Abstract
Polycystic ovarian syndrome (PCOS) is a typical disease of female endocrine and metabolic abnormalities. miR-155, famous as a multifunctional miRNA, promotes the proliferation, migration and invasion of human cancer cells. Therefore, we aimed to explore its regulation mechanism in PCOS. BrdU incorporation and apoptosis assay were used to test KGN cell survival. Luciferase activity experiment was employed to test targeting link between miR-155 and programmed cell death 4 (PDCD4). Migration and invasion assay were operated to examine the influence of miR-155 and PDCD4 in migration and invasion of KGN cells. In addition, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay and western blot analysis were utilized to measure expression of miR-155 and other relative factors. We found that expression of miR-155 was high in PCOS patients’ tissues and it promoted proliferation, migration and invasion in KGN cells. Further studies found that PDCD4 was down-regulated by miR-155 and was a target of miR-155. Overexpression of PDCD4 promoted cell apoptosis to mitigate PCOS. Besides, up-regulation of PDCD4 suppressed PI3K/AKT and JNK signal pathways. To sum up, miR-155 promoted proliferation, migration, invasion and the activation of PI3K/AKT and JNK pathways in KGN cells through negatively regulating PDCD4.
Introduction
Polycystic ovary syndrome (PCOS) is a complicated disease caused by endocrine and metabolic abnormalities, which is common in women of childbearing age, with an incidence rate is about 5–10% [Citation1]. The main clinical manifestations are irregular menstrual cycle, infertility, hairy and haemorrhoids [Citation2]. Maybe due to the presence of viciousness permanent pathological cycle in which excess androgen favours the distribution of fat in the visceral belly and helps to increase androgen secretion from ovaries and adrenal glands [Citation3]. Resistance to insulin causes compensatory hyperinsulinemia, which has different influences in adipose tissue and androgen secretion. This is the normal characteristic of PCOS, but it is not the diagnostic criteria of PCOS [Citation4].
MicroRNAs (miRNA) are a class of small non-coding endogenous molecular with 22 nucleotides in length [Citation5]. miRNAs can silence genes through binding to 3′ untranslated region (UTR) of objective mRNA to suppress a translation process or degrading the target gene, participating in processes of gene transcription and post-transcriptional expression regulation [Citation6]. Previous studies have demonstrated that expression of many miRNAs is firmly related to the appearance and growth of human diseases [Citation7]. miRNAs act as regulators to be involved in pathological process, like cell proliferation, apoptosis, migration, invasion and the activation and inhibition of relative signal pathways [Citation8].
A lot of miRNAs were found to be up-regulated in ovaries and serum of patients with PCOS, such as miR-222, miR-146a, miR-30c, miR-21, miR-27b, miR-103 and miR-155 [Citation9–12]. Among these miRNAs, the regulatory mechanisms of other miRNAs are unknown except miR-222 [Citation4]. miR-155 is an exemplary multifunctional miRNA, which locates in chromosome 21q21 that encoded B cell integration cluster (BIC) gene [Citation13]. miR-155 stimulates cancer cells proliferation, invasion and metastasis via targeting SHIP1 [Citation14], C/EBPbeta [Citation15] and SOCS1 [Citation16]. miR-155 works as a biomarker in hyperandrogenic PCOS patients to detect influence in antiandrogen therapy [Citation17]. However, the effects of miR-155 on PCOS pathogenesis are unknown. Additionally, the important role of PI3K/AKT and JNK pathways in PCOS pathogenesis has been proved to be involved in the occurrence and growth of tumours and inflammation [Citation18,Citation19], and the inhibition of PI3K is considered as a new potential goal for PCOS’s treatment [Citation20]. Therefore, it will be interesting to explore the relationship between these pathways and miR-155 in PCOS. In this research, our purpose is to study the effects and the underlying regulation mechanism of miR-155 to provide a possible molecular target for the therapy of PCOS.
Materials and methods
Tissue sampling
The test group was lesion ovarian cortical tissues from 20 female patients who were diagnosed with PCOS, collecting between 2014 and 2015 from No.1 People’s Hospital of Jining (Jining, China). No.1 People’s Hospital of Jining ethics committee had agreed with our research. We got written informed consent from every adult so that our study could use their ovarian cortical tissues. All programmes had been consented to China Ethics Committee and were conducted on the basis of ethical standards. Our study did not include such patients with Cushing’s syndrome and androgen-secreting tumours. We got samples during laparoscopic ovarian biopsy. All patients received no treatment prior to the experiment, including no diabetes, high blood pressure or mental illness, and no oral contraceptives, cholesterol or antihypertensive drugs. Experimental procedure was to anaesthetize patients, puncture abdomen with a trocar and insert a laparoscope (diameter = 3.3 mm). Use biopsy forceps to collect ovarian cortical tissue samples from multiple locations in the ovary. Samples were quickly frozen in liquid nitrogen and then kept in −80 C to extract miRNA.
The control group was normal ovarian cortical tissues of women (n = 20) with normal menstruation and normal androgen levels. The indicators which we tested are as follows: body mass index (BMI) 25.0 kg/m2 was considered as overweight, testosterone 30.75 ng/ml was considered to be hyperandrogenemia and insulin 318.0 μU/ml was considered to be hyperinsulinism.
Cell culture
Based on the previous studies [Citation21,Citation22], human granular tumour cell line KGN (Suer, Shanghai, China) is the central cell type for androgen production in ovary. KGN cells are selected and used in this study because they are undifferentiated and keep the physiological characteristics of ovarian cells, such as expression of functional FSH receptor and activities of steroid production [Citation23,Citation24]. Besides, they display standards of detectable basal secretion of pregnenolone and progesterone (P) and show a relatively high aromatase activity [Citation21,Citation22]. KGN cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F-12 medium (Gibco, Carlsbad, CA, USA) complemented with 10% foetal bovine serum (FBS, Gibco), 100 U/ml penicillin G, and 0.1 mg/ml streptomycin sulphate (Invitrogen, Carlsbad, CA, USA) in a damp environment with 5% CO2 at 37 °C.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA from tissues and cells was extracted through Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) following manufacturer’s instruction. The manufacturer’s protocol was referred to do qRT-PCR. Using One-Step SYBR® PrimeScript® PLUS RT-RNA PCR Kit (TaKaRa Biotechnology, Dalian, China) to detect the expression level of miR-155. Besides, the SuperScript RT kit (Invitrogen) and the 7500c real-time PCR detection system (Applied Biosystems, Carlsbad, CA, USA) with SYBR premix EX Taq (TaKaRa Biotechnology) were used to detect the expression level of PDCD4. U6 and GAPDH were employed as the internal control of miRNA and mRNA, respectively. 2−△△Ct method was applied to calculate the relative expression level.
Transfection
Construct the full-length programmed cell death 4 (PDCD4) sequences with pcDNA3.1 plasmids (GenePharma, Shanghai, China), referring to pc-PDCD4. Lipofectamine 3000 reagent (Life Technologies Corporation) was employed to do transfection. Use culture medium including 0.5 mg/ml G418 (Sigma-Aldrich, St Louis, MO, USA) to select calm transfected cells. G418-resistant clones were generated after about 4 weeks. Synthesized miR-155 mimics, inhibitor and respective NC (Life Technologies Corporation) were transfected into cells. 72 h post-transfection was recognized as the harvest time.
Cell proliferation assay
About 1 × 105 cells were seeded in 60-mm dishes in duplicate. BrdU incorporation was used to detect cell proliferation through a commercially available kit (Roche, Indianapolis, IN, USA). KGN cells with relative transfection were incubated with BrdU for 6 h. Then cells were rinsed and incubated with a fluorescein isothiocynate (FITC)-labelled antibody (Abcam, Cambridge, MA) against BrdU for 30 min. Collect and analyze stained cells through a fluorescence microplate reader (Millipore, Boston, MA, USA).
Apoptosis assay
Annexin V-FITC/PI cell Apoptosis Detection Kit (BD, Franklin Lakes, NJ, USA) was used to measure apoptotic cells following instructions. CellQuest software (BD) was used to analyze apoptotic rates.
Migration and invasion
Migration assay was performed through Transwell kit from BD. Seeded cells were suspended in 200 μl serum-free medium in the top well, and 600 μl of complete medium was added to the lower part. After 6 h cultivation at 37 C, methanol was added to lower chamber to fix cells. After taking a cotton swab to remove unpassed cells from the surface of filter, cells were stained through crystal violet and counted.
KGN cells invasion assay was done by using 24-well Millicell Hanging Cell Culture inserts with 8 mm PET membranes (Millipore). Incubate 5.0 × 104 cells in 200 μl serum-free DMEM medium onto BD BioCoatTM Matrigel TM Invasion Chambers (8 μM pore size polycarbonate filters; BD). Complete medium including 10% FBS was added to the lower part. After 48 h, non-invading cells were removed and methanol was added to fix invading cells, which were then stained with crystal violet and count. Data were expressed as average number of cells linked to the bottom surface from five randomly selected regions.
Western blot
Protein was gathered through RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) complemented with protease inhibitors (Roche). BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA) was employed to quantitated protein samples. Protein sample was separated via SDS-PAGE. Then gel was transferred to the polyvinylidene fluoride (PVDF) membrane, which was blocked via a protein-free blocking solution (Sangon Biotech, Shanghai, China). Primary antibodies specific against c-Myc (ab39688, Abcam), cyclinD1 (ab16663), p53 (ab1101), p21 (ab109520), pro-caspase-3 (ab183179), cleaved-caspase-3 (ab49822), pro-caspase-9 (ab219590), cleaved-caspase-9 (ab2324), β-actin (ab6276), MMP-2 (ab92536), MMP-9 (ab73734), vimentin (ab92547), PDCD4 (ab80590), p-PI3K (ab140307), t-PI3K (ab154598), p-AKT (ab8805), t-AKT (ab179463), p-JNK (ab179461) and t-JNK (ab76125) were maintained with membrane for 10 min in the room and kept at 4 °C overnight. Then the membrane was washed and incubated with secondary antibody, marking by horseradish peroxidase at room temperature for 1 h. Next, adding 200 μl Immobilon Western Chemiluminescent HRP Substrate (Millipore) to cover the membrane surface. Finally, Image Lab™ Software (Bio-Rad, Hercules, CA, USA) was employed to get the results.
Dual-luciferase activity assay
Luciferase reporter that constructed the PDCD4 3’UTR carrying an assumed miR-155 binding site was ligated into the pMIR-Report Luciferase vector (Promega, Madison, WI). 293T cells were co-transfected with miR-155 mimic and the PDCD4-wild-type (PDCD4-wt) or PDCD4-mutated-type (PDCD4-mut) through Lipofectamine 3000 (Life Technologies Corporation). Results were got through dual-luciferase experiment system (Promega) following manufacturer’s instruction. The sequences of PDCD4-wt and PDCD4-mut are as follows: PDCD4-wt: 5′-CAAGA-UAGCAUUA-3′; PDCD4-mut: 5′- ACAGGUUAACCCGA-3′.
Statistics analysis
Every experiment was repeated for three times. Differential significance analysis was performed via Graphpad version 6.0 statistical software (GraphPad Software Inc., La Jolla, CA). For the difference significance, t test or a one-way analysis of variance (ANOVA) was employed to calculate p values. It is generally considered that a p value of less than .05 is statistically significant.
Results
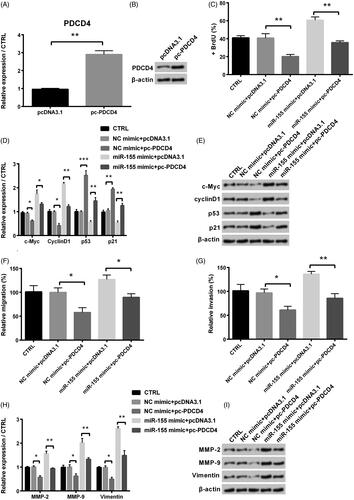
Overexpression of miR-155 occurred in PCOS tissues
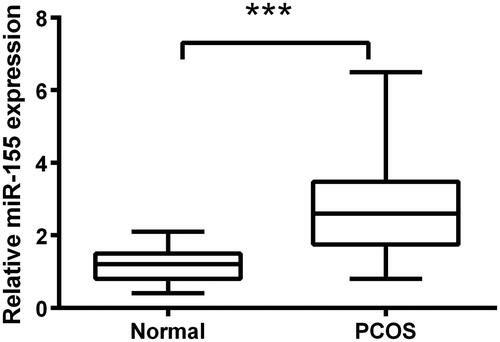
To explore the function of miR-155 in PCOS, qRT-PCR was performed to examine the expression of miR-155 in PCOS lesions ovarian cortical tissues (n = 20) and normal ovarian cortical tissues (n = 20). The mean ± SD of normal group and PCOS group were calculated through t-test that the values were 1.15 ± 0.45 and 2.79 ± 1.34, respectively. Besides, revealed the significance that the expression level of miR-155 in PCOS tissues was notably higher than that in normal tissues (p < .001).
miR-155 promoted KGN cells proliferation
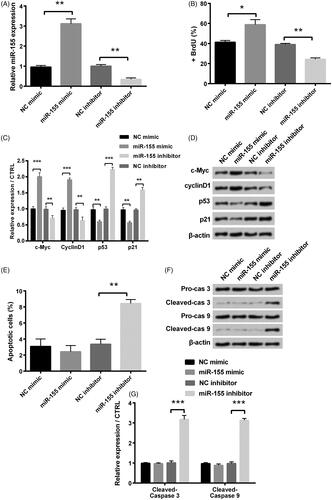
As shown in , we found that transfection with miR-155 mimic and inhibitor represented the overexpression and the suppression of miR-155, respectively. To further study, the influence of miR-155 in PCOS cells’ development, we performed BrdU incorporation to test cell proliferation. BrdU incorporation assay showed that miR-155 mimic notably enhanced cell proliferation in comparison with NC mimic (p < .05), while miR-155 inhibitor notably suppressed cell proliferation in comparison with NC inhibitor (p < .01, ). Besides, for the cell survival-related factors, levels of c-Myc and cyclinD1 were notably increased (p < .001), and levels of p53 and p21 were reduced in miR-155 mimic group (p < .01), whereas those were opposite in miR-155 inhibitor group (). In addition, flow cytometry analysis revealed that miR-155 inhibitor significantly increased the apoptosis rates in KGN cells (p < .01, ). Levels of cleaved-caspase-3 and cleaved-caspase-9 were notably raised in miR-155 inhibitor group (p < .001, ).
Figure 2. Influence of miR-155 in proliferation and apoptosis was measured in KGN cells, which were transfected with miR-155 mimic, miR-155 inhibitor and relative NC. (A) Level of miR-155 was measured via qRT-PCR. (B) Proliferation was detected via BrdU incorporation assay. (C,D) Cell survival related factors were measured via Western blot analysis. (E) Apoptosis was measured via flow cytometry. (F,G) Levels of apoptosis related factors were tested via Western blot analysis. *p < .05, **p < .01 and ***p < .001 in comparison with related NC. The data were shown as mean + SD.
miR-155 promoted KGN cells migration and invasion
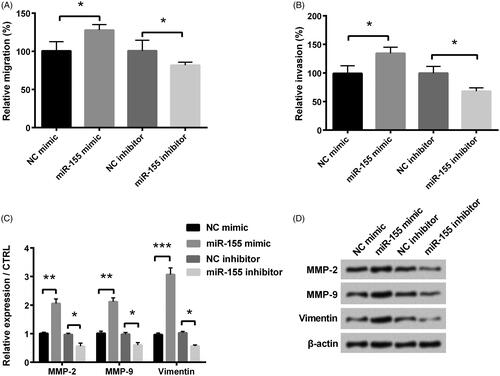
The migration and invasion experiments were used to detect the influence of miR-155 in KGN cells migration and invasion. Results revealed that miR-155 overexpression facilitated KGN cells migration and invasion in comparison with NC group (both p < .05, ). However, miR-155 knockdown inhibited KGN cells migration and invasion (both p < .05 and ). Furthermore, levels of matrix metalloproteinases (MMPs) (MMP-2 and MMP-9) and vimentin in miR-155 mimic groups were significantly higher than those in NC mimic groups (p < .01, p < .01 and p < .001), while their levels were notably reduced in miR-155 inhibitor group contrasted with those in NC inhibitor groups (all p < .05, ).
Figure 3. Influence of miR-155 in migration and invasion was measured in KGN cells, which were transfected with miR-155 mimic, miR-155 inhibitor, and relative NC. Cell migration (A) and invasion (B) were tested through a modified two-chamber migration experiment and BioCoatTM Matrigel TM Invasion Chambers, respectively. (C,D) Levels of MMPs and vimentin were examined via Western blot analysis. *p < .05, **p < .01 and ***p < .001 in comparison with related NC. The data were shown as mean + SD.
PDCD4 was the target of miR-155
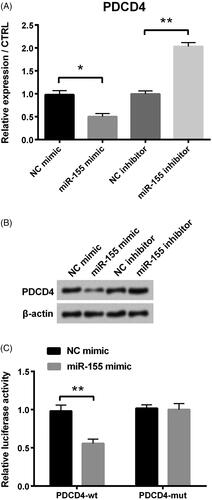
For the purpose of further studying the regulation mechanism of miR-155, we carried out qRT-PCR and western blot assays to detect regulation relationship between PDCD4 and miR-155, and dual luciferase activity assay to test whether PDCD4 could bind to miR-155. We found that PDCD4’s expression level was significantly decreased after miR-155 mimic transfection compared with negative control (p < .05, ), whereas that was significantly enhanced when miR-155 was inhibited (p < .01, ). Western blot assay revealed that expression of PDCD4 was weakened when cells were transfected with miR-155 mimic, while that was enhanced in miR-155 inhibitor group (). In addition, luciferase activity experiment revealed that luciferase activity was notably decreased in miR-155 mimic + PDCD4-wt group, while there was no difference in miR-155 mimic + PDCD4-mut group ().
Figure 4. PDCD4 was the target of miR-155 in PCOS. (A) PDCD4’s level was examined via qRT-PCR. (B) Protein level of PDCD4 was tested via Western blot. (C) Luciferase activity experiment determined target relationship between miR-155 and PDCD4. *p < .05 and **p < .01 in comparison with related NC. The data were shown as mean + SD.
Overexpression of PDCD4 inhibited KGN cells proliferation, migration and invasion
To further study, the effect of PDCD4 in process of PCOS, we conducted the following assays. Our results showed that KGN cells transfected with pc-PDCD4 had higher mRNA and protein levels of PDCD4 than those in NC group (). Cell proliferation was significantly decreased by the adding of pc-PDCD4 compared with NC (p < .01, ). Besides, levels of c-Myc and cyclinD1 were notably reduced (both p < .05), and levels of p53 and p21 were significantly increased by the adding of pc-PDCD4 (p < .001 and p < .01, ). In addition, from ,G), we found that migration and invasion of KGN cells were all significantly decreased by the adding of pc-PDCD4 (both p < .05), and the same trend was occurred for mRNA and protein levels of MMPs (MMP-2 and MMP-9) and vimentin ().
Figure 5. Influence of PDCD4 in proliferation, migration and invasion of KGN cells after co-transfection with miR-155 mimic and pc-PDCD4. (A) Level of PDCD4 was examined via qRT-PCR. (B) Protein level of PDCD4 was examined via Western blot. (C) Proliferation was detected via BrdU. (D-E) Levels of cell survival related factors were measured via Western blot analysis. Effects of PDCD4 on KGN cells’ migration (F) and invasion (G) were examined via a modified two-chamber migration experiment and BioCoatTM Matrigel TM Invasion Chambers, respectively. (H-I) Levels of MMPs and vimentin were examined via Western blot analysis. *p < .05, **p < .01 and ***p < .001 in comparison with related NC. The data were shown as mean + SD.
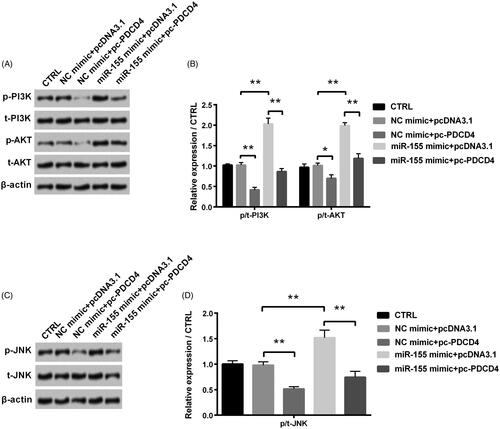
PI3K/AKT and JNK signal pathways
Further, we detected the PI3K/AKT and JNK signal pathways to study regulation mechanisms of miR-155 and PDCD4. miR-155 overexpression significantly raised levels of p-PI3K, p-AKT and p-JNK (all p < .01), while these promotion effects on p-PI3K, p-AKT and p-JNK were notably flattened by the adding of PDCD4 (all p < 0.01, ).
Figure 6. Regulation of PDCD4 and miR-155 in PI3K/AKT and JNK signal pathways was examined after co-transfection with miR-155 mimic and pc-PDCD4. (A,B) Levels of PI3K/AKT signal pathway associated proteins were tested via Western blot analysis. (C,D) Level of JNK signal pathway associated proteins was tested via Western blot analysis. *p < 0.05 and **p < 0.01 in comparison with related NC. The data were shown as mean + SD.
Discussion
PCOS, known as a syndrome of ovarian dysfunction [Citation25], occurs mostly in women of childbearing age. Many factors lead to PCOS, such as weight gain or abdominal obesity, genetic causes, familial ovulation dysfunction and ovarian polycystic changes [Citation26]. Therefore, determining the cause is a prerequisite for treatment of PCOS. In our study, miR-155 was overexpressed in PCOS patient tissues, indicating that miR-155 was involved in the pathogenesis of PCOS. Then further research found that it could promote KGN cells proliferation, migration, invasion and activate PI3K/AKT and JNK signal pathway by targeting PDCD4. These findings indicated that miR-155 played a crucial regulatory role in pathogenesis of PCOS.
miRNAs, a major scientific breakthrough [Citation27], are very important in the process of disease. Because of their stability and abundance in serum, miRNAs were used as non-invasive biomarker of PCOS [Citation25]. For example, expression of miR-21 and miR-155 might act as efficacious biomarkers for predicting poor prognosis [Citation28]. Murri et al. demonstrated a direct correlation between testosterone concentration and miR-155 expression levels and it was widely regulated between PCOS patients and healthy controls of different body mass indices [Citation11]. Consistent with our study that miR-155 was overexpressed in PCOS patient tissues, suggesting that miR-155 might act as a biomarker for monitoring PCOS’s occurrence. In addition, this is the first report of revealing the underlying functional mechanism of miR-155 in KGN cells that it promoted cells proliferation, migration, invasion and the activation of PI3K/AKT and JNK pathways by negatively regulating its target gene PDCD4. Our data may provide a new direction for the treatment of PCOS.
Previous studies showed that PDCD4 was considered as a tumour suppressor [Citation29], participating in cell apoptosis, inflammation, invasion and terminal differentiation [Citation30,Citation31]. As a regulator of apoptosis, PDCD4 played an important role through activator protein-1 (AP-1) to in cardiovascular disease [Citation32]. Besides, PDCD4 was reported to inhibit cell invasion via suppressing MMP-2 in breast cancer [Citation33]. Liu et al. found that PDCD4 was the target gene of miR-155, which inhibited proliferation and invasion in non-small cell lung cancer [Citation34]. Consistently, we also found that PDCD4 was a target of miR-155 and down-regulated by miR-155 in the process of PCOS. The suppression of PDCD4 by miR-155 contributes to the increase of KGN cells’ proliferation, migration and invasion that explains the enhanced function of miR-155 in PCOS, therefore, revealing a new miR-155/PDCD4 feedback regulatory mechanism.
Furthermore, the occurrence of disease is inseparable from the regulation of signalling pathways. PI3K/AKT signal pathway is participated in cell metastasis and firmly associated with the process of human tumours [Citation35]. Phosphorylation and activation of JNK is used to respond to various environments such as developmental and inflammatory stimulation [Citation36]. It has been reported that up-regulation of miR-155 elevates proliferation of nasopharyngeal carcinoma cells by positively targeting PI3K/AKT pathway [Citation37]. Here, we firstly found that miR-155 mimic could promote the arousal of PI3K/AKT and JNK pathways to enhance PCOS growth. Moreover, previous studies reported that PI3K/AKT signal pathway could constitutively restrain PDCD4 expression in acute myelocytic leukaemia [Citation38]. And PDCD4 could inhibit the phosphorylation of c-Jun at amino-acid residues, which are phosphorylated by JNK pathway [Citation39]. These findings revealed that PDCD4 restrained PI3K/AKT and JNK pathway. Consistently, we found that levels of p-PI3K, p-AKT and p-JNK were notably reduced in pc-PDCD4 group, suggesting the negative relationship between PDCD4 and PI3K/AKT and JNK pathways. Additionally, for the first time, we built up the regulatory mechanism among miR-155, PDCD4 and these pathways that miR-155 promoted the activation of these pathways through down-regulating PDCD4 in KGN cells. These findings may further provide theoretical basis for the clinical application of miR-155 in monitoring and treating PCOS in the future.
In conclusion, this study firstly demonstrated the regulation mechanism of miR-155 in PCOS. miR-155 was important in promoting the pathogenesis of PCOS that it promoted KGN cells proliferation, migration, invasion and the activation of PI3K/AKT and JNK signal pathways by negatively regulating PDCD4. This research provides a possible target for clinical treatment of PCOS.
Author contributions
Huanjun Xia and Yaxian Zhao: conception of the work; Huanjun Xia: collection of data and analysis of data; Huanjun Xia: writing of manuscript; Yaxian Zhao: revise of the article.
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure statement
The authors declare no conflict of interest.
References
- Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European society of endocrinology. Eur J Endocrinol. 2014;171(4):P1–29.
- Yildiz BO. Approach to the patient: contraception in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(3):794–802.
- Gonzalez F, Sia CL, Shepard MK, et al. Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(11):4071–4079.
- Sorensen AE, Wissing ML, Salo S, et al. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes. 2014;5(3):684–708.
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826.
- Murri M, Insenser M, Fernandez-Duran E, et al. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): effects of obesity and sex hormones. Metabolism. 2018;86:49–60.
- Cortez MA, Bueso-Ramos C, Ferdin J, et al. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477.
- Lao G, Liu P, Wu Q, et al. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumor Biol. 2014;35(12):11933–11938.
- Long W, Zhao C, Ji C, et al. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell Physiol Biochem. 2014;33(5):1304–1315.
- Sirotkin AV, Ovcharenko D, Grossmann R, et al. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219(2):415–420.
- Murri M, Insenser M, Fernandez-Duran E, et al. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab. 2013;98(11):E1835–E1844.
- Sirotkin AV, Laukova M, Ovcharenko D, et al. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol. 2010;223(1):49–56.
- Tam W. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene. 2001;274(1–2):157–167.
- Pedersen IM, Otero D, Kao E, et al. Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol Med. 2009;1(5):288–295.
- Costinean S, Sandhu SK, Pedersen IM, et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood. 2009;114(7):1374–1382.
- Jiang S, Zhang HW, Lu MH, et al. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70(8):3119–3127.
- Arancio W, Calogero Amato M, Magliozzo M, et al. Serum miRNAs in women affected by hyperandrogenic polycystic ovary syndrome: the potential role of miR-155 as a biomarker for monitoring the estroprogestinic treatment. Gynecol Endocrinol. 2018;34(8):704–708.
- Villavicencio A, Goyeneche A, Telleria C, et al. Involvement of Akt, Ras and cell cycle regulators in the potential development of endometrial hyperplasia in women with polycystic ovarian syndrome. Gynecol Oncol. 2009;115(1):102–107.
- Wu Y, Li P, Zhang D, et al. Metformin and pioglitazone combination therapy ameliorate polycystic ovary syndrome through AMPK/PI3K/JNK pathway. Exp Ther Med. 2018;15(2):2120–2127.
- Shah KN, Patel SS. Phosphatidylinositide 3-kinase inhibition: a new potential target for the treatment of polycystic ovarian syndrome. Pharm Biol. 2016;54(6):975–983.
- Huang X, Liu C, Hao C, et al. Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2016;151(6):643–655.
- Xiang Y, Song Y, Li Y, et al. miR-483 is down-regulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1). Med Sci Monit. 2016;22:3383–3393.
- Jiang L, Huang J, Li L, et al. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2015;100(5):E729–E738.
- Nishi Y, Yanase T, Mu Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–445.
- Song J, Luo S, Li SW. miRNA-592 is downregulated and may target LHCGR in polycystic ovary syndrome patients. Reprod Biol. 2015;15(4):229–237.
- Goodarzi MO, Dumesic DA, Chazenbalk G, et al. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233.
- Shibuya H, Iinuma H, Shimada R, et al. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79(3–4):313–320.
- Lankat-Buttgereit B, Goke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell. 2009;101(6):309–317.
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11(2):141–147.
- Rodrigues PM, Afonso MB, Simao AL, et al. Inhibition of NF-kappaB by deoxycholic acid induces miR-21/PDCD4-dependent hepatocellular apoptosis. Sci Rep. 2015;5(1):17528.
- Liu X, Cheng Y, Yang J, et al. An essential role of PDCD4 in vascular smooth muscle cell apoptosis and proliferation: implications for vascular disease. Am J Physiol Cell Physiol. 2010;298(6):C1481–C1488.
- Matsuhashi S, Hamajima H, Xia J, et al. Control of a tumor suppressor PDCD4: Degradation mechanisms of the protein in hepatocellular carcinoma cells. Cell Signal. 2014;26(3):603–610.
- Liu F, Song D, Wu Y, et al. MiR-155 inhibits proliferation and invasion by directly targeting PDCD4 in non-small cell lung cancer. Thorac Cancer. 2017;8(6):613–619.
- Chien CS, Shen KH, Huang JS, et al. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333(1–2):169–180.
- Lue H, Dewor M, Leng L, et al. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal. 2011;23(1):135–144.
- Zuo WN, Zhu H, Li LP, et al. MiR-155 promotes proliferation and inhibits apoptosis of nasopharyngeal carcinoma cells through targeting PTEN-PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(18):7935–7942.
- Hao Y, Huang J, Ma Y, et al. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol Lett. 2018;15(6):8223–8230.
- Bitomsky N, Bohm M, Klempnauer KH. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene. 2004;23(45):7484–7493.