Abstract
Instability of silk fibroin nanoparticles (SFNPs) in physiologic condition hinders its application as drug delivery vehicle. Herein, indocyanine green (ICG) loaded silk fibroin nanoparticles (ICG-SFNPs) was firstly prepared and then crosslinked by proanthocyanidins to obtain the stable ICG-CSFNPs for killing the residual tumour niche under near infra-red irradiation (NIR) after surgery. The particle size and zeta potentials of ICG-CSFNPs was 120.1 nm and -40.4 mV, respectively. Moreover, ICG-CSFNPs exhibited good stability of particle size in the physiological medium. Meanwhile, the stable photothermal properties of ICG-CSFNPs were not compromised even after several cycles of NIR. Few of the ICG-CSFNPs were phagocytized by RAW264.7 macrophage in vitro, while they were easily internalized by C6 glioma cells, resulting in their significant toxicity on tumour cells after NIR. The pharmacokinetic study showed that ICG-CSFNPs had a longer blood circulation time than ICG-SFNPs, making them more distribution in glioma after intravenous administration in vivo. Meanwhile, the pharmacological study showed the more effective inhibition of tumour growth was exhibited by ICG-CSFNPs in C6 glioma-bearing mice after NIR. Overall, the cross-linked nanoparticles of silk fibroin may be a promising vehicle of ICG for photothermal therapy of glioma after surgical resection.
Graphical Abstract
Introduction
Glioblastoma (GBM), accounting for more than 60% of the intracranial tumours in adults, is the most common and aggressive malignant tumour of the central nervous system [Citation1]. Despite the advances of the multimodal therapeutic regimen such as the intracranial surgical resection, radiotherapy, chemotherapy and gene therapy in the past few decades, patients suffering from GBM still only have a median survival of about 14 months with a 5-year survival rate of 4 − 5% after first diagnosis[Citation2]. Surgical resection remains the preferred approach for some patients with glioblastoma. Practically, it is difficult for surgical treatment to conduct the complete resection of the bulk GMB because of its invasive growth and the ambiguous boundaries between tumour and healthy brain parenchyma [Citation3]. In clinics, chemotherapeutic therapy or radiation therapy are usually required as an auxiliary treatment of GBM after surgical resection to kill the remaining tumour cells after surgical resection. However, the outcomes for these treatments were very limited because of the severe side effects [Citation4]. Thus, how to eradicate the residual tumour niche was very meaningful to prolong patient survival.
Photothermal therapy has been employed as an effective treatment for cancer [Citation5]. Indocyanine green (ICG) is a natural dye consisting of two polycyclic moieties and an intermediate carbon chain. ICG absorbs near infra-red light (650–900 nm) and produces thermal effect in vitro and in vivo. ICG has been approved by the US Food and Drug Administration (FDA) as a clinical near infra-red optical imaging contrast enhancer because of its low toxicity and being safe in the body. ICG has already been applied to cardiac output detection and liver function in clinical practice [Citation6]. Because of its unique infra-red photothermal (PTT) and photodynamic (PDT) effects, ICG has attracted the attention of many researchers in the field of photothermal therapy of cancer [Citation7]. However, ICG easily bonded to plasma protein in the body, and rapidly metabolized by the liver, causing its short half-life (about 2–4 min) in vivo. These drawbacks made ICG impossible to concentrate a sufficient dose inside tumour tissue for imaging or photothermal therapy purposes.
Silk fibroin, a natural biocompatible macromolecular protein has been widely used in many fields such as tissue engineering [Citation8] and drug delivery [Citation9] due to its good biocompatibility and high drug encapsulation efficiency. In our previous research, nanoparticle of silk fibroin (SFNPs) was found to firmly bind ICG with high loading efficiencies for imaging-guided photothermal therapy of GBM [Citation10]. However, because of its crystalline core and amorphous shell structure, SFNPs tended to easily aggregate in the physiologic condition, which exhibited a limit prolonging half-lives of ICG in vivo. Several crosslinking agents such as chitosan [Citation11], doxorubicin [Citation12] and cationic lipid layers [Citation13] had been used to improve the stability of SFNPs, but they did not show satisfactory results in vivo studies. Moreover, these strategies remained a safety issue of the residual crosslinking agents. By contrast, the natural pigment such as proanthocyanidins, genipin and tannic acid have been widely used as the cross-linking agents in tissue engineering because of their mild cross-linking properties and good safety [Citation14]. For example, proanthocyanidins, a polyphenol widely found in natural plants, was used as a safe crosslinking agent to improve stability of gelatine nanoparticles [Citation15].
In this study, proanthocyanidins (PC) was used as a natural crosslinking agent to improve the stability of ICG-SFNPs in physiologic medium. The physiochemical properties of ICG-SFNPs including size, zeta potential and morphology were first investigated and compared after crosslinking of PC. More importantly, the size stability and photo-stability of ICG-CSFNPs were carefully evaluated in physiologic solution. Thereafter, both the cellular uptake of ICG-CSFNPs and photothermal effect was further investigated on C6 glioma cells in vitro. Subsequently, the pharmacokinetic of ICG-CSFNPs in healthy rats was evaluated in comparison with that of ICG-SFNPs. Finally, the distribution of ICG-CSFNPs was detected in glioma-bearing mice by IVIS fluorescence imaging and the photo-thermal effect of ICG-CSFNPs was also confirmed.
Materials and methods
Materials
Bombyx mori Cocoons were collected from the Zhejiang Huzhou Shuanglin Huajie silk cotton opening factory. Na2CO3 and lithium bromide (LiBr) were purchased from Sigma-Aldrich Company (St Louis, MO, USA). Phosphotungstic acid and ICG were obtained from MeilunbioVR (Dianlian, China). Dialysis bag (MW 3500 Da) was provided by SolarbioVR (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM), foetal calf serum, and trypsin were provided by Gibco BRLVR (Grand Island, NY, USA). RAW264.7 and C6 cell line were obtained from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China).
Extraction of silk fibroin
Silk fibroin solution was extracted by using the method previously described [Citation10]. Briefly, B. mori cocoons were cut into pieces and immersed in Na2CO3 solution (0.02 M). The solution was then heated and boiled for 1 h at 100 °C in order to remove sericin. The boiled cocoons were withdrawn and rinsed with distilled water. After drying, the degummed fibres (1 g) were slowly dissolved in 20 ml of LiBr solution (9.3 M) at 60 °C for 4 h. the flocculent precipitation was then removed by centrifugation at 10,000 rpm and the supernatant was further filtrated to remove tiny undissolved substances. Finally, the dissolved silk solution was dialyzed using the dialysis bag (MWCO 3500) against deionised water (2 L) for 2 days to remove the LiBr, obtaining the dilute SF solution. The concentration of the resulting dilute SF solution was approximately 2%, which was calculated by weighing the residual dry SF after fan drying of 10 ml SF solution. The dilute SF solution was further concentrated in a vacuum drying oven at 65 °C and 4% of SF solution was finally obtained.
The crosslinking of ICG-SFNPs by proanthocyanidins
The ICG-SFNPs were prepared by self-assembly method [Citation16]. Briefly, SF solution (9 ml) with ICG (2 mg/ml) was added to acetone dropwise (50 μL/drop) and under vigorous stirring at room temperature to induce the desolvation process. After the addition of the SF-ICG solution, the reaction solution became a milk-green appearance and the resulting solution was further stirred overnight so that ICG-SFNPs was completely dehydrated. The resulting ICG-SFNPs solution was then further rotated for 3 h at room temperature to remove the acetone and the remaining ICG-SFNPs were reconstituted into distilled water followed by sonication at an amplitude of 200 W for 5 min (pulse of 2 s ON and 3 s OFF). The final aqueous ICG-CSFNPs solution was further added into an ultrafiltration device (Millipore, Billerica, MA, USA) to remove the unloaded ICG and detect the encapsulation rate.
For proanthocyanidins (PC) cross-linked ICG-SFNPs, the ICG-SFNPs were dispersed into the PC solution with various mass ratios of SFNPs/PC. The mixture was stirred for 24 h at 60 °C. Afterwards, the CSFNPs were dialyzed (MWCO 3500) against distilled water (2 L) for 48 h, removing excess PC. The dialyzed CSFNPs were filtered through a filter membrane (JinTeng, Tianjin, China) of 0.45 μm to remove contaminants. The filtered ICG-CSFNPs suspension was stored at 4 °C for further use. UV-Vis and FT-IR spectrum were used to confirm the crosslinking between PC and SF nanoparticles.
The stability of the cross-linked ICG-SFNPs
The colloidal stability of the cross-linked SFNPs
The particle size and zeta potentials of CSFNPs and SFNPs were measured by DLS (Litesizer, Anton Paar, Austria) in pH7.4PBS and DMEM medium. The particle sizes were measured at pre-determined time intervals (0–24 h).
After treating with saline or DMEM for 50 min, the morphologies of CSFNPs and SFNPs were assessed by Transmission Electron Microscopy (JEOLJEM-2000EX, JEOL, Japan) at 200 kV. Briefly, 10 μL of SFNPs or CSFNPs solution wasdropped onto a carbon-coated grid followed by staining with 1% phosphotungstic acid.
In vitro photo-stability of ICG-CSFNPs
To compare the photo-stability of ICG-CSFNPs, ICG-SFNPs and free ICG, the samples were irradiated with 808 nm NIR laser for 5 min (laser on), followed by naturally cooling to room temperature without NIR for 15 min (laser off). The cycle was repeated for three times. The photothermal imaging of ICG-CSFNPs was also taken at pre-determined time intervals (0–40 min). The thermal picture was taken by Thermal Imager (DT-9868, CEM) in vitro.
In vitro release of ICG from ICG-CSFNPs
In vitro release of ICG from ICG-CSFNPs was investigated by the dynamic dialysis method. Briefly, ICG-CSFNPs or ICG-SFNPs (2 ml) were placed in the dialysis tube with a cut-off molecular weight of 3500 Da and dialyzed against 50 ml pH 4.5 or pH7.4 phosphate buffer at 37 °C under the stirring speed of 100 rpm. At each predetermined time intervals, the release medium was withdrawn for UV-Vis analysis and the equivalent fresh buffer was replenished. The cumulative release was calculated by the following formula: the cumulative release percentage (%)=(C1×V + C2×V+⋯Ct×V)×100/(2 × Cs), Ct was the concentration of ICG in release medium at t time point, V was the volume of the release medium, Cs was ICG concentration in silk fibroin nanoparticles.
In vitro cell experiments
Cell culture
RAW 264.7 and C6 glioma cells were cultured in DMEM culture medium containing 10% foetal bovine serum, 1% penicillin and 1% streptomycin at 37 °C under a condition containing 5% CO2.
In vitro cells uptake
RAW 264.7 and C6 cells were seeded into six-well chambered cover glasses at the density of 5 × 104 in 1 ml of DMEM medium. After 24 h of culturing at 37 °C under 5% CO2, the culture medium was replaced by new medium containing ICG-SFNPs or ICG-CSFNPs (40 μg/ml ICG). After 4 h and 8 h of incubation, the cells were rinsed with PBS (0.01 M, pH = 7.4) for three times, fixed with 4% paraformaldehyde and finally, analyzed under confocal laser microscopy.
In vitro photo-thermal therapy
The cytotoxicity of photothermal treatment was evaluated by MTT method. C6 cells were seeded into the 96 well at density of 6 × 103/well. The ICG-CSFNPs were added into the well at 10 μg/mL ICG of final concentration. After 24 h and 48 h incubation, each well was exposed at laser (808 nm, 1 W/cm2) for 3 min and the cell viability was measured by MTT method.
Calcein-AM/PI staining was used to confirm the photothermal therapy of ICG-CSFNPs. C6 cells were seeded into 48-well plates (1 × 104 cells/well) in 500 μL DMEM. After 48 h incubation at 37 °C, the previous medium was replaced by a new medium of ICG-CSFNPs containing 10 μg/mL ICG and the three media, namely, medium without ICG, medium with free ICG and medium with ICG-SFNPs were used as controls. The cells were irradiated with laser (808 nm, 1 W/cm2) for 3 min using the same procedures and then washed with PBS and stained with Calcein-AM for live cell and PI for dead cell visualisation. The results were obtained through the inverted fluorescence microscope.
In vivo study of ICG-CSFNPs
Constructing glioma BalB/c mice model
Male BalB/C nude mice (6–8 weeks) were purchased from Shanghai, China. All animal experiments were performed under the approval and guidance of the Institutional Animal Care and Use Committee of Wenzhou Medical University (SPF level). Briefly, C6 cells at the density of 1 × 106 were subcutaneously injected in the right flanks of each nude mice.
Pharmacokinetic of ICG-CSFNPs
SD rats were fasted for one night before the pharmacokinetics experiment. The body weights were accurately weighed before i.v. administration. Each rat was administered with ICG dose of 0.5 mg/Kg. Blank blood was collected as a control. Blood was taken at predetermined time points and each time the blood collection volume was 0.1–0.2 ml. After centrifugation at 12,000 rpm for 10 min, the supernatant was withdrawn and added to 900 μL DMSO for mixing on vortex set, followed by centrifugation at 12,000 rpm. The fluorescence spectrum of the supernatant was detected by using microplate reader at an excitation wavelength of 745 nm and an emission wavelength range of 800–850 nm. The ICG concentration was calculated by the standard fluorescence curve and the ICG-SFNPs were studied as a control.
In vivo fluorescence imaging
When the tumour reached the size of 60mm3, in vivo fluorescence imaging of tumour-bearing mice were taken by IVIS (IVISVR Lumina II) after intravenous injection of free ICG, ICG-SFNPs or ICG-CSFNPs (100 μg/mL). Filter set (Excitation Filter: 710 nm. Emission filter: ICG) was used for in vivo fluorescence imaging.
In vivo photo-thermal therapy
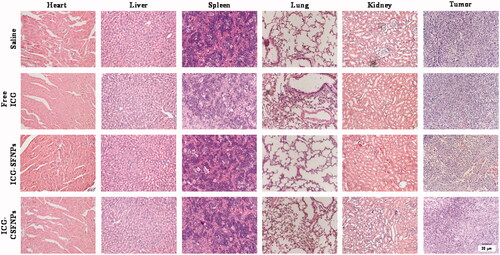
When the tumour reached the size of 60 mm3, tumour-bearing mice were injected with ICG-CSFNPs (100 μg/mL ICG). NIR (808 nm, 1 W/cm2) was applied to irradiate the tumour for 3 min. The photothermal imaging of tumour-bearing mice was taken by the thermal imager (DT-9868, CEM). Meanwhile, the tumour volume was measured by a calliper every day during the whole therapeutic process and calculated by the formula: tumour volume=((tumour length) × (tumour width)2)/2. Relative tumour volume was calculated as V/V0 (V0 was the initial tumour volume before therapy; V was the practical tumour volume at each test time). The mice survival rate was also counted during the whole therapy. At the end of photothermal therapy, the mice were sacrificed and the major organs (heart, liver, spleen, lung, kidney and tumours) were collected for haematoxylin and eosin (H&E) staining.
Statistical analysis
The level of significances in all statistical analyses was set at a probability of p<.05. Data are presented as mean ± SD. Analysis of variance and t-tests were used to analyze the data.
Result and discussion
Preparation and characterization of ICG-CSFNPs
ICG-SFNPs were first prepared by inducing the self-assembly of silk fibroin in organic solvents. For this, ICG was added to silk fibroin aqueous solution as aqueous phase, followed by dropping the ICG/SF aqueous phase into acetone to self-assemble into nanoparticle. The formation of nanoparticles was attributed to the change in conformation of SF from α-helix/random coil to β-sheet. PC is a natural polyphenol widely found in plants, which easily reacted with polymer containing amino groups through Michael addition or Schiff-mediated crosslinking reaction. Therefore, ICG-SFNPs was further cross-linked with PC to obtain the stable ICG-CSFNPs in the physiological solution. By adjusting the ratio of PC/ICG-SFNPs, series of ICG-CSFNPs were prepared and their macroscopic appearances were exhibited in . As the ratio of PC/ICG-SFNPs increased, the appearance of ICG-CSFNPs gradually became brown-grey because of crosslinking reaction between amide groups of the ICG-SFNPs and the phenolic group of PC. Due to the strong affinity of SF and ICG, the encapsulating efficiency of ICG in SFNPs highly reached to 95.69 ± 0.54%. After crosslinking with PC, the encapsulating efficiency slightly changed from 95.03 to 97.77%. These indicated the crosslinking of PC had no significant effect on the encapsulate efficiency of ICG in SF nanoparticles.
Figure 1. Preparation and characterization of ICG-CSFNPs: (A) The macroscopic appearance of ICG-CSFNPs cross-linked with the different ratio of PC/ICG-SFNPs; (B) particle sizes (red bar) and Zeta potentials (grey dot) of ICG-CSFNPs; (C) UV-Vis spectra; and (D) FT-IR spectra of PC, ICG-SFNPs and ICG-CSFNPs.
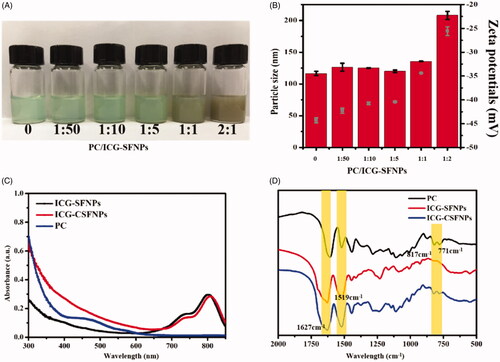
The particle size and zeta potential of these ICG-CSFNPs was detected and compared with that of ICG-SFNPs (without crosslinking). Results were shown in . When the ratio of PC/ICG-SFNPs increased from 1:50 to 1:5, Dh and zeta potentials of ICG-CSFNPs (Dh ranging from 128 to 130 nm and zeta potential of -42∼-40 mV) were slightly changed in comparison with that of ICG-SFNPs (Dh of 126.45 nm and zeta potential of -45 mV). However, Dh of ICG-CSFNPs was obviously increased to 208 nm and its zeta potential decreased to -25 mV when ratio of PC/ICG-SFNPs was at 2:1. The increase in Dh and zeta potential of ICG-CSFNPs was partially due to the self-crosslinking of PC on surface of ICG-CSFNPs besides its crosslinking with SF molecules at the high ratio of PC/ICG-SFNPs. Considering that the sufficient crosslinking with SF nanoparticles at low ratio of PC/ICG-SFNPs but excessive crosslinking at its high ratio might occur, the ratio of PC/ICG-SFNPs at 1:5 was thus used to prepare ICG-CSFNPs for the following studies.
To test whether the spectral properties of ICG-CSFNPs was compromised after PC crosslinking, the spectrum of UV-Vis of ICG-CSFNPs were monitored and compared with free PC and ICG-SFNPs. As shown in , the absorption peak of ICG at 800 nm for ICG-CSFNPs was not affected after crosslinking with PC. Moreover, the absorption peak at 450 nm attributed to PC was not in sight for ICG-CSFNPs, indicating the successful crosslinking of PC. The crosslinking of PC inside ICG-CSFNPs was also confirmed by FT-IR. As shown in , ICG-SFNP exhibited the typical β-sheet structure of SF characterised by groups of peaks such as 1627 cm−1 and 1519 cm−1. Besides these peaks, ICG-CSFNPs showed a group of peaks at 817 cm−1 and 771 cm−1 attributed to C–H of PC but the peak at 1600 cm−1 for benzene ring disappeared.
The stability of ICG-CSFNPs in vitro
To confirm the improvement of ICG-CSFNPs in the physiological solution, the particle size stabilities of ICG-CSFNPs (1:5) were firstly examined by monitoring Dh after incubation with PBS at 37 °C for different pre-defined time intervals. As shown in , Dh of ICG-SFNPs rapidly increased from 116.39 ± 1.45 nm to 1852.78 ± 752.00 nm only after 5 min of incubation in PBS and finally aggregated within 24 h. By contrast, no significant change in Dh of ICG-CSFNPs was observed after incubation with PBS within first 30 min. Although Dh of ICG-CSFNPs exhibited an increasing trend as the incubation time extended, there was not any aggregate in sight for ICG-CSFNPs and its Dh maintained in the range of 200–580nm during 24 h of incubation with pH 7.4 PBS. These results showed the particle size stability of ICG-CSFNPs was significantly improved in pH 7.4 PBS. Moreover, there was also no significant change of Dh for ICG-CSFNPs in DMEM even after 24 h of incubation, indicating the better stability of ICG-CSFNPs in comparison with ICG-SFNPs.
Figure 2. Stability of ICG-CSFNPs: (A) Particle size changes of ICG-CSFNPs and ICG-SFNPs in pH7.4 PBS or DMEM; (B) TEM of ICG-SFNPs and ICG-CSFNPs in pH7.4 PBS or DMEM after 50 min of incubation at 37 °C; (C) temperature changes of ICG-CSFNPs after the repeated NIR (808 nm, 1 W/cm2, 5 min on, 15 min off); and (D) thermal image of free ICG solution, ICG-SFNPs and ICG-CSFNPs after irradiation.
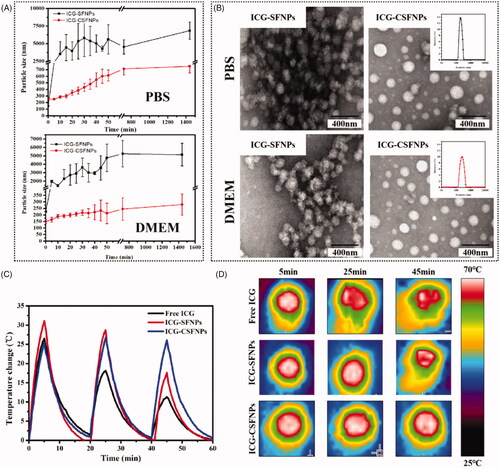
The stability improvement of ICG-CSFNPs was also confirmed by TEM. As shown in , the flocculate composed of the fine sphere-like nanoparticle was displayed in TEM of ICG-SFNPs after incubation with pH 7.4 PBS or DMEM medium, whereas the regular spherical morphology with good dispersion was observed for ICG-SFNPs after incubation with the mediums.
Besides, the photothermal stability of ICG-CSFNPs was also evaluated by detecting the temperature increase after continuous NIR laser irradiation in vitro. As shown in , the potential of temperature elevation for free ICG or ICG-SFNPs decayed gradually after NIR radiation. The decaying of temperature elevation for free ICG or ICG-SFNPs might be due to the gradual degradation of ICG in the aqueous environment after NIR irradiation. By contrast, the temperature elevation of ICG-CSFNPs for each NIR irradiation was stable at the level of about 23–26 °C. These results indicated that ICG-CSFNPs improved the photothermal stability of ICG because of its stabile encapsulating in nanoparticles. Although the tumour cells were not durable after exposure to NIR-induced thermal response of ICG [Citation17], the usage of free ICG solution alone was difficult to produce the insufficient temperature raising and completely kill the tumour cells in vivo, because of the decaying photothermal effect after irradiation [Citation18]. Thus, in this study, the stabile temperature rising for ICG-CSFNPs was beneficial for the effective photothermal therapy of tumour.
In vitro release of ICG from ICG-CSFNPs
The release profiles of ICG from ICG-SFNPs or ICG-CSFNPs were exhibited in Figure S1. The pH-dependent release profile was presented for both of the nanoparticles Approximately, 58 and 42% of ICG from ICG-SFNPs and ICG-CSFNPs were released in pH 4.5 medium within 24 h, respectively, higher than releases observed at pH values of 7.4 (ca.44% for ICG-SFNPs and ca.28% for ICG-CSFNPs). The pH-dependent release is favourable in specific distribution of therapeutic candidate towards tumour because lower pH environments favour the growth of tumour cells but have the opposite effect on normal cells [Citation19]. However, ICG-CSFNPs showed the more sustained-release profiles in comparison with ICG-SFNPs at pH7.4 medium because of the crosslinking of proanthocyanidins, which was also beneficial for passively targeting tumour tissue [Citation20].
In vitro cell uptake and the cytotoxicity of ICG-CSFNPs after NIR irradiation
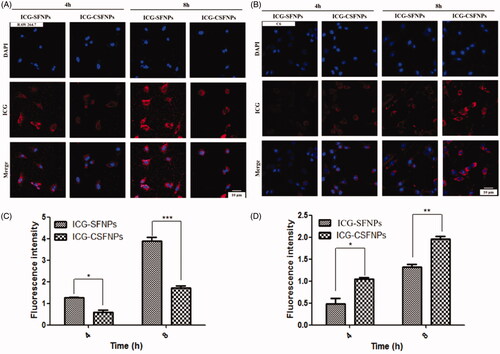
In order to investigate whether cell uptake of ICG was enhanced by ICG-CSFNPs in vitro, the cellular uptake of ICG-SFNPs and ICG-CSFNPs by both RAW264.7 and C6 cells model were conducted and compared by confocal microscopy after 4 h and 8 h of incubation. Results were exhibited in . Interestingly, the internalization of ICG-CSFNPs by RAW264.7 was significantly inhibited in comparison with that of the unstable ICG-SFNPs, regardless of the incubation time of 4 h or 8 h (). These might be due to the stealthy potential of SF and its stable particle size for ICG-CSFNPs. By contrast, the obvious red fluorescence of ICG was localized in the cytoplasm of glioma C6 cells treated with ICG-CSFNPs after just 4 h of incubation. Moreover, the cellular uptake of ICG-CSFNPs became more obvious as incubation time extended to 8 h. Inversely, the uptake of ICG-SFNPs by C6 cells was less obvious than that of ICG-CSFNPs (). The enhanced cellular uptake of ICG-CSFNPs by C6 cells may be due to the specific tumour cell endocytosis and the improved stability of ICG-CSFNPs [Citation17].
Figure 3. In vitro cell uptake: the cellular uptake of ICG-SFNPs and ICG-CSFNPs by Raw 264.7 cells (A and C) and C6 cells (B and D) after 4 h or 8 h incubation (***p<.001, **p<.01, *p<.05, n = 5).
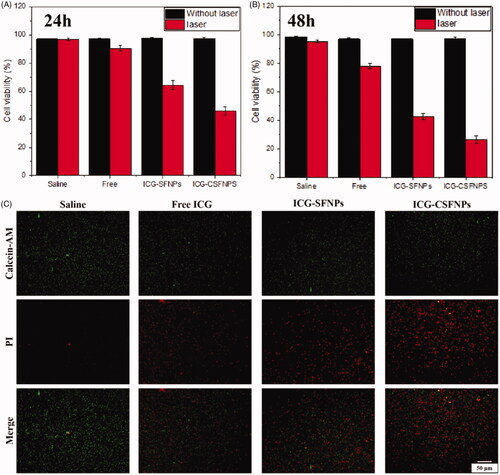
Alternatively, the effective photothermal cytotoxicity of ICG-CSFNPs against C6 cells was confirmed by MTT test. MTT results showed that ICG combined with light had obvious cell-killing effect compared to the saline control group (). Moreover, ICG-CSFNPs showed the more effective photothermal cytotoxicity than free ICG or ICG-SFNPs, probably because of their enhanced internalization inside C6 cells. The enhanced cellular uptake of ICG-CSFNPs could evidently increase intracellular ICG concentrations in C6 cells, thus significantly improving the photothermal efficiency after NIR irradiation. Meanwhile, the living/dead cells of C6 cells treated with these ICG formulations were further detected by calcein-AM/PI staining. Results were shown in . Similarly, among these treatments, ICG-CSFNPs displayed the most obvious dead cells, which indicates the effective photothermal cytotoxicity. Thus, ICG-CSFNPs combined with laser irradiation were further tested as photothermal therapy for tumour-bearing mice in vivo.
Figure 4. C6 cell viability after treatment with ICG-CSFNPs for 24 h (A) or 48 h (B) in the presence or absence of NIR irradiation; (C) calcein-AM staining of C6 cells treated with ICG-containing formulation followed by 3 min of NIR irradiation (808 nm, 1 W/cm2): viable cells were stained with green fluoresce but dead/late apoptosis cells were stained red with PI.
In vivo pharmacokinetics and distribution of ICG-CSFNPs in C6 bearing mice
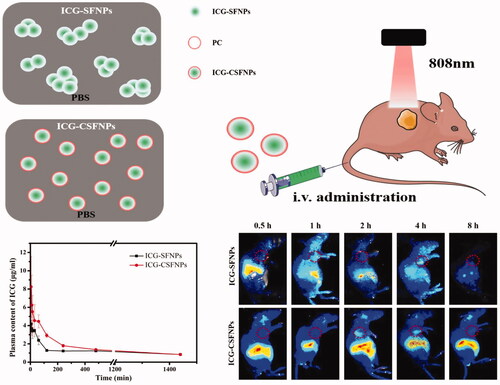
In vivo pharmacokinetic profile of ICG-CSFNPs was conducted in healthy SD rats and results were displayed in . ICG-CSFNPs showed an extending retention time in blood and a larger AUC0-t. Only within 10 min after intravenous administration, the plasma ICG centration rapidly decreased to 3.67 ± 0.48 μg/mL for ICG-SFNPs group, while it was high at 6.23 ± 3.29 μg/mL for ICG-CSFNPs group. Even at 400 min after administration, the plasma ICG centration in ICG-CSFNPs group was significantly higher than that in ICG-SFNPs group. Furthermore, AUC0-t of ICG-CSFNPs was calculated to be 2220.5 μg*min/mL, significantly larger than that of ICG-SFNPs (AUC0-t of 1726.9 μg*min/mL)
Figure 5. (A) Plasma ICG concentrations profile against time in SD rats after intravenously injected with ICG-CSFNPs or ICG-SFNPs; (B) in vivo fluorescence imaging of glioma-bearing nude mice after intravenously injected with ICG-CSFNPs or ICG-SFNPs, respectively (red circle indicated the tumour site).
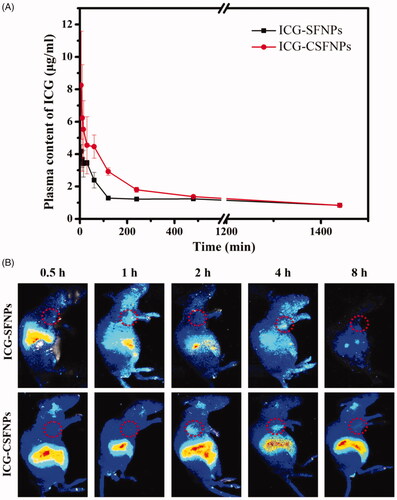
C6 glioma-bearing xenograft nude mice were constructed by subcutaneous injections to evaluate the in vivo distribution of ICG-CSFNPs. When the sizes of the tumours reached 60 mm3, the mice were intravenously injected with either ICG-SFNPs or ICG-CSFNPs and in vivo imaging was acquired at different time points. As shown in , ICG-SFNPs was rapidly distributed to the liver of the mice within 0.5 h after intravenous administration, where a strong fluorescence signal was displayed. As time extended, the fluorescence of ICG inside liver decreased gradually in vivo and almost disappeared at 8 h after administration. Hwever, there was merely no fluorescence in sight inside the tumour zone at each time points, indicating the poor distribution of ICG-SFNPs in the tumours. On the contrary, tumour tissues, as well as the liver tissues, showed strong fluorescent signals of ICG after administration with ICG-CSFNPs and the intensity became stronger with time. The fluorescence inside the tumour zone was strongest at 2 h after intravenous injection and could still be detected in the tumour site even at 4 h. This result indicated the promotion of ICG-CSFNPs retention in the tumour through the EPR effect in comparison with ICG-SFNPs. The enhancing penetration and retention effect (EPR effect) might allow ICG-CSFNPs entry into the tumour site through the blood circulation [Citation21].
In vivo photo-thermal therapy of ICG-CSFNPs
The photo-thermal effect of ICG-CSFNPs was confirmed in C6 glioma bearing nude mice by thermal imaging after NIR irradiation. The photo-thermal imaging of mice at different time point after NIR irradiation were captured and showed in . There was no obvious temperature change inside the tumour zone in the saline group even after 3 min of NIR irradiation. No significant temperature rising at tumour zone was observed within 1.5 min of NIR irradiation for free ICG group, but a little temperature elevation was in sight after 2 min of NIR irradiation, displaying the ambiguous tumour border. Interestingly, there was a fast temperature rise inside tumour zone within 0.5 min of NIR irradiation after intravenous administration of ICG-loaded nanoparticles including ICG-SFNPs and ICG-CSFNPs, indicating their fast distribution towards tumour tissues. However, the tumour border presented by thermal image for ICG-SFNPs groups was not clear and accurate to delineate the practical tumour border. These might be due to the fact that their instability in blood resulted in the limited distribution inside tumour zone by EPR effect but more distribution in normal tissue such as liver. By contrast, the temperature at the tumour site was obviously raised and the tumour border became very clear after intravenous injection of ICG-CSFNPs, which indicated its effective photothermal effect.
Figure 6. Therapeutic effect of ICG-CSFNPs for the tumour bearing mice: (A) Thermal picture of mice after NIR; (B) imaging of C6 bearing nude mice after NIR photo-thermal therapy; (C) relative tumour growth inhibition curves and survival rates (D) of the different groups.
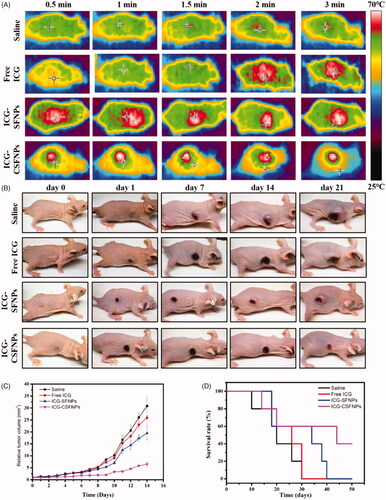
The anti-tumour efficacy of saline, free ICG solution (free ICG), ICG-SFNPs and ICG-CSFNPs was further evaluated on C6 tumour-bearing mice. The relative volume of the tumours and the survival were real-time monitored. As shown in ), the tumours grew rapidly in mice treated with saline or free ICG and all the mice in these groups died within 30 days. In contrast, the obvious inhibition of tumour growth was observed in mice treated with ICG-SFNPs or ICG-CSFNPs after NIR irradiation. However, the most obvious inhibition of tumour growth was observed after treatment with ICG-CSFNPs. The relative tumour volume in ICG-CSFNPs group slowly grew to 6.55 ± 1.03 after 2 weeks while the relative tumour volume in ICG-SFNPs group reached to 19.48 ± 2.78. Moreover, the survival rates of the mice treated with ICG-CSFNPs extended to 45 days while the survival rates of the mice treated with ICG-SFNPs was only 35 days. Furthermore, the remaining tumour tissues after 2 weeks of treatments were collected and stained by HE. Results were displayed in . The residual tumour cells were severely destroyed, presenting the shrunk cells nuclei after treatment with ICG-CSFNPs. The toxicity of these treatments against normal organs including heart, liver, spleen, lung and kidney were also evaluated by HE staining after treatment. No obvious abnormalities were found in the major organs of mice treated with ICG-CSFNPs (), which indicated its safety for in vivo application.
Conclusions
ICG-SFNPs were prepared by the silk fibroin and its physiological stability was improved by cross-linking with PC. ICG-CSFNPs showed stable particle size in physiologic medium and photothermal property after NIR irradiation. Moreover, ICG-CSFNPs showed pH-dependent and sustained release profiles in in vitro study. Because of its good stability in the PBS and DMEM medium, the internalization of ICG-CSFNPs by RAW 264.7 was significantly inhibited in comparison with ICG-SFNPs. However, cellular uptake of ICG-CSFNPs by C6 glioma cells was significantly promoted, which resulted in its stronger anti-tumour effect after NIR irradiation. Moreover, the pharmacokinetic study showed that ICG-CSFNPs had a longer blood circulation time. Meanwhile, in vivo fluorescent imaging showed that the more ICG-CSFNPs were distributed to the tumour of C6 bearing nude mice in comparison with ICG-SFNP, exhibiting stronger tumour-specific fluorescence distribution in the tumour region after intravenous injection. Furthermore, the temperature inside the tumour zone in mice treated with ICG-CSFNPs was more significantly increased than those treated with ICG-SFNPs after 808 nm NIR, leading to more effective inhibition of tumour growth. Overall, the cross-linked SFNPs may be a promising vehicle of ICG for photothermal therapy of glioma after surgical resection.
Supporting_information-2019-09-07.doc
Download ()Additional information
Funding
References
- Bush NAO, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14.
- Batash R, Asna N, Schaffer P, et al. Glioblastoma multiforme, diagnosis and treatment; recent literature review. CMC. 2017;24(27):3002–3009.
- Goode B, Mondal G, Hyun M, et al. A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat Commun. 2018;9(1):810–818.
- Eisele G, Weller M. Targeting apoptosis pathways in glioblastoma. Cancer Lett. 2013;332(2):335–345.
- Liu CG, Han YH, Zhang JT, et al. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem Eng J. 2019;370:1188–1199.
- Andreas R, Jürgen B, Rüdiger G, et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132–139.
- Han YH, Kankala RK, Wang SB, et al. Leveraging engineering of indocyanine green-encapsulated polymeric nanocomposites for biomedical applications. Nanomaterials. 2018;8(6):360–387.
- Chen BQ, Kankala RK, Chen AZ, et al. Investigation of silk fibroin nanoparticle-decorated poly(l-lactic acid) composite scaffolds for osteoblast growth and differentiation. IJN. 2017;12:1877–1890.
- Biao-Qi C, Kumar KR, Geng-Yi H, et al. Supercritical fluid-assisted fabrication of Indocyanine green-encapsulated silk fibroin nanoparticles for dual-triggered synergistic cancer therapy. ACS Biomater Sci Eng. 2018;4:3487–3497.
- Xu HL, Zhuge DL, Chen PP, et al. Silk fibroin nanoparticles dyeing indocyanine green for imaging-guided photo-thermal therapy of glioblastoma. Drug Deliv. 2018;25(1):364–375.
- Collado-González M, Montalbán MG, Peña-García J, et al. Chitosan as stabilizing agent for negatively charged nanoparticles. Carbohydr Polym. 2017;161:63–70.
- Ye T, Xuejiao J, Xin C, et al. Doxorubicin-loaded magnetic silk fibroin nanoparticles for targeted therapy of multidrug-resistant cancer. Adv Mater. 2015;26:7393–7398.
- Kim WJ, Kim BS, Cho YD, et al. Fibroin particle-supported cationic lipid layers for highly efficient intracellular protein delivery. Biomaterials. 2017;122:154–162.
- Nie X, Zhao L, Wang N, et al. Phenolics-protein interaction involved in silver carp myofibrilliar protein films with hydrolysable and condensed tannins. LWT Food Sci Technol. 2017;81:258–264.
- Zou T, Percival SS, Cheng Q, et al. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins–gelatin–chitosan nanoparticles. Eur J Pharm Biopharm. 2012;82:36–42.
- Kundu J, Chung YI, Kim YH, et al. Silk fibroin nanoparticles for cellular uptake and control release. Int J Pharm. 2010;388(1-2):242–250.
- Pengfei Z, Mingbin Z, Caixia Y, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014;35:6037–6046.
- Ma Y, Tong S, Bao G, et al. Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy. Biomaterials. 2013;34(31):7706–7714.
- Xu H, Yang D, Cai C, et al. Dual-responsive mPEG-PLGA-PGlu hybrid-core nanoparticles with a high drug loading to reverse the multidrug resistance of breast cancer: an in vitro and in vivo evaluation. Acta Biomater. 2015;16:156–168.
- Xu H, Yao Q, Cai C, et al. Amphiphilic poly(amino acid) based micelles applied to drug delivery: the in vitro and in vivo challenges and the corresponding potential strategies. J Control Release. 2015;199:84–97.
- Jiang Q, Luo Z, Men Y, et al. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials. 2017;143:29–45.