Abstract
Bacillus subtilis is a Gram-positive probiotic bacterium that successfully colonises plant roots due to its ability to utilise various sugars. The vast probiotic potential of B. subtilis has been recently demonstrated in numerous host organisms under different environmental conditions. We examined the probiotic potential of B. subtilis against the pathogenic bacterium Streptococcus mutans, which is involved in various oral disorders due to its robust biofilm-forming capability. B. subtilis cells attenuated biofilm formation by S. mutans during their dual growth in the presence of sugar alcohols. Transcription of genes encoding key enzymes in the metabolism of sugar alcohols by B. subtilis were highly induced. Moreover, growth-curve analysis suggested that B. subtilis is more efficient at early utilising sugar alcohols than S. mutans, as supported by the bacterial metabolic activity rates. Similarly, a comparison of secondary metabolites of mono and mixed cultures of B. subtilis and S. mutans indicated that B. subtilis is more active metabolically in the dual culture. Finally, knock-out mutations of the genes encoding key enzymes in the central metabolic pathway significantly reduced B. subtilis' ability to mitigate biofilm formation by S. mutans. We conclude that effective metabolism of sugar alcohols by B. subtilis reinforces the probiotic potential of this bacterium against pathogenic species such as S. mutans.
Introduction
Bacillus species have been used as probiotics for at least 50 years, and scientific interest in characterising this potential was sparked about 15 years ago [Citation1–3]. Bacillus subtilis has two major advantages as a probiotic bacterium. First, in-vitro and in-vivo assessments have found it to be safe in food supplements [Citation4]. Second, B. subtilis forms dormant and highly resistant endospores in response to harsh environmental conditions. Thus the bacterium remains in a dormant state under various environmental stresses without any deleterious effect on its viability [Citation2,Citation5,Citation6].
Probiotics have been associated mainly with gastrointestinal tract health. However, recent studies have suggested the benefits of probiotic bacteria for oral health as well [Citation7–9]. Probiotic bacteria can be administered to the oral cavity to achieve their probiotic effect through their consumption in food for long or short periods [Citation10,Citation11], or in tablet [Citation12], chewing gum [Citation13,Citation14] or mouthwash form [Citation15]. The most prevalent oral disorder—dental caries, which is caused mainly due to oral biofilm-forming bacteria, has also been shown to be highly dependent on diet [Citation16].
One of the most cariogenic bacteria in the oral cavity is Streptococcus mutans [Citation17]. S. mutans can adhere to dental surfaces using adhesion-like proteins. After the initial adhesion, S. mutans secretes enzymes producing extracellular polysaccharides (EPS), which are considered important for further bacterial adhesion and acceleration of biofilm formation [Citation16,Citation18]. S. mutans accumulation as a dental biofilm is the result of the bacteria's self-adhesion mechanisms, but is also highly dependent on dietary components. The correlation between sucrose, biofilm formation and caries has been well documented in the literature [Citation19,Citation20]. Sucrose increases biofilm biomass, since it serves as a substrate for EPS production [Citation19], and its fermentation by cariogenic bacteria generates organic acids [Citation20]. One approach to controlling biofilm formation and the development of caries is replacement of sucrose with sugar alcohols [Citation21]. Nonetheless, the frequent use of sugar alcohols in various products has been shown to result in bacterial adaptation [Citation22]. That leads to insufficient decrease in biofilm biomass and adhesiveness of bacterial plaque or the growth of mutans streptococci [Citation23]. Moreover, it was shown that sugar alcohols induce an expression of the biofilm-related genes in S. mutans [Citation24].
In the current study, we examined the probiotic potential of B. subtilis against the cariogenic bacterium S. mutans. It was hypothesized that the efficiency of B. subtilis in metabolising sugar alcohols could affect biofilm formation and, presumably, cariogenicity associated with S. mutans.
Materials and methods
Bacterial strains and growth media
Cultures of S. mutans strain UA159 were grown overnight in brain heart infusion (BHI) broth (Acumedia, Lansing, MI) at 37 °C in 95% air/5% CO2. B. subtilis wild-type (WT) strain NCIB3610 and mutant strains were routinely maintained in Lysogeny broth (LB, Neogen, Lansing, MI). Two carbohydrate-metabolism mutant strains of B. subtilis: ΔgutB (BKK06150) and ΔmtlD (BKK03990), were obtained from the Bacillus Genetic Stock Centre (Ohio State University, Columbus, OH). To transfer these mutations to the NCIB3610 background, SPP1 phage transduction was performed as described previously [Citation25]. To ensure that the mutant cells could not metabolise the sugar alcohols, cells were grown in M9 medium [Citation26] supplemented with either sorbitol or mannitol. Growth curves were compared to those of the WT as well as to growth with glucose (Figure S1). To generate starter cultures, one colony of B. subtilis from a fresh LB agar plate was grown as a suspension in LB by incubating at 37 °C/150 rpm for 5 h. All experiments were conducted with bacteria towards the late exponential phase.
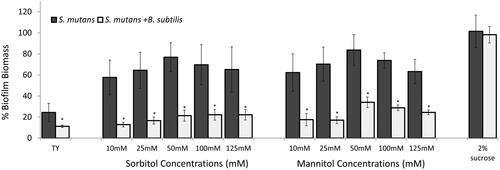
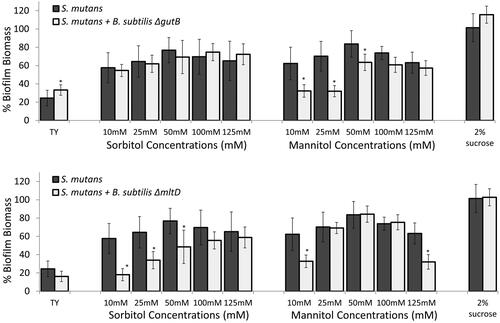
Figure 1. B. subtilis mitigates biofilm formation by S. mutans in the presence of sorbitol and mannitol.
S. mutans cells were grown with or without B. subtilis (dual- and mono-species suspension, respectively) in TY medium supplemented with different concentrations of either sorbitol or mannitol. TY medium alone and TY with 2% sucrose served as positive and negative controls, respectively. Mono- and multi-species suspensions of the bacterial cells were incubated at 37 °C in 95% air/5% CO2 for 24 h. Biofilm biomass was quantified by the Crystal Violet staining. Data are displayed as relative biofilm biomass compared to the biomass of S. mutans biofilm formed in TY supplemented with 2% sucrose (set to 100%). Data present the means ± SD from at least three independent biological experiments, each performed in triplicate. *p <.01 compared to the S. mutans mono-species biofilm at the same sugar concentration.
All experiments were conducted in either TY medium (1.4% w/v tryptone and 0.8% w/v yeast extract (Acumedia) [Citation27,Citation28] or M9 minimal medium (M9 salts, per L: 12.8 g Na2HPO4·7H2O, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl). The final working medium consisted of adding micronutrient components to the M9 salts at final concentrations of: 1 mM MgSO4, 100 µM CaCl2, 3 × 10−9 M (NH4)6Mo7O24·4H2O, 4 × 10−7 M H3BO3, 3 × 10−8 M CoCl2·6H2O, 1 × 10−8 M CuSO4·5H2O, 8 × 10−8 M MnCl2·4H2O, 1 × 10−8 M ZnSO4·7H2O and 1 × 10−6 M FeSO4·7H2O.
Mono- and Dual-Species biofilm formation
For B. subtilis mono-species biofilm, a starter culture (generated as described above) was diluted 1:100 (to a final OD600nm ∼ 0.07) in TY or TY supplemented with either 2% (w/v) sucrose (∼58 mM) or sorbitol or mannitol at various final concentrations (10, 25, 50, 100 or 125 mM) in a 96-well plate (Nunc, Roskilde, Denmark). The plate was incubated at 37 °C in 95% air/5% CO2 for 24 h.
For S. mutans mono-species biofilm, overnight cultured S. mutans (OD600 nm ∼ 1) was diluted 1:10 in either TY or TY supplemented with 2% sucrose (controls), or TY supplemented with different concentrations of sorbitol or mannitol (10, 25, 50, 100 or 125 mM) in a 96-well plate. The plate was incubated at 37 °C in 95% air/5% CO2 for 24 h.
For the dual-species biofilm, a starter culture of S. mutans (diluted 1:10, approximately 2.5 × 107 CFU) and B. subtilis (diluted 1:100, approximately 2.5 × 105 CFU) was added to fresh TY or TY supplemented with either 2% sucrose (control), or sorbitol or mannitol at different concentrations (10, 25, 50, 100 or 125 mM). The ratio of B. subtilis to S. mutans cells seeded to obtain the dual-species biofilm was approximately 1:100. The plate was incubated at 37 °C in 95% air/5% CO2 for 24 h [Citation29].
Quantification of biofilm biomass using crystal violet (CV) staining
The generated submerged biofilms were carefully washed twice with saline solution. The biofilms were stained with 0.1% (w/v) CV (Merck, Darmstadt, Germany) solution as described previously [Citation30,Citation31]. Briefly, following 20 min of incubation with the tested biofilm, the CV was washed away twice with saline and the stained biofilms were dried overnight at room temperature (RT). Next, 33% acetic acid was added to elute the CV for 30 min while shaking at RT. The extract was placed in a 96-well plate and OD595 nm was measured in a plate reader (Infinite PRO2000, Neotec Scientific Instrumentation Ltd. Camspec, Cambridge, UK) [Citation30,Citation31].
Growth-Curve analysis
For the growth-curve analyses, B. subtilis and S. mutans cultures were grown as described above, and diluted (1:100 and 1:10, respectively) in TY or TY supplemented with ether 2% sucrose or different concentrations of sorbitol or mannitol (10, 25, 50, 100 or 125 mM). When needed, the bacteria were diluted in M9 medium with 0.2% (w/v) glucose or 50 mM sorbitol or mannitol. The cultures were then incubated at 37 °C overnight with slight shaking. Growth was recorded every hour at OD595 nm in the plate reader.
Gene expression for key enzymes in the sorbitol/mannitol metabolic pathway
RNA extraction
Cultures of B. subtilis and S. mutans grown as described above were diluted (1:100 and 1:10, respectively) in TY medium alone or TY supplemented with 50 mM sorbitol or mannitol. Next, B. subtilis and S. mutans were grown, respectively, at 37 °C, 150 rpm in 95% air/5% CO2. Samples for each treatment were taken after 3, 6, 12, 18 and 24 h.
A 1-ml aliquot of RNAprotect (Qiagen, Hilden, Germany) was added to each sample and incubated at room temperature for 5 min. The cells were then centrifuged at 4,000 g for 10 min. RNA was extracted with the GenUP Total RNA Kit (biotechrabbit, Hennigsdorf, Germany) according to the manufacturer’s protocol. RNA purity and concentration were determined using a Nanodrop (NanoVue, GE Healthcare Life Science, Buckinghamshire, UK).
Reverse transcription and Real-Time PCR
Reverse transcription was performed using a qScript cDNA Synthesis Kit (Quantabio, Beverly, MA). The synthesised cDNA was used for real-time PCR analysis of key enzyme-encoding genes in the metabolic pathways of S. mutans and B. subtilis (sorbitol dehydrogenase (gutB) and mannitol-1-phosphate 5-dehydrogenase (mtlD)). RT-PCR was performed as described previously [Citation32]. Briefly, the reaction mixture (20 µL) contained 1X SYBR Green (Invitrogen, Carlsbad, CA), 1 µL cDNA sample and 25 µM of the appropriate forward and reverse PCR primers (). PCR conditions included an initial denaturation at 95 °C for 10 min, followed by 40 cycles of amplification, consisting of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. Residual genomic DNA contamination was determined from control reactions devoid of reverse transcriptase. The expression levels of all genes tested by real-time PCR compared to the expression in TY medium supplemented with either sorbitol or mannitol vs. TY medium alone. The relative expressions were normalised using the 16S rRNA gene of S. mutans as an internal standard [Citation33] or the sigA gene for B. subtilis.
Table 1. Real-time PCR primers used in this study.
Metabolic activity
Microbial metabolic activity by MTT assay
The assay to measure cell-proliferation rate was performed as described previously with some modifications [Citation34]. Briefly, B. subtilis or S. mutans cultures were grown as described above, and diluted (1:100 or 1:10, respectively) in TY or M9 supplemented with 50 mM sorbitol or mannitol. The bacterial cultures were grown at 37 °C in 95% air/5% CO2. Samples for each treatment were taken at 3, 6, 9, 12, 18 and 24 h of incubation and placed in a 96-well plate. To determine planktonic cell viability, 20 μL of 100 mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) was added to each well. The plate was incubated for 1 h at 37 °C. The MTT dissolved by adding 20 μL DMSO to each well for 15 min while shaking at RT. Absorbance values were measured at 570 nm.
Secondary metabolite analysis
B. subtilis and S. mutans starter cultures were diluted in TY supplemented with 50 mM sorbitol (1:100 and 1:10, respectively) in mono-species cultures and were grown together in dual-species culture at the same cell ratios. All cultures were incubated at 37 °C in 95% air/5% CO2 for 6 h. Metabolite extraction for GC–MS was performed as described by Urbanczyk-Wochniak et al. [Citation35] with some modifications. A 2-ml aliquot of each culture medium was centrifuged at 4000 g for 10 min and passed through a 0.45-µm filter (MillexGV, Merck). The samples were immediately frozen in liquid nitrogen, lyophilised and kept at −80 °C until use. The samples were then homogenised using a ball mill precooled with liquid nitrogen and extracted in 700 µL methanol; 30 µL internal standard (0.2 mg ribitol/mL water) was subsequently added for quantification. Extraction, derivatization, standard addition, and sample injection were exactly as described previously [Citation36]. The GC–MS system was comprised of an Agilent 7693 autosampler, Agilent J&W DB-35ms column, Agilent 7200B gas chromatograph, and quadrupole time-of-flight (TOF) MS with removable electron-ionization source. Metabolites were identified in comparison to commercial standard compounds purchased from Sigma-Aldrich (St. Louis, MO). Chromatograms and mass spectra were evaluated using MassHunter Data Analysis by Agilent, quantitative and qualitative analysis (TOF). A list of the identified secondary metabolites is provided in the Supplementary Table S1.
Statistical analysis
The data were statistically analysed by t-test. All applied tests were two-tailed, and p ≤ 0.05 was considered statistically significant. For the metabolomics assay, principal component analysis (PCA) was conducted. Statistical analysis was performed using the Analyse-it add-in tool for Microsoft Excel software.
Results
B. subtilis mitigates biofilm formation by S. mutans in the presence of sugar alcohols
The initial phenotypic observations in this study demonstrated the ability of B. subtilis cells to reduce biofilm formation by S. mutans in the presence of the sugar alcohols - sorbitol and mannitol. TY medium was selected as a rich medium without external addition of glucose in order to evaluate the effect of the added sugar. Enhanced biofilm formation by S. mutans (compared to the TY control) in the presence of either sorbitol or mannitol during mono-species cultivation was suppressed upon addition of B. subtilis cells to the system. For instance, the biofilm biomass of S. mutans was significantly reduced, by more than 50%, at all tested concentrations of the sugar alcohols compared to the S. mutans mono-culture biofilm at the same concentrations ( and ). However, in the presence of sucrose (a non-alcohol sugar), B. subtilis cells had no effect on the S. mutans biofilm biomass in the mixed culture. Importantly, B. subtilis monoculture did not adhere to the surface and could not form a submerged biofilm (Figure S2).
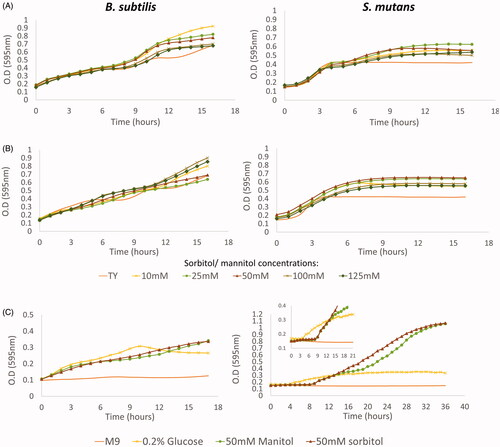
Figure 2. B. subtilis and S. mutans growth curves in the presence of sorbitol and mannitol. The bacteria were incubated at 37 °C. O.D (595nnm) measurements were taken every 1 h. A. S. mutans or B. subtilis cells were grown in TY medium supplemented with different concentrations of sorbitol, TY medium alone served as control. B. S. mutans or B. subtilis cells were grown in TY medium supplemented with different concentrations of mannitol, TY medium alone served as control. C. B. subtilis and S. mutans cells were grown in M9 minimal medium or M9 supplemented with 0.2% glucose, 50mM sorbitol or 50 mM mannitol. In both media, B. subtilis cells entered immediately to the log stage in all tested sugar concentrations. However, S. mutans had a long lag phase in M9 in the presence of either sorbitol or mannitol. The data are the mean values of two independent experiments, each formed in duplicate.
B. subtilis grows better than S. mutans in the presence of sugar alcohols
Next, we examined the growth dynamics of the tested bacterial species in the presence of sugar alcohols. Growth-curve analysis indicated that B. subtilis cells actively move to the log phase, whereas S. mutans cells required a relatively prolonged lag phase in the presence of either sorbitol () or mannitol ().
Since TY medium is considered a rich nutrient medium, we decided to check the bacterial growth in M9 medium (a minimal medium that enables controlling the type of carbon source and its amount) (). Growth-curve analysis confirmed that B. subtilis cells can grow immediately in the presence of either sorbitol or mannitol, even in minimal medium (). Moreover, B. subtilis growth required the addition of a carbon source to M9 medium (). Conversely, the cells of S. mutans could entered the log phase faster in the presence of glucose compared to sorbitol or mannitol as the sole carbon source which required a long lag phase.
Enrichment of growth media with sugar alcohols up-regulates genes encoding key enzymes in cellular metabolism
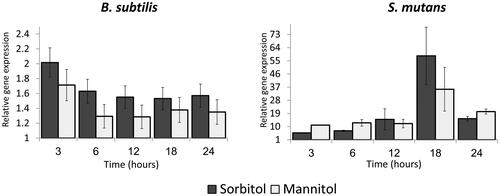
Differences between the abilities of Bacillus and Streptococcus cells to grow in the presence of different sugars raised a question about the regulation of key enzymes involved in sorbitol or mannitol metabolism. Therefore, we tracked the regulation profile of genes encoding sorbitol dehydrogenase (gutB), which catalyses the oxidation of sorbitol to fructose [Citation37], and mannitol-1-phosphate 5-dehydrogenase (mtlD), which catalyses the oxidation of mannitol 1-phosphate to fructose 6-phosphate [Citation38] (). The effect on these genes' expression was determined by comparison to the same growth time point in TY medium alone. Both genes, gutB and mtlD, were up-regulated in the presence of sorbitol and mannitol (respectively) compared to TY medium alone (the expression value for the TY control was adjusted to 1). The induction occurred in the early stages of bacterial growth after addition of either sugar to the medium. For B. subtilis, maximal induction was reached at 3 h of growth—2-fold for gutB (in the presence of sorbitol) and 1.6-fold for mtlD (in the presence of mannitol) (). From 6 h onward, induction was similar and stable for gutB (around 1.6-fold) and for mtlD (around 1.2-fold) (). In contrast, the regulation pattern of both genes differed in S. mutans. Although upregulation of their expression was observed after 3 h of incubation (), maximal upregulation occurred only 18 h after incubation in the presence of the sugar alcohols, with moderate induction thereafter (). Our results thus indicate diverse regulation profiles of the key metabolism enzymes in the tested bacteria.
Figure 3. Relative expression of gutB and mtlD genes during bacterial growth in the presence of sugar alcohols. B. subtilis or S. mutans cells were grown in TY medium with or without 50 mM of either sorbitol or mannitol. At each time point, a sample was taken for RNA extraction and relative gene expression was determined by real-time RT-PCR. In both bacteria, the genes were up-regulated in the presence of the sugars; up-regulation was lower in B. subtilis than in S. mutans. Expression results present mean ± SD of two independent experiments each performed in triplicate.
B. subtilis cells metabolize sugar alcohols more effectively than do S. mutans cells
Since S. mutans cells could not grow in the presence of sugar alcohols in minimal medium for 16 h, but we could nevertheless detect gene-expression upregulation for the metabolic enzymes, we hypothesised that although there is transcription of the gene, there might be a delay in the translation, leading to the low metabolic activity in S. mutans. To test this hypothesis, we estimated the metabolic activity of the bacteria by MTT assay. The metabolic activities of B. subtilis and S. mutans increased during 9 h of incubation in the presence of the sugars, with no significant difference between the bacteria ( and ). However, MTT not only reflects the metabolic activity of the bacteria; it can also be influenced by the number of viable cells [Citation39]. Therefore, we repeated the assay in M9 after 6 h of growth when CFU counts where similar in both of the bacteria (). In M9, B. subtilis cells exhibit a significantly higher metabolic activity in both of the sugar alcohols. Importantly, in M9 media containing sorbitol or mannitol, S. mutans cells were alive and dividing, albeit at a slower rate than the B. subtilis cells that started less and grew extensively (Figure S4).
Figure 4. B. subtilis demonstrates extend potential to metabolise sorbitol and mannitol compare to S. mutans in earlier stages of growth. A. B. subtilis or S. mutans cells were grown in TY medium with 50mM sorbitol (A1) or mannitol (A2). At each time point, a sample was taken and the culture metabolic activity was determine using the MTT assay. Although the metabolic activity of B. subtilis was higher than S. mutans in all time points, the difference wad not significant. Results represent mean ± SD of two independent experiments each performed in triplicate. B. B. subtilis or S. mutans cells were grown in M9 medium with 50mM or 100mM sorbitol or mannitol. After 6 h the culture metabolic activity was determine using MTT assay. The metabolic activity of B. subtilis was significantly higher than S. mutans in the presence of sorbitol or mannitol. results represent mean ± SD of two independent experiments each performed in triplicate. C. B. subtilis or S. mutans cells were grown alone or in a co-culture in TY medium with 50mM sorbitol. After 6 h growth, a sample of a media was collected for secondary metabolomics analysis using GCMS. The secondary metabolic profile of B. subtilis is more similar to the profile of the mixed culture than S. mutans secondary metabolic profile. The results represent PCA analysis of three biological experiments each performed in duplicate.
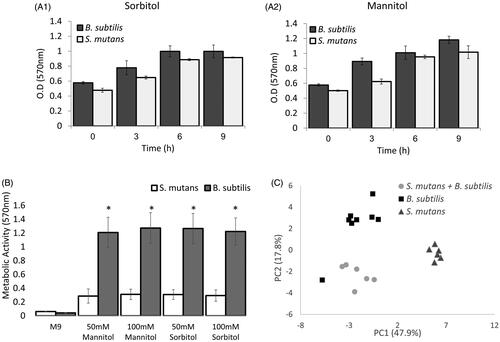
Lastly, to assess the metabolic activities of B. subtilis and S. mutans in a dual-species culture in the presence of sorbitol, we performed a secondary metabolic analysis using GC–MS on the growth media of each bacterium alone and that of the dual-species culture. To compare the secondary metabolites identified in the three groups, PCA analysis was conducted () [Citation40]. The scatter plot shows that the metabolite profiles of B. subtilis and the mixed culture were more alike than the metabolite profile of S. mutans mono-culture. Although the GC-MS results suggested that B. subtilis is more active metabolically in the dual culture, it seems thatS. mutans retainssome metabolic activity.
Metabolic effectiveness of B. subtilis cells at early stages of growth has an important role in mitigating biofilm formation
According to our results, it is conceivable that B. subtilis is better able to utilise the sugar alcohols due to early expression of the key enzymes sorbitol dehydrogenase and mannitol-1-phosphate 5-dehydrogenase (encoded by gutB and mtlD, respectively). To prove this, we tested the ability of B. subtilis ΔgutB and ΔmtlD knock-out mutants to mitigate biofilm formation by S. mutans in the presence of either sorbitol or mannitol. The inhibitory effect of the B. subtilis mutant cells on biofilm formation by S. mutans was notably reduced in the presence of either sorbitol or mannitol (at most of the concentrations) (). Interestingly, the deletion mutations also had an effect in the presence of the other sugar (i.e. ΔgutB in the presence of mannitol and ΔmtlD in the presence of sorbitol) at high concentrations, where the reduction in biofilm biomass in the dual-species culture was lower or absent (). Importantly, the deletion mutations did not affect B. subtilis growth (Figure S5).
Figure 5. Key enzymes for metabolising either sorbitol or mannitol are required for mitigating S. mutans biofilm. S. mutans cells were grown with or without B. subtilis mutant cells (ΔgutB or ΔmtlD) in TY medium supplemented with different concentrations of sorbitol or mannitol. TY medium alone and TY with 2% sucrose served as controls. Bacteria were incubated at 37 °C in 95% air/5% CO2 for 24 h. Biofilm biomass was quantified by Crystal Violet staining. Data are displayed as relative biofilm biomass compared to S. mutans biofilm formed in TY supplemented with 2% sucrose (set to 100%). B. subtilis mutant strains did not significantly affect biofilm biomass in either case compared to S. mutans biofilm with the related tested sugar. Data are the means ± SD of data from at least three independent biological experiments, each performed in triplicate.*p <.05 compared to S. mutans biofilm alone at the same sugar concentration.
Discussion
We developed a unique system to explore the potential role of B. subtilis against the cariogenic bacterium S. mutans. Our key finding was potent anti-biofilm activity of B. subtilis cells against the cariogenic bacterium S. mutans in the presence of sugar alcohols. Our results strongly indicate the superiority of B. subtilis cells in utilising sugar alcohols, sorbitol as well as mannitol, compared to S. mutans, which could explain the significant reduction in S. mutans biofilm formation during its co-culture with B. subtilis cells. Taken together, these findings further confirm the probiotic potential of B. subtilis by emphasising its metabolic superiority to human pathogens such as Streptococcus.
Since biofilm mostly represents a multispecies community, interactions between the different species are influenced by different factors, including production of antibacterial agents, metabolic requirements, and environmental conditions. Any change in one or more of these factors can dramatically affect the structure and dynamics of the biofilm community [Citation41,Citation42]. Although it has been shown that cells of B. subtilis and S. mutans are capable of forming dual-species biofilm in the presence of sucrose [Citation29], changing the carbohydrate source to sugar alcohols such as sorbitol or mannitol resulted in a significant decrease in biofilm biomass. This result indicates that although B. subtilis is capable to metabolise sucrose [Citation43], it is not competing with S. mutans on sucrose a carbon source. An evaluation of the amount of each bacterial species showed that S. mutans is the predominant bacterium in the dual-species biofilm (Figure S6). However, the DNA quantitation indicates on small amount of S. mutans cells that adhere to the surface in the presence of B. subtilis. Growth-curve analysis suggested that B. subtilis readily enters the log phase through metabolism of either sorbitol or mannitol (). However, S. mutans demonstrated a prolonged lag phase during which it adjusted to the growth medium. The ability of B. subtilis to rapidly metabolise sugar alcohols might be related to its origin as a soil bacterium and its accumulation in plant roots [Citation44–46]. In this regard, it is known that many plants produce polyhydric alcohols such as sorbitol and mannitol to control osmoregulation [Citation47]. Therefore, the ability to metabolise those sugars could present a large advantage for root-colonizing bacteria.
Further investigating the regulatory profiles of the key metabolic enzymes in the tested bacteria led us to the assumption that B. subtilis cells express those genes (at least at a basal level) even prior to their exposure to the sugars. Notably, even though there was major induction in the expression of the genes gutB and mtlD in S. mutans even after 3–6 h, the cells started to grow in minimal media with addition of sorbitol or mannitol at a very low rate which is correlated to the findings by Dills and Seno [Citation48]. The growth of S. mutans cells in TY medium containing sorbitol or mannitol is maybe due to sucrose derivatives, which has been found in GCMS analysis of the medium (), and may not be related to the presence of the sugar alcohols. These results support the findings of Brown and Wittenberger [Citation49] who demonstrated that sorbitol or mannitol dehydrogenase activities are almost completely absent in S. mutans cells grown in a mixture of sorbitol or mannitol and another carbon source. Moreover, the sorbitol transport by S. mutans is mediated by the phosphotransferase system that could be inhibited by glucose residues, which disables the sorbitol uptake [Citation48,Citation50,Citation51].
Due to the preferences of each bacterium for different types of sugar, we assume that the significant decrease in biofilm biomass in the mixed culture in the presence of sugar alcohols is a result of rapid use of these sugars by B. subtilis. Our assumption is also supported by previous studies, which found that the expression of biofilm-related genes in S. mutans in the presence of sugar alcohol is mostly related to late exponential growth phases, and that biofilm formation occurs at a later stage of growth than in the presence of sucrose [Citation24,Citation52]. Indeed, deletion mutations in sorbitol dehydrogenase (gutB) and mannitol-1-phosphate 5-dehydrogenase (mtlD) significantly reduced the inhibitory effect of B. subtilis cells on biofilm formation by S. mutans. This suggests that rapid utilisation of sugar alcohols in the mixed biofilm is responsible for this inhibitory effect. Once again, we wanted to confirm that the observed phenotype is due to a disruption in the metabolic pathway; thus, the cells were grown in TY or TY supplemented with various concentrations of sorbitol or mannitol (Figure S4). While the B. subtilis ΔgutB mutant strain grew similarly to the control in TY medium, growth of the B. subtilis ΔmtlD mutant was delayed in this medium, even in the presence of the sugar alcohols, compared to the WT strain. These results are in agreement with previous studies which found that mannitol-1-phosphate 5-dehydrogenase also cooperates with the gut operon and that mutant stains of B. subtilis in the mtlD gene are also incapable of growing on sorbitol as the only carbon source [Citation53,Citation54]. Yet, although there was a slight delay in the growth of the ΔmtlD strain, the reduction in biofilm-formation inhibition was still observed in the presence of mannitol at most concentrations, supporting our assumption that the reduction is not only due to rapid growth but also due to the rapid metabolism of sugar alcohols. However, in lower concentrations of mannitol or sorbitol (10 mM), a slight inhibition was still observed in the presence of the mutant strains of B. subtilis. A possible explanation for this observation could be that the deletion of the gene encoding to the dehydrogenase did not affect the sugar uptake. Similar result was found in high amount of mannitol but here it could resulted in lysis of B. subtilis cells which might harmed the ability of S. mutans to form biofilm [Citation55].
Overall, the results of this research indicate that B. subtilis is more effective at metabolising sugar alcohols, and therefore has an advantage relatively to S. mutans in the presence of sugar alcohols. This environmental advantage of B. subtilis resulted in mitigating S. mutans in a mixed biofilm. Therefore, B. subtilis may serve as a probiotic bacterium against the cariogenic bacterium S. mutans in products containing sorbitol or mannitol as an alternative for sucrose.
Efficiency_of_Bacillus_subtilis-_supplementary_29.3.2020.docx
Download MS Word (487.9 KB)Acknowledgments
This study forms part of Danielle Duanis-Assaf’s Ph.D. project. Danielle Duanis-Assaf is a recipient of Scholarship of Excellency for outstanding Ph.D. students from The ARO. We would like to thank Dr. Avin-Wittenburg and Ms. Hila Mizrahi from the Hebrew University for excellent assistance with GCMS measurements and analysis. The authors of the paper declare no competing interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
- Hong HA, Duc LH, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev. 2005;29(4):813–835.
- Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28(2):214–220.
- Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm. 1994;133(1):3–18.
- Hong H, Huang JM, Khaneja R, et al. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J Appl Microbiol. 2008;105(2):510–520.
- Spinosa MR, Braccini T, Ricca E, et al. On the fate of ingested Bacillus spores. Res Microbiol. 2000;151(5):361–368.
- Sanders M, Morelli L, Tompkins T. Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Comp Rev Food Sci Food Safety. 2003;2(3):101–110.
- Haukioja A. Probiotics and oral health. Eur J Dent. 2010;4(3):348–355.
- Saxelin M, Tynkkynen S, Mattila-Sandholm T, et al. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol. 2005;16(2):204–211.
- de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi:10.1007/10_2008_097
- Näse L, Hatakka K, Savilahti E, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35(6):412–420.
- Ahola A, Yli-Knuuttila H, Suomalainen T, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002;47(11):799–804.
- Caglar E, Kavaloglu Cildir S, Ergeneli S, et al. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006;64(5):314–318.
- Caglar E, Kavaloglu S, Kuscu O, et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Invest. 2007;11(4):425–429.
- Twetman S, Derawi B, Keller M, et al. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 2009;67(1):19–24.
- Tsubura S, Mizunuma H, Ishikawa S, et al. The effect of Bacillus subtilis mouth rinsing in patients with periodontitis. Eur J Clin Microbiol Infect Dis. 2009;28(11):1353–1356.
- Burne R. Oral streptococci. products of their environment. J Dent Res. 1998;77(3):445–452.
- Ajdic D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen [Research Support, U.S. Gov't, P.H.S.]. Proc Natl Acad Sci Usa. 2002;99(22):14434–14439.
- Lemos JA, Quivey RG, Jr Koo H, et al. Streptococcus mutans: a new Gram-positive paradigm? Microbiology (Reading)). 2013;159(Pt 3):436–445.
- Muñoz-Sandoval C, Muñoz-Cifuentes MJ, Giacaman RA, et al. Effect of bovine milk on Streptococcus mutans biofilm cariogenic properties and enamel and dentin demineralization. Pediatric Dentistry. 2012;34(7):197E–201E.
- Liu C, Niu Y, Zhou X, et al. Hyperosmotic response of streptococcus mutans: from microscopic physiology to transcriptomic profile. BMC Microbiol. 2013;13(1):275
- Giacaman R. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Dis. 2018;24(7):1185–1197.
- Boyd DA, Thevenot T, Gumbmann M, et al. Identification of the operon for the sorbitol (glucitol) phosphoenolpyruvate: sugar phosphotransferase system in Streptococcus mutans. Infect Immun. 2000;68(2):925–930.
- Mäkinen KK. Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med Princ Pract. 2011;20(4):303–320.
- Shemesh M, Tam A, Feldman M, et al. Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr Res. 2006;341(12):2090–2097.
- Yasbin RE, Young FE. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol. 1974;14(6):1343–1348.
- Harwood C, Cutting S. Chemically defined growth media and supplements. Chichester, UK: Wiley; 1990.
- Assaf D, Steinberg D, Shemesh M. Lactose triggers biofilm formation by Streptococcus mutans. Int Dairy J. 2015;42:51–57.
- Belli W, Marquis R. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol Immunol. 1994;9(1):29–34.
- Duanis-Assaf D, Duanis-Assaf T, Zeng G, et al. Cell wall associated protein TasA provides an initial binding component to extracellular polysaccharides in dual-species biofilm. Sci Rep. 2018;8(1):9350
- Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30(2):285–293.
- Stepanović S, Vuković D, Dakić I, et al. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179.
- Tam A, Shemesh M, Wormser U, et al. Effect of different iodine formulations on the expression and activity of Streptococcus mutans glucosyltransferase and fructosyltransferase in biofilm and planktonic environments. J Antimicrob Chemother. 2006;57(5):865–871.
- Shemesh M, Tam A, Steinberg D. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J Med Microbiol. 2007;56(Pt 11):1528–1535.
- Feldman M, Shenderovich J, Lavy E, et al. A sustained-release membrane of thiazolidinedione-8: effect on formation of a candida/bacteria mixed biofilm on hydroxyapatite in a continuous flow model. BioMed Res Int. 2017;2017:1–9.
- Urbanczyk-Wochniak E, Leisse A, Roessner-Tunali U, et al. Expression of a bacterial xylose isomerase in potato tubers results in an altered hexose composition and a consequent induction of metabolism. Plant Cell Physiol. 2003;44(12):1359–1367.
- Lisec J, Schauer N, Kopka J, et al. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc. 2006;1(1):387–396.
- Ng K, Ye R, Wu X-C, et al. Sorbitol dehydrogenase from Bacillus subtilis. Purification, characterization, and gene cloning. J Biol Chem. 1992;267(35):24989–24994.
- Horwitz SB. D-Mannitol 1-phosphate dehydrogenase and d-sorbitol dehydrogenase from Bacillus subtilis. Methods Enzymol. 1966;9:155–159. Vol. Elsevier;
- Grela E, Kozłowska J, Grabowiecka A. Current methodology of MTT assay in bacteria–A review. Acta Histochem. 2018;120(4):303–311.
- Cueva C, Moreno-Arribas MV, Martín-Álvarez PJ, et al. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol. 2010;161(5):372–382.
- Elias S, Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol Rev. 2012;36(5):990–1004.
- Burmølle M, Ren D, Bjarnsholt T, et al. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 2014;22(2):84–91.
- Fouet A, Arnaud M, Klier A, et al. Bacillus subtilis sucrose-specific enzyme II of the phosphotransferase system: expression in Escherichia coli and homology to enzymes II from enteric bacteria. Proc Natl Acad Sci Usa. 1987;84(24):8773–8777.
- Fall R, Kinsinger RF, Wheeler KA. A simple method to isolate biofilm-forming Bacillus subtilis and related species from plant roots. Syst Appl Microbiol. 2004;27(3):372–379.
- Vullo DL, Coto CE, Sineriz F. Characteristics of an inulinase produced by Bacillus subtilis 430A, a strain isolated from the rhizosphere of Vernonia herbacea (Vell Rusby). Appl Environ Microbiol. 1991;57(8):2392–2394.
- Beauregard PB, Chai Y, Vlamakis H, et al. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci Usa. 2013;110(17):E1621–E1630.
- Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008;54(No. 3):89–99.
- Dills SS, Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983;153(2):861–866.
- Brown A, Wittenberger C. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973;18(1):117–IN19.
- Slee A, Tanzer J. The repressible metabolism of sorbitol (D-glucitol) by intact cells of the oral plaque-forming bacterium Streptococcus mutans. Arch Oral Biol. 1983;28(9):839–845.
- Svensäter G. Sorbitol transport and metabolism by oral streptococci. Swed Dent J Suppl. 1991;79:1–103.
- Durso SC, Vieira L, Cruz J, et al. Sucrose substitutes affect the cariogenic potential of Streptococcus mutans biofilms. Caries Res. 2014;48(3):214–222.
- Lopez J, Thoms B. Role of sugar uptake and metabolic intermediates on catabolite repression in Bacillus subtilis. J Bacteriol. 1977;129(1):217–224.
- Watanabe S, Hamano M, Kakeshita H, et al. Mannitol-1-phosphate dehydrogenase (MtlD) is required for mannitol and glucitol assimilation in Bacillus subtilis: possible cooperation of mtl and gut operons. J Bacteriol. 2003;185(16):4816–4824.
- Heravi KM, Wenzel M, Altenbuchner J. Regulation of mtl operon promoter of Bacillus subtilis: requirements of its use in expression vectors. Microb Cell Fact. 2011;10(1):83.
