?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study is focussed on evaluating and comparing two mediators of osteoclast, osteoprotegerin (OPG) and nuclear factor-κB ligand (RANKL), in plasma and tissue levels in patients with steroid-induced osteonecrosis of femoral head (SIONFH). Subjects were included in this cross-sectional case-control study in 2016. Bone histomorphology, immunohistochemistry, Western blotting, OPG and RANKL plasma levels, post-hoc statistical power and receiver-operating characteristic (ROC) curves were evaluated. Eighty-six patients diagnosed with SIONFH and 51 healthy subjects were included. OPG expression levels in bone samples increased with ARCO stage, and RANKL expression levels decreased with ARCO stages. Plasma OPG and RANKL levels were significantly higher in the SIONFH group compared with the healthy control group. The plasma OPG level and ratio of OPG and RANKL were positively associated with ARCO stages and significantly higher in stages III and IV. Plasma RANKL levels were negatively associated with ARCO stage and were significantly higher in ARCO stages II and III. Plasma OPG and RANKL may represent potential biomarkers during SIONFH at different stages. Higher plasma OPG levels indicated late-stage SIONFH, and higher plasma RANKL levels indicated early stage. Our findings may provide a clue for the development of diagnostic tools and therapies for SIONFH.
Introduction
Osteonecrosis of femoral head (ONFH), which is an increasing worldwide health problem, is a serious joint disorder [Citation1,Citation2]. Steroids, which can cause death of the dynamic component of bone, are the most common cause of ONFH [Citation3]. ONFH involves progressive changes in hip structures initiated by various risk factors, including high-dose corticosteroids, which have been confirmed [Citation4]. Although the aetiology of ONFH is unclear, abnormal bone metabolism has played an intensive role in the progression of the disease accompanied with clinical symptoms, including severe pain and a limping gait. The features, including head collapse, lesion size, presence and/or degree of head depression, and acetabular involvement, can be observed from radiographic evaluation [Citation5]. Common staging and classification systems are obtained from the Association Research Circulation Osseous (ARCO) [Citation6].
Recently, efforts had been made to identify biomarkers in ONFH [Citation7–9]. Osteoprotegerin (OPG) can inhibit differentiation of osteoclast cells and mediate the activity of osteoclast [Citation10]. Given the special role of OPG gene in osteoclastogenesis and remodelling, previous studies have also identified OPG gene polymorphisms associated with multiple cancers, vertebral fractures and bone mineral density (BMD) [Citation11]. RANKL can interact with a receptor on osteoclast precursors called receptor activator of NF-kappa B (RANK) and the interaction results in activation, differentiation of the osteoclast lineage so that they can start the process of resorption as well as prolonging osteoclast survival by suppressing apoptosis [Citation12–15]. RANKL can specifically bind to OPG; enhance differentiation of bone marrow cells into osteoclasts in an in vitro osteoclast coculture system, which can be inhibited by OPG; and activates mature osteoclasts to resorb bone both in vitro and in vivo [Citation16]. An effort was made to apply laboratory biomarkers for diagnosis, but the result was disappointing [Citation17]. The repaired mechanism of ONFH may function as temporally active osteoclasts in necrotic zone at the early stage. However, the persistent activation of osteoclasts may lead to the breakage of trabecular and subchondral bone [Citation18]. We hypothesized that OPG and RANKL in plasma may be associated with patients’ severity in SIONFH. In this study, we aimed to compare tissue and plasma OPG and RANKL levels in patients with SIONFH with healthy individuals to determine the correlation between plasma OPG and RANKL concentrations and radiographic disease severity as well as other clinical features to assess the roles of these proteins.
Materials and methods
Study population
This was a prospective clinical control study. All of the patients were previously diagnosed with SIONFH based on clinical evidence and radiological data (Supplementary materials). A total of 86 SIONFH patients in case groups were recruited from May 2016 to November 2016 from the First Affiliated Hospital of Guangzhou University of Chinese Medicine. Fifty-one healthy subjects were recruited as control group.
Inclusion and exclusion criteria
The inclusion criteria were patients between 17 and 70 years and ARCO stage II, III or IV SIONFH. The exclusion criteria were history of trauma; patients with a history of previous operation, including core decompression, bone grafting and proximal femoral osteotomy in the affected hip; patients who underwent any previous potential treatments for avascular necrosis in the affected hip; or patients taking medications. The control group included healthy subjects without abnormal bone metabolism and hip pain and anteroposterior and frog-leg lateral pelvic radiographs from these patients did not show any lesions. Necrotic bone samples were collected after total hip arthroplasty (THA) (n = 16 total, 5 in Stage II, 5 in Stage III, 6 in Stage IV, respectively). Bone samples in the control group (n = 6 total) from femoral neck fractures were also collected during the same period. Bone samples were harvested from healthy tissue or subchondral necrotic zone at 1–4 mm below the cartilage.
Clinical data, demographic information and radiographic progression assessment
The demographic information was collected before any treatment was performed. The stage and type of the osteonecrosis was classified according to the ARCO stage [Citation6,Citation19]. Staging is based on AP and frog-leg views of the femoral head on X-ray images. Clinical features, such as the length of disease history and ages, were collected to identify any potential relation with plasma OPG and RANKL levels. All of the participants signed an informed consent agreement. The First affiliate Hospital of Guangzhou University of Chinese Medicine Human Research Committee for Approval of Research Involving Human Subjects approved the use of humans in this study.
The normal AP and frog-leg X-ray radiographs were performed for each patient. The severity of radiographic progression of FHN was evaluated by the ARCO stage system as follows [Citation6]:
Stage II: diffuse or localized areas of sclerosis and/or lucencies in the femoral head.
Stage III: characterized by the crescent sign (subchondral fracture) or collapse and fracture involving the articular surface along with segmental flattening of the femoral head but no acetabular involvement.
Stage IV: characterized by the development of acetabular changes and advanced degenerative changes of the joint. The stage used for analysis was the higher of the two hips.
Patients with Stage ≥ II disease were enrolled in this study. The results of radiographs were read and evaluated by two experienced radiologists in our hospital who were blinded to the selection of subjects.
H&E staining of bone tissue
Bone samples from necrotic areas and normal samples from healthy control femoral heads were harvested and fixed in 4% formaldehyde for at least 24 h at room temperature. Samples were embedded in paraffin wax after being decalcified in EDTA (10%) for at least 4 weeks and dehydrated. Specimens were cut into 5-μm sections (Leica 2135, Wetzlar, Germany) and stained by haematoxylin and eosin (H&E). Images were observed and captured using a microscope (BX53, Olympus Corp., Tokyo, Japan). Ten images of HE staining from each sample were randomly chosen, and the ratio of empty lacunae (empty lacunae/total lacunae) were calculated.
Micro-CT scan
Micro-CT analyses were carried out through a high-resolution device, SkyScan 1176 (Bruker, Billerica, MA). The coronal plane of the bone samples was placed in the chamber of a micro-CT set with the following parameters: resolution 9 μm, combined aluminium and copper filter and rotation step 0.4°. Metrological analysis and 3D reconstruction were performed by CT-vox (Bruker, Kontich, Belgium). The microarchitecture parameters of trabecular bone, such as bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were calculated by CTAn (CTAn, Skyscan, Kontich, Belgium).
Immunohistochemistry for OPG and RANKL
Immunohistochemistry was performed using standard procedures. Five micron-thick tissue sections were baked in a 60 °C oven for 30 min, deparaffinized in xylene and rehydrated in a descending series of ethanol in distilled water for 2 min. The tissue sections were treated with Tris-buffered saline and tween for 30 min at 37 °C. After quenching the endogenous peroxidase activity and blocking the non-specific binding, the sections were incubated overnight at 4° with the rabbit anti-human OPG antibody (Zen BioScience, Chengdu, China) or the rabbit anti-human receptor activator of nuclear factor-κ B ligand (RANKL) antibody (Zen BioScience, Chengdu, China). Appropriate secondary biotinylated antibody was applied for 30 min at room temperature. The sections were counter-stained with haematoxylin and cover with mounting medium, observed under microscope (BX53, Olympus Corp., Tokyo, Japan). The integrated optical density (IOD) values were quantified using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD).
Western blotting for OPG and RANKL
Samples were subjected to agarose-SDS-PAGE, transferred to PVDF membranes, blocked (3% bovine serum albumin (BSA) in PBS), and detected with specific rabbit anti-human he rabbit anti-human OPG antibody (Zen BioScience, Chengdu, China) or the rabbit anti-human RANKL antibody (Zen BioScience, Chengdu, China) as the primary antibody and anti-rabbit immunoglobulins (Dako Cytomation, Glostrup Denmark) as horseradish peroxidase-conjugated secondary antibodies. Semi-quantitative analysis of the enhanced chemiluminescence (ECL) western blot detection system was used to visualize particular proteins.
Enzyme-linked immunosorbent assay to determine the protein levels of OPG and RANKL
Venous blood samples collected from the patients and stored at −80 °C until the day of measurement. Detection of OPG and RANKL in plasma was performed by sandwich enzyme-linked immunosorbent assay (ELISA) by a commercially available test kit according to the manufacturer’s protocol (Human OPG ELISA Kit, Human soluble receptor activator of nuclear factor-kB ligand, sRANKL ELISA Kit Cusabio Biotech Co, Wuhan, Hubei Province, PR China). Both OPG and RANKL were used to generate a linear standard calibration curve (range 7.8–500 pg/ml). The manufacturer-reported precision was <8% (intra-assay) and <10% (inter-assay). The sensitivity of this assay was 1.95 pg/ml.
Post-hoc statistical power calculation
Power analysis and sample size (PASS) 2008 Statistical Software (Kaysville, UT) was used to calculate the statistic power (1–β). The formula according to the obtained data of different mean levels, standard error and enrolled numbers of patients in each group was written below. Statistic power was regarded strong when >0.8. The ratio between the sample sizes of the two groups is,
Formulas to compute sample size and power, respectively:
κ=nA/nB is the matching ratio; σ is standard deviation; Φ is the standard; normal distribution function; Φ − 1 is the standard normal quantile function; α is Type I error; β is Type II error, meaning 1−β is power.
Statistical analysis
All values are expressed as the mean ± standard error of the mean, unless otherwise indicated. Group comparisons were performed using Student’s t-test (two-sample test) or one-way analysis of variance (one-way ANOVA) and then LSD. A Mann–Whitney U test was used when the variance was not normally distributed. p < .05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL).
Results
Demography of cases and radiographic progression assessment in two groups
In total, 86 patients were included in the case group with a mean age of 43.9 ± 12.7 years. The control group included 51 patients, and the mean age was 39.1 ± 11.9 years. No significant difference in age was identified (Student’s T test, p > .05). There were 59 males and 27 females in the case group and 32 males and 19 females in the control group. Regarding the ARCO stages, 21 patients were ARCO Stage II, 39 patients were ARCO Stage III and 26 patients were ARCO Stage IV.
Radiographic and H&E staining of bone tissue
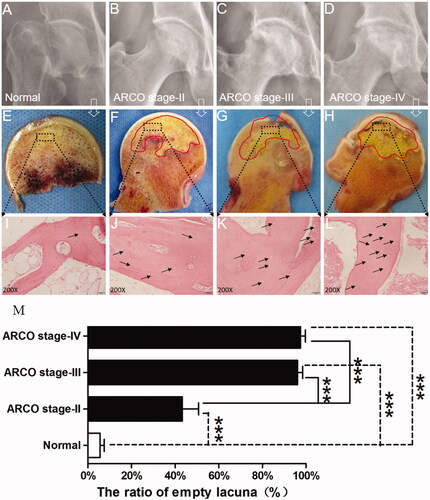
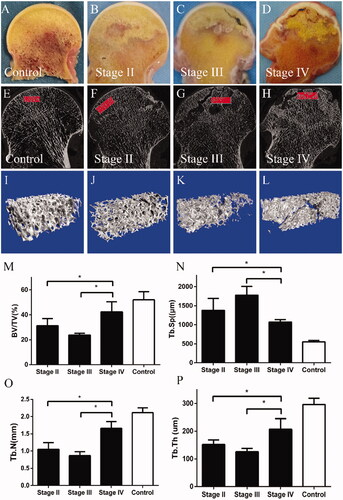
D) presents the radiographic results from the SIONFH group and control group. depicts a spherical femoral head and normal joint gap. presents a typical Stage II necrotic femoral head characterized by no crescent sign but the presence of sclerosis, osteolysis and focal porosis. shows a necrotic femoral head with a flattening articular surface indicative of stage III. General appearances of femoral head sections are shown in H). No pathological sign was found in . shows the femoral head does not collapse but a clear necrotic area could be found in the femoral head (red line). reveals an obvious subchondral bone fracture with a relatively rough cartilaginous surface. shows that the cartilage structure becomes thinner, deteriorated and severely destroyed. HE staining images are shown in L). presents a healthy trabecular, and the lacunae are filled with osteocytes. L) shows that osteocytes were absent in the lacunae and the number of empty lacunae increases as the stage increases. shows that the ratio of empty lacunae in the SIONFH groups in each stage was dramatically increased compared with the control group (p < .001). The ratio of empty lacunae in stages III and IV was higher than that in Stage II (p < .001); however, no differences were found between Stage III and Stage IV (p > .05).
Figure 1. (A–D) X-ray images of control subject and SIONFH patients with different ARCO stages. Subchondral bone fracture, flattened femoral head, articular gap narrowing and cartilage destruction were closely related to ARCO stage. (E–H) Bone samples from the control group and SIONFH group with three ARCO stages. The black frame indicates that the regions were collected for further analysis. (I–L) Histopathological features of control and SIONFH bone. (M) The number of empty lacuna increased with ARCO stage and was significantly higher in the SIONFH group compared with control subjects. *** Represents p<.001.
Micro-CT scan
The results of micro-CT images and evaluation are shown in L). The structure and quantity of trabecular bone differed obviously in necrotic area in different stages. In Stage III, decreased value of BV/TV, Tb.Th and Tb.N and increased value of Tb.Sp was found. Moreover, higher mean values of BV/TV, Tb.N, Tb.Th and lower Tb.Sp than Stage II and IV indicated that the structural characteristics of trabeculeas in Stage IV were significantly changed ().
Figure 2. (A–D) General images of bone samples of control subject and SIONFH patients with different ARCO stages. (E–H) Micro-CT images of bone samples from the control group and SIONFH group with three ARCO stages. (I–L) The region of interest in bone samples from control and SIONFH bone samples. (M–P) Decreased value of BV/TV, Tb.Th and Tb.N and increased value of Tb.Sp were found in stage III. Higher mean values of BV/TV, Tb.N, Tb.Th and lower Tb.Sp than Stage II and IV indicated that the structural characteristics of trabeculeas in Stage IV were significantly changed. *Represents p<.05.
Immunohistochemistry for OPG and RANKL
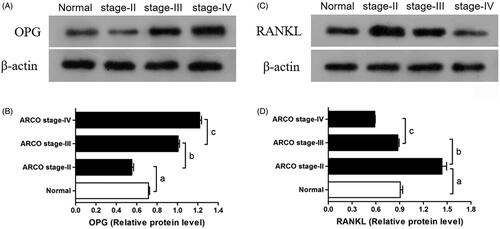
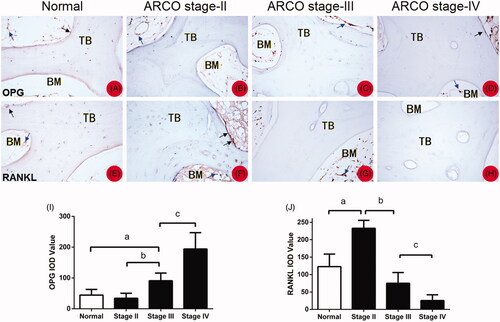
Positivity for OPG and RANKL immunohistochemical staining was assessed in both necrotic and healthy regions (). OPG was detected on the trabecular bone, osteoblast and bone marrow. OPG levels decreased as ARCO stage decreased and was accompanied by an increase in empty bone lacuna (). RANKL was also detected at similar locations. However, the amount of RANKL was decreased as ARCO stage increased (). The result is further quantified and shown in J).
Figure 3. (A–D) OPG was detected on the trabecular bone, osteoblast, and bone marrow (blue arrows). OPG levels decreased as ARCO stage decreased and was accompanied by an increase in empty bone lacuna. (E,F) RANKL was also detected at similar locations (blue arrows). The amount of RANKL was decreased as ARCO stage increased. (I,J) The IOD value of immunohistochemistry for OPG and RANKL *represents p<.05. TB: trabecular bone; BM: bone marrow.
Western blotting OPG and RANKL levels
OPG and RANKL expression in bone samples of SIONFH patients and the control group were significantly different (). Quantitative results reveal that OPG expression in bone samples from the control group was significantly higher compared with stage II SIONFH patients but lower than that from stage III and IV SIONFH patients (p < .001). OPG expression significantly increased with the ARCO stage (). RANKL expression in bone samples from the control group was significantly higher than that from Stage IV SIONFH patients, similar to that of Stage III patients and lower compared with Stage II patients (p < .001) ().
Plasma OPG and RANKL levels
Plasma OPG and RANKL levels in SIONFH patients and controls are presented in ,B). Plasma OPG and RANKL concentrations were both higher in SIONFH patients compared with controls (34.6 ± 18.7 vs. 12.0 ± 5.4 pg/ml, p < .05; 15.5 ± 21.3 vs. 2.5 ± 2.9 pg/ml, p < .05). Plasma OPG levels were 22.5 ± 9.8 pg/ml in ARCO Stage II patients, 36.7 ± 21.8 pg/ml in Stage III patients, and 41.2 ± 14.6 pg/ml in Stage IV patients (). Significant differences in plasma OPG levels were noted among the three stages (p < .05). RANKL plasma levels differ significantly among SIONFH patients at different ARCO stages (Stage II: 30.6 ± 33.9 pg/ml, Stage III: 14.4 ± 16.3 pg/ml, stage IV: 6.9 ± 4.7 pg/ml, p < .05, ). Patients with collapsed femoral heads had higher plasma OPG levels but lower RANKL levels compared with those with pre-collapsed femorad heads (22.5 ± 9.8 vs. 38.5 ± 19.2 pg/ml, p < .05; 30.7 ± 33.9 vs. 11.3 ± 13.3 pg/ml, p < .05, ). Significant differences in OPG/RANKL ratios were noted among the SIONFH groups and the ratios increased as stage increased (Stage II: 2.1 ± 2.3, Stage III: 7.1 ± 7.9, Stage IV: 9.8 ± 7.1, p <.05, , ).
Figure 5. (A–D) Overview of plasma OPG and RANKL levels in the SIONFH patients and control group. (A,B) OPG and RANKL levels in the SIONFH group were markedly increased compared with the control group. (C) OPG levels in Stage III and IV SIONFH patients were increased compared with controls and the Stage II group. (D) RANKL levels in Stage II and III SIONFH patients were increased compared with controls and the Stage II group. (E,F) OPG/RANKL level in the femoral head collapsed groups were significantly different compared to pre-collapsed group. (G) OPG/RANKL ratios generally increased.*Represents p<.05.
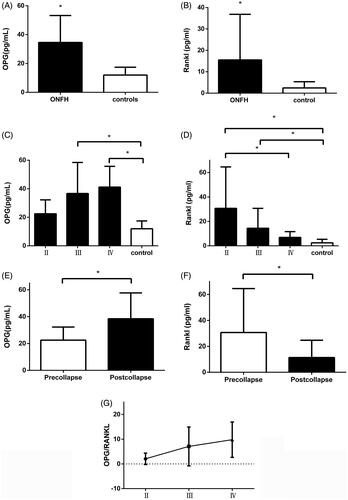
Table 1. Levels of plasma OPG and RANKL in SIONFH patients and control subjects and relation between ARCO stages.
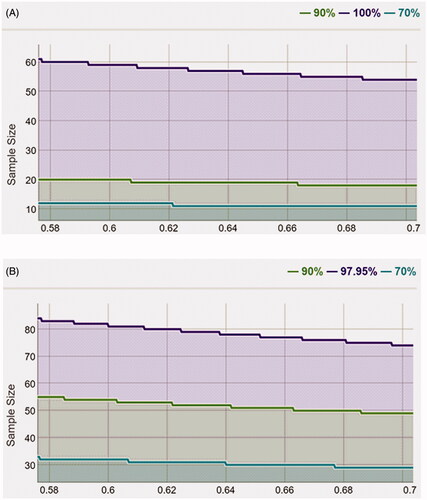
Statistical power
After calculation, the statistical power of OPG was 0.9, suggesting that the sample size of 86 and the sampling ratio of 0.64 were sufficient to obtain the conclusion (). The statistical power of RANKL was 0.98, suggesting that the sample size of 86 and the sampling ratio of 0.64 were sufficient to obtain the conclusion ().
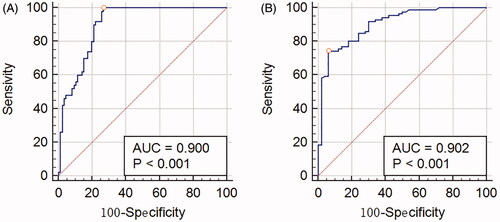
ROC curve analysis
ROC curve analysis was performed to investigate the predictive value of plasma OPG and RANKL levels for ONFH patients. Area under curve (AUC) of plasma OPG was 0.9 (p < .001, ) and RANKL was 0.902 (p < .001, ). The results demonstrate plasma OPG and RANKL show predictive value for diagnosis of ONFH.
Discussion
The purpose of this study was to reveal the relationship between OPG/RANKL and the occurrence of SIONFH as well as disease progression in local bone tissue and plasma. In plasma, both OPG and RANKL levels were significantly increased in the SIONFH group. The increased plasma OPG level was related to higher ARCO stages (Stages III and IV) and the decreased RANKL level was related to lower ARCO stages (Stages II and III). OPG and RANKL expression in necrotic bone samples were similar to trends noted in plasma levels. Moreover, result from MicroCT suggested that the microstructure properties of trabeculae in necrotic area were significantly changed during the progression of ONFH.
X-ray, general view and HE staining results revealed that SIONFH characteristics included osteocyte apoptosis and an increased ratio of empty lacunae. Higher ratios of empty lacunae were revealed as SIONFH progressed, demonstrating that the bone samples objectively reflect the pathological changes.
The pathogenesis of SIONFH is complex and remains to be fully elucidated. Several molecular pathways (such as adenosine monophosphate kinase (AMPK), βcatenin/Wnt, OPG/RANKL/RANK) determine the balance between osteoclasts and osteoblasts [Citation20,Citation21]. Bone metabolism requires a precise relationship between bone resorption and formation. Osteoblasts, which are derived from marrow mesenchymal stem cells, play a critical role in the synthesis, secretion and mineralisation of bone matrix. Osteoclasts, which are derived from the mononuclear precursors of haematopoietic stem cells, are multinucleated cells that are responsible for bone resorption [Citation22]. The functions and numbers of osteoblasts and osteoclasts are regulated by different factors and pathways that maintain the balance of bone formation and resorption [Citation23,Citation24]. We believe that changes in osteoblast and osteoclast activity play an important role in SIONFH progression and the occurrence of femoral head collapse. Cao et al. reported that osteoclast increased significantly in the necrotic region while osteoblast increased significantly in the sclerotic region. Due to bone resorption overactivity, the mechanical properties and structure of trabecular bone changes significantly [Citation25]. Combined with the continuous load pressure and stress, the subchondral trabecular bone eventually generates a large number of microfractures when the pressure is greater than the bone trabeculae can withstand [Citation26]. The RANKL/RANK/OPG pathway is a key loop regulator of osteoblast and osteoclast differentiation and bone resorption, thus preventing bone loss and ensuring the renewal of bone [Citation27,Citation28]. To our knowledge, this study is the first to identify the relation between SIONFH and OPG and RANKL levels in tissue and plasma, especially in different stages of SIONFH. OPG is a member of the family of soluble TNF receptors that is produced by osteoblasts and binds RANKL to prevent it from binding to RANK on osteoclasts and its subsequent activation [Citation29]. Previous studies report that there was a trend but not statistically significant elevation of mRNA levels of OPG in necrotic tissues compared to normal tissues. However, the expression of RANKL was significantly elevated [Citation30,Citation31]. We have reported a different result compared to previous studies. One of the most possible reasons may be the tissues we collected for testing. We have harvested necrotic tissue from different stages because the bone metabolism in necrotic zone is not constant. In this study, immunohistochemistry and Western blot results demonstrated that OPG expression levels decreased in the early stages of SIONFH but increased in the late stages. In addition, RANKL expression increased in the early stages of SIONFH but decreased in the late stages. This result suggested that osteoblast and osteoclast activity changed significantly during the development of SIONFH in necrotic bone tissue in necrotic femoral heads. Osteoclast activity was increased dramatically in the early stages of SIONFH. This high osteoclastic level potentially resulted in changes in the mechanical properties and structure of the trabeculae in the necrotic region [Citation25]. On the other hand, repair of the necrotic region also occurred as the disease progressed. New blood vessels grew into the necrotic region from the edge of the sclerotic band between necrotic region and normal bone tissue, and new bone formation required breaking the sequestrum [Citation22]. Osteogenesis occurs in the necrotic region only if bone resorption occurs. However, this procedure could be time-consuming, and it is possible that the necrotic region was not completely repaired before the femoral head collapses. Approximately, three months are required to build new bone with good mechanical performance, whereas the osteoclastic process requires only approximately 3 weeks to weaken the bone structure [Citation22]. Eventually, given the reduced macromechanical strength of femoral heads due to whole and repeated loading, a large region and number of microfractures occurred, causing the femoral heads to collapse. Once a collapsed femoral head is observed in X-rays, the disease has progressed to Stage III. Due to continued osteogenic activities in the centre of necrotic region, OPG levels increased and RANKL levels decreased as SIONFH progressed. Additionally, the results of micro-CT also revealed that a higher osteoclastic level in Stage II and III could weaken the bone microstructure in trabecular scale, which might eventually causing the femoral heads to collapse. Even though the microstructure properties could not be completely recovered to healthy level, trabecular quantity increased as bone reconstruction took place in Stage IV.
Plasma OPG and RANKL levels were similar to protein expression levels in local bone samples. SIONFH patients with ARCO III and IV had significantly higher plasma OPG levels compared with Stage II patients and the control group. Higher plasma OPG levels in late stages may result from necrotic region repair. This result indicates that plasma OPG may represent a potential biomarker for late-stage SIONFH. Patients with ARCO II and III had a significantly higher plasma RANKL levels compared with Stage IV patients and the control group. This finding indirectly illustrated that increased plasma RANKL levels are related to the occurrence of SIONFH and might act as a predictive factor for SIONFH diagnosis. The ratio of OPG and RANKL increased as SIONFH progressed, and relevant analysis results also revealed that the ratio of OPG and RANKL was positively related to ARCO stage.
Many studies have focussed on OPG/RANKL expression in bone samples from different regions but not in the progression of SIONFH. Samara S et al. showed that the OPG mRNA levels were higher in the necrotic areas compared with the normal site; however, the results were not significant. Moreover, OPG protein levels showed no remarkable divergence [Citation17]. A similar result was noted in another study from Samara S et al. [Citation31]. Western blotting analysis was performed to quantify OPG protein levels in different parts of the necrotic femoral head, including necrotic bone tissues, breakage of cartilage and normal tissues. According to both studies mentioned above, OPG protein levels were not significantly different. However, the findings were not compared with normal femoral heads, and the sample was not divided according to different stages. Xiong et al. indicated that OPG mRNA levels are higher but not significantly different in necrotic tissue compared with normal tissue [Citation32]. Patients with chronic autoimmune diseases, such as glomerulonephritis and systemic lupus erythematosus, are administered high dosages of steroids, but whether the various dosages of glucocorticoid can affect plasma OPG concentrations remains unknown.
There are some limitations in this study. First, the control group was not matched by steroid consumption, only age and sex. Second, even RANKL levels were higher in early stages of SIONFH, but we did not observe elevated levels of OPG in Stage II. Thus, it is difficult to predict early-stage SIONFH only using OPG. Second, OPG and RANKL levels in other joint diseases, such as rheumatoid arthritis, should be compared. Third, a relatively small sample limits the accuracy of the study. Despite these limitations, our study first demonstrated that plasma OPG and RANKL levels were abnormal in patients at certain stages. These findings suggest that plasma OPG and RANKL levels are indicative of disease progression. Fourth, our research did not include Stage I SIONFH. ARCO Stage I was difficult to observe unless MRI was used, while the high cost of MRI and the asymptomatic morbidity also made it difficult to include patients at this stage.
In conclusion, our results indicated that plasma OPG and RANKL may represent potential biomarkers during different stages of SIONFH. Higher plasma OPG levels indicated late-stage SIONFH, and higher plasma RANKL levels indicated early stage. Our findings may provide a clue for the development of SIONFH diagnosis and therapies. However, further experiments, including those evaluating Stage I patients, are need to investigate OPG and RANKL levels in the pathogenesis of SIONFH.
Conclusion
Plasma OPG and RANKL may represent potential biomarkers during SIONFH at different stages. Higher plasma OPG levels indicated late-stage SIONFH, and higher plasma RANKL levels indicated early stage. Our findings may provide a clue for the development of diagnostic tools and therapies for SIONFH.
supplemental.2.tif
Download TIFF Image (1.3 MB)supplement_data_1.xlsx
Download MS Excel (13.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yu K, Tan H, Xu Y. [Research progress of experimental animal models of avascular necrosis of femoral head]. Zhon Xiu Chong Jian Wai Zhi. 2015;29(12):1564–1569.
- Kubo T, Ueshima K, Saito M, et al. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci. 2016;21(4):407–413.
- Liu L, Gao F, Sun W, et al. Investigating clinical failure of core decompression with autologous bone marrow mononuclear cells grafting for the treatment of non-traumatic osteonecrosis of the femoral head. Int Orthop. 2018;42(7):1575–1583.
- Griffith JF, Antonio GE, Kumta SM, et al. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235(1):168–175.
- Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am Vol. 2010;92(12):2165–2170.
- Mont MA, Marulanda GA, Jones LC, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(3):16–26.
- He M, Gong SD, Chen XJ, et al. Plasma C-terminal cross-linking telopeptide of type II collagen as a biomarker in advanced stages of femoral head osteonecrosis. Biomed Pharmacother. 2019;111:1213–1220.
- Gong SD, Chen XJ, Chen ZQ, et al. Elevated plasma cartilage oligomeric matrix protein (COMP) level are associated with the progression of non-traumatic osteonecrosis of femoral head. Clin Chim Acta. 2019;490:214–221.
- Chen XJ, Yang F, Chen ZQ, et al. Association of reduced sclerostin expression with collapse process in patients with osteonecrosis of the femoral head. Int Orthop. 2018;42(7):1675–1682.
- Zhou X, Yishake M, Li J, et al. Genetic susceptibility to prosthetic joint infection following total joint arthroplasty: a systematic review. Gene. 2015;563(1):76–82.
- Bonfa AC, Seguro LP, Caparbo V, et al. RANKL and OPG gene polymorphisms: associations with vertebral fractures and bone mineral density in premenopausal systemic lupus erythematosus. Osteoporos Int. 2015;26(5):1563–1571.
- Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos Int. 2000;11(11):905–913.
- Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5(6):618–625.
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176.
- Wada T, Nakashima T, Hiroshi N, et al. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25.
- Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–150.
- Rushbrook JP. The effects of alcohol in orthopaedic patients. Orthopaed Trauma. 2013;27:7.
- Celik A, Tekis D, Saglam F, et al. Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant Proc. 2006;38(2):512–516.
- Sugano N, Atsumi T, Ohzono K, et al. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7(5):601–605.
- Floerkemeier T, Hirsch S, Budde S, et al. Bone turnover markers failed to predict the occurrence of osteonecrosis of the femoral head: a preliminary study. J Clin Lab Anal. 2012;26(2):55–60.
- Rathinavelu S, Guidry-Elizondo C, Banu J. Molecular modulation of osteoblasts and osteoclasts in type 2 diabetes. J Diabetes Res. 2018;2018:6354787.
- Wang C, Wang X, Xu XL, et al. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLOS One. 2014;9(5):e96361.
- Boyan BD, Schwartz Z, Lohmann CH, et al. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21(4):638–647.
- Perez-Amodio S, Beertsen W, Everts V. (Pre-)osteoclasts induce retraction of osteoblasts before their fusion to osteoclasts. J Bone Miner Res. 2004;19(10):1722–1731.
- Cao HJ, Zheng LZ, Wang N, et al. Src blockage by siRNA inhibits VEGF-induced vascular hyperpemeability and osteoclast activity – an in vitro mechanism study for preventing destructive repair of osteonecrosis. Bone. 2015;74:58–68.
- Kim HKW, Aruwajoye O, Du J, et al. Local administration of bone morphogenetic protein-2 and bisphosphonate during non-weight-bearing treatment of ischemic osteonecrosis of the femoral head: an experimental investigation in immature pigs. J Bone Joint Surg Am Vol. 2014;96(18):1515–1524.
- Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97(2):105–114.
- Sung B, Prasad S, Yadav VR, et al. RANKL signaling and osteoclastogenesis is negatively regulated by cardamonin. PLOS One. 2013;8(5):e64118.
- Mandelin J, Li TF, Liljestrom M, et al. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. J Bone Joint Surg Br Vol. 2003;85-B(8):1196–1201.
- Miao Q, Hao S, Li H, et al. Expression of osteoprotegerin, RNAK and RANKL genes in femoral head avascular necrosis and related signaling pathway. Int J Clin Exp Pathol. 2015;8(9):10460–10467.
- Samara S, Dailiana Z, Chassanidis C, et al. Expression profile of osteoprotegerin, RANK and RANKL genes in the femoral head of patients with avascular necrosis. Exp Mol Pathol. 2014;96(1):9–14.
- Xiong MY, Liu LQ, Liu SQ, et al. Effects of osteoprotegerin, RANK and RANKL on bone destruction and collapse in avascular necrosis femoral head. Am J Translat Res. 2016;8(7):3133–3140.