;157(2):175–186.
- Guo C, Hu F, Yi H, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2014;75(1):278–285.
- Egelston C, Kurkó J, Besenyei T, Tryniszewska B, et al. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012;64(10):3179–3188.
- Romas E, Gillespie M, Martin T. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002;30(2):340–346.
- Zhao X, Rong L, Zhao X, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094–4104.
- Cornish AL, Campbell IK, McKenzie BS, et al. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):554.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356.
- Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25(4):305–312.
- Kurkó J, Vida A, Glant TT, et al. Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. 2014;15(1):281.