Abstract
A rapid, one-pot synthesis of eight benzo[a]furo[2,3-c]phenazine derivatives has been achieved in moderate to good yields in good yields via a multi-component of 2-hydroxynaphthalene-1,4-dione, arylglyoxal, indole (H, CH3) in the presence of H3PW12O40@Fe3O4-ZnO magnetic core-shell nanoparticles (MCNPs) under solvent-free conditions using microwave irradiation. The catalyst was synthesised and characterised by X-ray diffraction, EDX, TEM, FESEM, TGA, VSM and atomic force microscope. As an application for the synthesised nanocatalyst, degradation of methylene blue as heavy-mass organic pollution was measured. These results showed advantages for synthesis, such as mild reaction conditions, low energy consumption and economically affordable.
Graphical Abstract
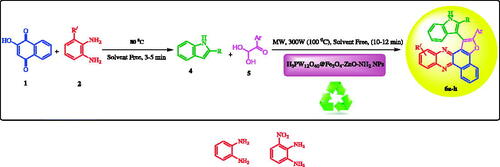
Introduction
Multi-component reactions are one of the most successful methods in increasing structural diversity and molecular complexity using a simple process [Citation1,Citation2]. This method, like an evolving process for the preparation of pharmaceutical compounds, allows the expansion of many chemical compounds, with greater structural diversity. The advantages of this method compared to other methods are the use of available raw materials, low expensive, easy working method, a wide range of goods, creating a high variety in several stages [Citation3–5].
Organic reactions under microwave radiation have attracted the attention of scientists due to their high reaction rate and clean products. Microwave-assisted reactions required short reaction times hence become a more popular route for organic chemists [Citation6–8]. Mild reaction conditions, shorter reaction time, and excellent yields are the main advantages of this method [Citation9,Citation10].
Recently, semiconductor heterogeneous photocatalysts such as TiO2 and ZnO have attracted a lot of attention due to their excellent performance in destroying organic compounds and purifying water. Among them, pure nano-ZnO has important research and application value because of its bandgap width of 3.2 eV [Citation11,Citation12]. Also, due to the recyclability of the catalyst, Fe3O4 has been considered by many researchers as a ferromagnetic material due to its good ability [Citation13,Citation14]. Based on the above study, we completed the effective combination of ZnO and Fe3O4–ZnO and designed an H3PW12O40@Fe3O4–ZnO core-shell photocatalyst with magnetic separation capability. Core-shell nanoparticles are used for medical applications, and due to their high stability, this magnetic nanoparticle can be useful for hyperthermia magnetic therapy (MHT) for the treatment of cancer [Citation15,Citation16]. Furthermore, nanomaterial applications as a heterogeneous catalyst in place of homogeneous catalysts in MCRs make the process greener due to easy handling, non-corrosiveness, high surface area, surface modification ability, excellent thermal and chemical stability, simple work-up procedures, environmentally benign nature, reusability, low cost and ease of synthesis and isolation [Citation17,Citation18]. Among them, non-toxic ZnO and ZnO nanoparticles catalyzed organic processes are often considered to follow the principles of green chemistry, that is, these catalyzed processes consume a minimum of energy and reagents or auxiliaries and minimize waste [Citation19].
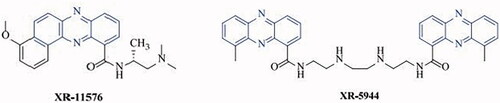
Phenazine-based compounds are nitrogen-containing heterocycles that have been illustrated to possess numerous biological functions including antimicrobial [Citation20], antimycobacterial [Citation21], antifungal [Citation22] and antitumor [Citation23] activities. Phenazines such as XR-5944 and XR-11576 () have been reported to exhibit anti-cancer effects [Citation24]. Benzo[a]phenazine derivatives are widely used in photodynamic therapy; which selectively target tumours [Citation25].
Herein, we report a very green synthesis of functionalized benzo[a]furo[2, 03-c]phenazine derivatives catalyzed by H3PW12O40@Fe3O4–ZnO magnetic core-shell nanoparticles (MCNPs) under solvent-free conditions via microwave irradiation (Scheme 1). Degradation of methylene blue was studied as a sample of organic pollutants by the core-shell structure of H3PW12O40@Fe3O4–ZnO.
Scheme 1. Synthesis of benzo[a]furo[2, 3-c]phenazine derivatives in the presence of H3PW12O40@Fe3O4-ZnO as heterogeneous catalyst.
![Scheme 1. Synthesis of benzo[a]furo[2, 3-c]phenazine derivatives in the presence of H3PW12O40@Fe3O4-ZnO as heterogeneous catalyst.](/cms/asset/97275c34-c005-45e1-98f2-e208c47190f7/ianb_a_1894163_sch0001_c.jpg)
Experimental
General
All melting points were determined on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded on a Shimadzu IR-470 spectrometer. Elemental analyses for C, H, and N were performed using a Costech ECS 4010 CHNS-O analyzer. Mass spectra were recorded on an Agilent Technology (HP) spectrometer operating at an ionization potential of 70 eV. The 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on Bruker DRX-400 Advance instruments with DMSO as a solvent. All reactions were carried out using a laboratory microwave oven (MicroSYNTH, Milestone Company, Italy). Thin-layer chromatography (TLC) was performed on silica-gel Polygram SILG/UV 254 plates.
All reagents and solvent were purchased from Merck and Aldrich and used without further purification. Scanning electron microscope (SEM) micrographs were taken using a VPLEO–Germany 1450 microscope. TEM measurements were performed on a Philips 120 CM electronic microscope. Atomic force microscope (AFM) was taken using an Easy scan 2 Nanosurf microscopes. Thermal analysis (TGA-DTA) was carried out using a Bahr STA-503 instrument at a heating rate of 10 °C min−1 in the air. Powder X-ray diffraction (XRD) patterns were obtained on an STOE diffract metre using Cu K radiation (=0.15418 nm).
Preparation of nano-Fe3O4
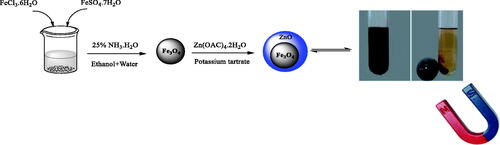
To prepare the iron nanoparticles, 2 g of ferric chloride II (FeCl2·4H2O) were dissolved with 5.4 g of ferric chloride III (FeCl3·6H2O) in 522 ml of deionized water, and then 62 ml of ammonia was added dropwise under mechanical stirring. The colour of the solution immediately changed to black. The resulting solution is stirred at 72 °C for 92 min and then rinsed with deionised water. Due to the high saturation magnetization of the nanoparticles, Fe3O4 absorbs the magnet well after each washing step and facilitates the washing operation. When rinsed by placing a magnet under the fuzzy separator, the transparent solution is discarded and discarded. After three rinses, the precipitates were dried under a vacuum ().
Preparation of ZnO nanoparticle
First, 0.1 mol of zinc nitrate was dissolved in 0.15 mol of oxalic acid in 100 cc of deionized water and stirred for 30 min at 45 °C with a magnetic stirrer. The obtained solution was then transferred to a two-way balloon for reflux and homogenization. The reflux lasted for 15 h at 75 °C. The solution was then centrifuged for 10 h at 90 °C in the oil bath and then placed directly on the hot plate at 130 °C for two hours. It was then incubated in the oven at 500 °C for 1 h.
Preparation of Fe3O4/ZnO core/shell MNPs
Fe3O4 superparamagnetic cores were synthesized by co-precipitation of FeCl3⋅6H2O and FeCl2·4H2O at the 2:1 molar ratio of Fe3+ and Fe2+, and they were dissolved in deionized water. After stirring for 10 min, the calculated amount of NH4OH was added to the solution. The Fe3O4 precipitates were separated using a centrifuge and washed rigorously with deionized water. Fe3O4/ZnO core/shell nanoparticles were synthesized using a previously reported method with minor modifications. The as-prepared magnetite sample was dispersed into deionized water and the pH value was regulated to 5.0. Then, Zn(NO3)2 dissolved in deionized water was dripped into the solution with a molar ratio of magnetite to zinc source adjusted to 1:2. After 1 h of contact, the pH value was increased up to 9.0 at 80 °C to promote the dehydration and atomic rearrangement involved with the formation of ZnO on the surface of the magnetite. At the end of the contact stage, the solids were magnetically collected, washed with ethanol and abundant deionized water, and dried (Scheme 2).
Preparation of H3PW12O40@Fe3O4–ZnO nanoparticles
1.0 g Fe3O4–ZnO nanoparticles were suspended in a methanolic solution of phosphotungstic acid (1.0 g in 50 ml), and the mixture briefly sonicated and microwave irradiation then stirred at ambient temperature for 9 h. The resulting solid was separated and repeatedly washed with ethanol and water, then dried under reduced pressure and designated PTA@Fe3O4–ZnO. The bulk W loading from ICP-OES was 12.9 wt% (equivalent to 16.9 wt% PTA), with loadings of other elements as follows: Fe 29.1 wt%.
General experimental procedure for the synthesis of benzo[a]furo[2, 3-c]phenazine derivatives (6a–h)
Initially, 2-hydroxynaphthalene-1,4-dione 1 (1 mmol), aromatic 1,2-diamines 2 (1 mmol) were mixed at 80 °C until an orange solid of benzo[a]phenazine 3 was formed within 3-5 min [Citation13].
The microwave was programmed to give a maximum internal temperature of 100 °C. Then, arylglyoxal (1 mmol), indole (1 mmol), and 0.03 g of 60% H3PW12O40@Fe3O4–ZnO nanoparticles were added to the above reaction mixture which was irradiated further at the same temperature (300 W, max. 100 °C) for an appropriate time as shown in . Upon completion of the reaction, monitored by TLC, the reaction mixture was allowed to cool to room temperature. Then, 5 ml of water was added to the mixture and washed several times. The catalyst was separated from the solvent by a magnet and finally dried under vacuum overnight. The separated product was washed twice with ethanol (2 × 5 ml). The crude solid product gives pure product 6 after washing and filtration ().
Table 1. Optimization of reaction conditions of compound 6a.
Table 2. Sequential one-pot four-component synthesis of benzo[a]furo[2, 3-c]phenazine derivatives.
The analytical and spectroscopic data for the unknown compounds
1-(1h-indol-3-yl)-2-(4-methoxyphenyl)-10-nitrobenzo[a]furo[2,3-c]phenazine (6a)
Red powder; yield 0.51 g (97%), m.p 232 − 234 °C; IR (KBr): νmax= 3315, 3030, 2850, 1661, 1606, 1573, 1521, 1471, 1445, 1326, 1211, 1148, 1113, 1025, 1000, 945, 808, 755 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 3.79 (s, 3H), 7.06 − 7.13 (m, 2H), 7.23 − 7.32 (m, 2H), 7.44 − 7.55 (m, 2H), 7.63 (td, J=7.8, 1.2 Hz, 1H), 7.75 − 7.81 (m, 2H), 8.03 (d, J=2.5 Hz, 1H), 8.06 − 8.13 (m, 1H), 8.30 − 8.41 (m, 3H), 8.61 (dd, J=7.8, 1.3 Hz, 1H), 9.21 (d, J=2.0 Hz, 1H), 9.46 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 55.3, 107.0, 112.2, 114.3, 120.4, 120.9, 121.7, 122.3, 122.5, 123.3, 124.1, 124.9, 125.6, 125.8, 126.1, 126.5, 128.1, 128.6, 128.6, 129.1, 129.2, 129.4, 136.8, 137.7, 138.2, 141.4, 143.2, 148.2, 151.4, 155.9, 160.2; MS (m/z, %): 536 (M+, 83); Anal. Calcd for C33H20N4O4: C, 73.87; H, 3.76; N, 10.44%. Found: C, 73.89; H, 3.74; N, 10.42%.
1-(1h-indol-3-yl)-10-nitro-2-(p-tolyl)benzo[a]furo[2,3-c]phenazine (6 b)
Orange powder; yield 0.48 g (94%), m.p 292 − 294 °C; IR (KBr): νmax=3245, 3135, 2675, 2340, 1673, 1629, 1576, 1510, 1478, 1434, 1404, 1317, 1210, 1165, 1116, 1084, 1002, 952, 834, 755 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 2.36 (s, 3H), 7.21 − 7.32 (m, 4H), 7.45 − 7.56 (m, 2H), 7.63 (td, J = 7.7, 1.5 Hz, 1H), 7.74 − 7.81 (m, 2H), 8.05 − 8.12 (m, 2H), 8.30 − 8.41 (m, 2H), 8.42 (d, J = 8.4 Hz, 1H), 8.61 (dd, J=7.8, 1.2 Hz, 1H), 9.21 (d, J=2.1 Hz, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 21.2, 107.0, 112.2, 120.5, 120.8, 121.7, 122.3, 123.2, 124.0, 124.9, 125.5, 125.6, 125.7, 126.1, 126.5, 127.7, 128.1, 128.6, 129.2, 129.7, 136.8, 137.7, 138.2, 140.0, 141.3, 143.3, 148.2, 151.4, 155.7; MS (m/z, %): 520 (M+, 86); Anal. Calcd for C33H20N4O3: C, 76.14; H, 3.87; N, 10.76%. Found: C, 76.15; H, 3.85; N, 10.74%.
1-(1h-indol-3-yl)-2-(p-tolyl)benzo[a]furo[2,3-c]phenazine (6c)
Brown powder; yield 0.40 g (85%), m.p 251 − 253 °C; IR (KBr): νmax=3420, 3250, 3030, 2875, 2695, 2340, 1617, 1582, 1528, 1481, 1443, 1404, 1348, 1312, 1222, 1130, 1063, 1001, 814, 761, 734, 706 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 2.37 (d, J=0.8 Hz, 3H), 7.22 − 7.32 (m, 4H), 7.46 − 7.55 (m, 2H), 7.55 − 7.62 (m, 1H), 7.58 − 7.69 (m, 2H), 7.75 − 7.82 (m, 2H), 8.01 − 8.13 (m, 3H), 8.14 − 8.22 (m, 1H), 8.36 (dd, J=8.1, 1.5 Hz, 1H), 8.58 − 8.64 (m, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 21.2, 107.0, 112.1, 120.4, 120.9, 121.7, 122.3, 123.2, 124.1, 125.0, 125.5, 125.7, 126.0, 127.7, 128.0, 128.6, 128.7, 128.9, 129.2, 129.2, 129.5, 129.7, 136.7, 136.9, 139.8, 141.7, 142.4, 142.5, 151.5, 155.0; MS (m/z, %): 475 (M+, 90); Anal. Calcd for C33H21N3O: C, 83.35; H, 4.45; N, 8.84%. Found: C, 83.33; H, 4.42; N, 8.82%.
1-(1h-indol-3-yl)-2-phenylbenzo[a]furo[2,3-c]phenazine (6d)
Brown powder; yield 0.42 g (93%), m.p 249 − 251 °C; IR (KBr): νmax= 3440, 3240, 3050, 2890, 1612, 1581, 1518, 1484, 1444, 1400, 1327, 1200, 1161, 1111, 1084, 1025, 1002, 943, 821, 734 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 7.23 − 7.32 (m, 2H), 7.40 − 7.48 (m, 1H), 7.46 − 7.56 (m, 4H), 7.58 (dd, J=7.2, 5.7 Hz, 1H), 7.58 − 7.68 (m, 2H), 7.83 − 7.90 (m, 2H), 8.01 − 8.13 (m, 3H), 8.14 − 8.21 (m, 1H), 8.36 (dd, J=7.9, 1.3 Hz, 1H), 8.61 (dd, J=8.1, 1.3 Hz, 1H), 9.46 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 107.0, 112.1, 120.4, 120.9, 121.7, 122.3, 123.2, 124.1, 125.0, 125.7, 126.0, 127.3, 127.9, 128.0, 128.6, 128.7, 128.9, 129.1, 129.3, 129.4, 129.5, 129.6, 136.8, 136.9, 141.8, 142.4, 142.5, 151.5, 155.2; MS (m/z, %): 461 (M+, 91); Anal. Calcd for C32H19N3O: C, 83.28; H, 4.15; N, 9.10%. Found: C, 83.30; H, 4.12; N, 9.12%.
1-(2-Methyl-1H-indol-3-yl)-2-(p-tolyl)benzo[a]furo[2,3-c]phenazine (6e)
Brown powder; yield 0.44 g (91%), m.p 240–242 °C; IR (KBr): νmax= 3460, 3280, 3160, 3045, 1583, 1515, 1483, 1443, 1402, 1328, 1269, 1227, 1201, 1169, 1112, 1025, 1002, 944, 826, 736, 690 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 2.37 (s, 3H), 2.70 (s, 3H), 7.14 (td, J=7.4, 1.2 Hz, 1H), 7.21 (td, J=7.5, 1.9 Hz, 1H), 7.26 (d, J=8.3 Hz, 2H), 7.51 (ddd, J=9.3, 7.8, 1.6 Hz, 2H), 7.55 − 7.65 (m, 2H), 7.65 (td, J=7.8, 1.2 Hz, 1H), 7.73 − 7.79 (m, 2H), 7.89 (dd, J=7.8, 1.4 Hz, 1H), 8.02 − 8.10 (m, 1H), 8.13 − 8.22 (m, 1H), 8.31 (dd, J=7.8, 1.4 Hz, 1H), 8.62 (dd, J=7.8, 1.3 Hz, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 12.3, 21.2, 105.5, 112.3, 120.9, 121.7, 121.8, 123.2, 124.5, 124.9, 125.0, 125.4, 126.4, 126.9, 127.7, 128.1, 128.6, 128.7, 128.8, 128.9, 128.9, 129.5, 129.67, 135.7, 136.5, 136.7, 137.5, 140.0, 142.4, 142.5, 151.0, 160.1; MS (m/z, %): 489 (M+, 79); Anal. Calcd for C34H23N3O: C, 83.41; H, 4.74; N, 8.58%. Found: C, 83.38; H, 4.76; N, 8.56%.
2-(4-Ethoxyphenyl)-1-(2-methyl-1H-indol-3-yl)benzo[a]furo[2,3-c]phenazine (6f)
Brown powder; yield 0.45 g (90%), m.p 255 − 257 °C; IR (KBr): νmax= 3425, 3185, 3045, 2865, 1668, 1606, 1582, 1517, 1482, 1440, 1325, 1274, 1199, 1169, 1114, 1065, 1002, 942, 890, 810, 744, 675 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 1.44 (t, J=6.6 Hz, 3H), 2.70 (s, 3H), 4.02 (q, J=6.6 Hz, 2H), 7.00 − 7.06 (m, 2H), 7.14 (td, J=7.5, 1.4 Hz, 1H), 7.21 (td, J=7.6, 1.9 Hz, 1H), 7.51 (ddd, J=7.6, 6.3, 1.5 Hz, 2H), 7.55 − 7.67 (m, 3H), 7.74 − 7.80 (m, 2H), 7.90 (dd, J=8.0, 1.5 Hz, 1H), 8.02 − 8.11 (m, 1H), 8.16 − 8.22 (m, 1H), 8.34 (dd, J=7.9, 1.4 Hz, 1H), 8.62 (dd, J=7.8, 1.3 Hz, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 12.3, 14.6, 63.5, 105.5, 112.2, 114.4, 120.7, 121.7, 121.8, 122.2, 123.2, 124.6, 124.9, 125.3, 126.4, 127.0, 128.1, 128.6, 128.6, 128.7, 128.9, 129.2, 129.3, 129.5, 129.5, 135.7, 136.6, 136.7, 137.6, 142.4, 142.5, 150.9, 159.6, 160.3; MS (m/z, %): 505 (M+, 73); Anal. Calcd for C34H23N3O2: C, 80.77; H, 4.59; N, 8.31%. Found: C, 80.72; H, 4.61; N, 8.34%.
1-(2-Methyl-1H-indol-3-yl)-10-nitro-2-(p-tolyl)benzo[a]furo[2,3-c]phenazine (6 g)
Brown powder; yield 0.46 g (87%), m.p 279 − 281 °C; IR (KBr): νmax= 3425, 3185, 3045, 2865, 1668, 1606, 1582, 1517, 1482, 1440, 1325, 1274, 1199, 1169, 1114, 1065, 1002, 942, 890, 810, 744, 675 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 2.36 (d, J=0.9 Hz, 3H), 2.70 (s, 3H), 7.13 (td, J=7.4, 1.3 Hz, 1H), 7.17 − 7.29 (m, 3H), 7.52 (ddd, J=7.7, 6.2, 1.6 Hz, 2H), 7.63 (td, J=7.8, 1.2 Hz, 1H), 7.74 − 7.80 (m, 2H), 7.90 (dd, J=8.0, 1.6 Hz, 1H), 8.32 (d, J=8.4 Hz, 1H), 8.41 − 8.33 (m, 2H), 8.59 − 8.65 (m, 1H), 9.21 (d, J=1.9 Hz, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 12.3, 21.2, 108.3, 112.3, 120.9, 121.7, 121.7, 123.2, 124.8, 124.9, 125.0, 125.4, 125.7, 126.4, 126.5, 127.0, 127.6, 128.1, 128.6, 129.2, 129.3, 129.7, 135.7, 136.5, 137.5, 138.1, 140.0, 141.3, 143.3, 148.2, 150.9, 160.3; MS (m/z, %): 534 (M+, 73); Anal. Calcd for C34H22N4O3: C, 76.39; H, 4.15; N, 10.48%. Found: C, 76.36; H, 4.12; N, 10.45%.
2-(4-Methoxyphenyl)-1-(2-methyl-1H-indol-3-yl)-12-nitrobenzo[a]furo[2,3-c]phenazine (6 h)
Brown powder; yield 0.51 g (93%), m.p 253 − 255 °C; IR (KBr): νmax= 3425, 3185, 3045, 2865, 1668, 1606, 1582, 1517, 1482, 1440, 1325, 1274, 1199, 1169, 1114, 1065, 1002, 942, 890, 810, 744, 675 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 1.43 (t, J=6.6 Hz, 3H), 2.70 (s, 3H), 4.02 (q, J=6.6 Hz, 2H), 7.00 − 7.06 (m, 2H), 7.13 (td, J=7.5, 1.4 Hz, 1H), 7.21 (td, J=7.6, 2.0 Hz, 1H), 7.47 − 7.55 (m, 2H), 7.63 (td, J=7.8, 1.2 Hz, 1H), 7.74 − 7.80 (m, 2H), 7.90 (dd, J=7.8, 1.4 Hz, 1H), 8.30 − 8.42 (m, 3H), 8.61 (dd, J=7.7, 1.5 Hz, 1H), 8.72 (d, J=1.9 Hz, 1H), 9.47 (s, 1H) ppm; 13C NMR (125 MHz, DMSO-d6) δ 12.3, 14.6, 63.5, 109.0, 112.1, 114.5, 120.9, 121.7, 121.7, 122.2, 123.3, 124.9, 125.3, 125.4, 125.5, 126.4, 126.5, 127.0, 128.1, 128.6, 129.2, 129.5, 135.7, 136.5, 137.5, 138.1, 141.3, 143.3, 148.2, 150.9, 159.6, 160.4; MS (m/z, %): 550 (M+, 81); Anal. Calcd for C34H22N4O4: C, 74.17; H, 4.03; N, 10.18%. Found: C, 74.15; H, 4.06; N, 10.08%.
To optimise the catalytic system, the reaction of benzo[a]phenazine (1 mmol), arylglyoxal (1 mmol), indole (1 mmol) under solvent-free was used as a model reaction for the synthesis of compound 6a. We studied the type of catalyst, catalyst amount, solvent, and the influence of microwave irradiation. The results are summarised in . The reaction gave the highest yield using 60% of H3PW12O40@Fe3O4–ZnO under microwave irradiation (300 W, max. 100 °C) (, entry 7). Increasing the amount of 60% H3PW12O40@Fe3O4–ZnO to more than 0.06 g showed no substantial improvement in the yield, whereas the yield decreased by decreasing the amount of the catalyst to 0.015 g. The IR spectrum of compound 6a exhibits two absorption bands, one at 1648 cm−1 for the (O=C) and the other at 1331 and 1506 cm−1 for the (2NO2) groups. The 500 MHz 1H NMR spectrum of 6a exhibits multiplets at δ 7.08–9.21 ppm for aromatic protons. The 13C NMR spectrum of compound 6a shows 27 distinct resonances, in agreement with the proposed structure.
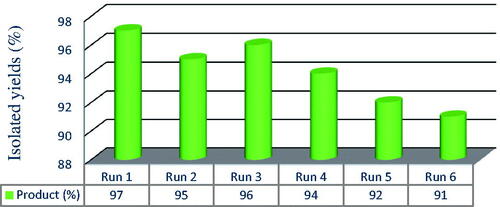
As shown in , employing magnetic separation of the catalyst and subsequent ethanol washing and drying at 90 °C for 4 h in between each reaction. We found that the catalyst could be used at least five times with only slight reduction inactivity, for example, the yield (6a) typically declined from 88 to 85%. It can be seen that the activity of the recovered catalyst with the same function is very appropriate during the two stages. In the later stages, the activity of the magnetic catalyst is reduced, which can be expressed due to the weight of the catalyst in the later stages of synthesis during the recovery process. This proper performance of the catalyst indicates its excellent performance in the reaction under solvent-free conditions and microwave radiation.
With the optimised conditions in hand, the scope and efficiency of the reaction were explored for the synthesis of benzo[a]furo[2,3-c]phenazine derivatives. Thus, 2-hydroxynaphthalene-1,4-dione, arylglyoxal, indole in the presence of a catalyst (60 mol%) at microwave irradiation. The results are shown in .
To show the merit of the present work in comparison with the reported results in the literature, we compared the results of SiO2–SO3H as catalyst with PTSA [Citation26], H2SO4 [Citation27], phosphotungstic acid [Citation27], [NMP]H2PO4 [Citation27] and SiO2–SO3H [Citation28] in the synthesis benzo[a]furo[2,3-c] phenazine derivatives from 2-hydroxynaphthalene-1,4-dione 1, aromatic 1,2-diamines 2, arylglyoxal and indole. As shown in , entry 7, this new catalytic system compared to previous similar works has a number of advantages such as: very shorter reaction time, higher yields, catalyst recovery, and most importantly, avoidance of hazardous or toxic catalysts and organic solvents. In all these cases, our work has introduced green and more economically cost-effective relative to previous similar works.
Table 3. Comparison of results obtained using H3PW12O40@Fe3O4–ZnO with results obtained using other catalyst reported in the literature for the synthesis.
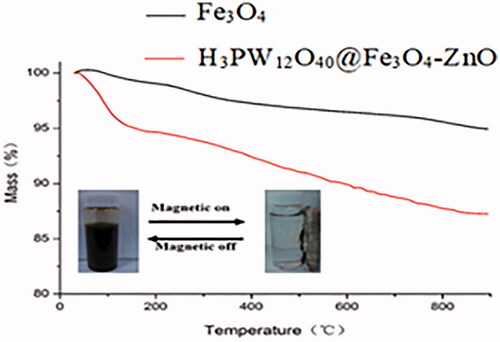
Thermal analysis of H3PW12O40@Fe3O4–ZnO nanoparticles is given in . According to , the TGA curve of naked Fe3O4 nanoparticles showed a weight loss of 5.05% from 25 to 900 °C. While the H3PW12O40@Fe3O4–ZnO nanoparticles showed a weight loss of 12.76%. The reason was possible that compared with naked Fe3O4 nanoparticles there was the release of hydroxyl ions and decomposition of aminopropyl groups on the H3PW12O40@Fe3O4–ZnO nanoparticles except for water thermo-desorption. It was also confirmed that the Fe3O4 nanoparticles were successfully decorated by APTMS. Also, it could be easily concluded that the magnetic content of H3PW12O40@Fe3O4–ZnO nanoparticles was 8.12% less than that of naked Fe3O4 nanoparticles.
Results and discussion
Structural characterization
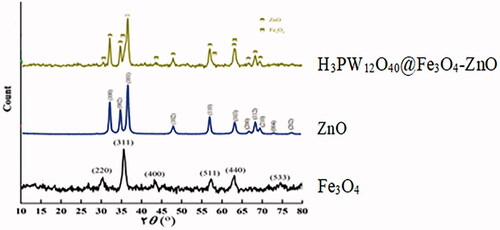
The X-ray diffraction pattern for the samples of Fe3O4, ZnO, and H3PW12O40@Fe3O4–ZnO is shown in . Structural characterization of X-ray diffraction patterns of samples including crystal structure type, spatial band, and cellular parameter calculation was performed by Xpert High Score and Fullprof software. The results show that the best fit is done with the least possible difference. As you can see in , the characteristic diffraction peaks for Fe3O4 at angles 2θ (30.2, 35.3, 43.4, 53.1, 57.2 and 74.6) corresponding to the plates (220), (311), (400), (400), (511) and (533), respectively. Structural investigations using the Xpert commercial package confirm the formation of a cubic spinel structure with the Fd-3m space group without the presence of the impure phase, which is consistent with the values recorded in standard JCPDS cards number 0206-79. As can be seen, most of the diffraction is from the plate (311).
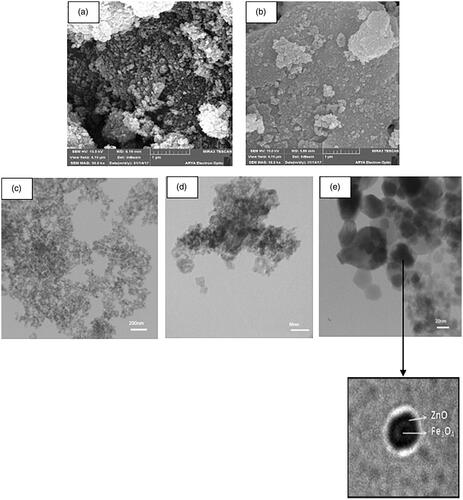
The SEM and TEM images of the Fe3O4 and H3PW12O40@Fe3O4–ZnO were shown in . Fe3O4 nanoparticles have a spherical shape and have a smooth surface without porosity. Magnetite particles also have a very strong tendency to accumulate plasma and form larger particles. Core-shell particles tend to accumulate plasma, which is an expected event. Because the zinc oxide particles, which are located in the outermost part of the core-shell structure, tend to shrink. The diameter of the H3PW12O40@Fe3O4–ZnO core-shell particle varies in the range of 75 to 89 nm.
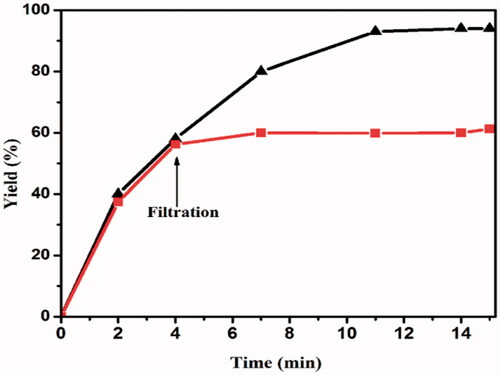
A hot filtration test was performed by separating the H3PW12O40@Fe3O4–ZnO catalyst from the reaction mixture after 5 min in the first cycle of reaction. The recovered nanocatalyst was collected and then dried at 100 °C. This may lead to a slight increase of the yield to 59% from 55% after the hot filtration test. Furthermore, disruptions, such as hot filtration, may deactivate an existing active dissolved metal, leading to the incorrect conclusion that there are no active homogeneous catalytic species before the filtration ().
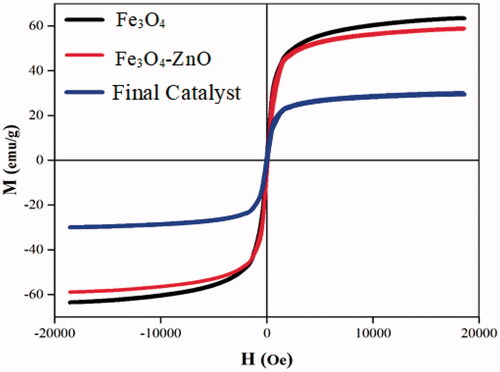
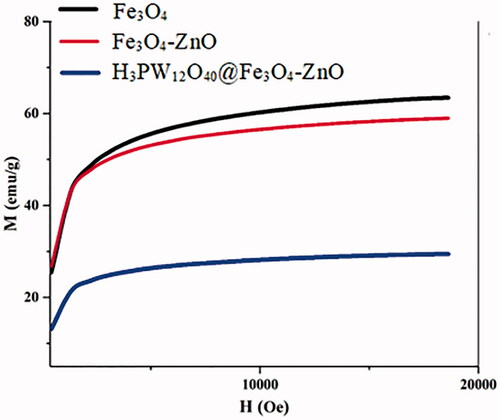
Magnetic Properties of Fe3O4, H3PW12O40@Fe3O4–ZnO nano-particles by the vibrating sample magnetometer was examined. Magnetization diagram in terms of the magnetic field applied to each specimen in . As you can see in all three samples, there is a very narrow residual loop and a sudden increase in magnetization with a slight increase in field magnetically, thereby having a paramagnetic cloud property. For all three samples, the magnetization in the fields does not reach saturation and still has a slight increase.
As can be seen in , the slope of the magnetization diagrams at large magnetic fields decreases for the samples of Fe3O4, Fe3O4/EN-MIL-101 and nano TiO2-PTA@Fe3O4/EN-MIL-101(Cr), respectively, which is another evidence of increasing size. Particles. The values obtained for the forcing field, saturation magnetization, and residual magnetization are reported in .
Table 4. Coercion field values, saturation magnetization and residual magnetization.
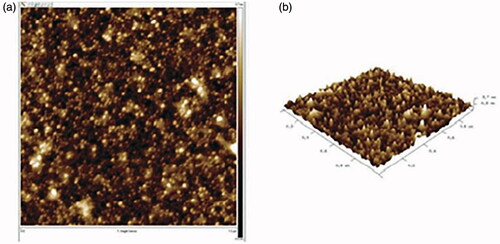
To further characterize the H3PW12O40@Fe3O4-ZnO nanocomposite, the AFM analysis was applied. AFM is a beneficial instrument for investigating various structural features and parameters and has the advantage of probing in deep insights of surface topography qualitatively due to its both straight and vertical nanometer-scale spatial resolution. shows an AFM image of the Nano H3PW12O40@Fe3O4-ZnO and its surface morphology. As observed in , AFM image revealed the presence of sphere-like nanoparticles and their respective particle size and morphology were close to those determined by the SEM images. In , the surface of the composite showed an approximately uniform structure indicating that the size (height) of the particles on the nanocomposite sheet was 75 nm.
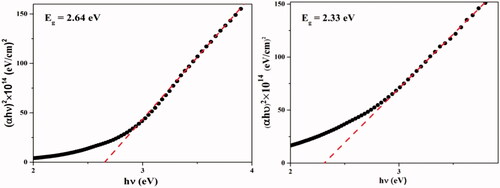
The energy gaps of H3PW12O40@Fe3O4–ZnO sample are shown in , respectively. As can be seen, the energy gap of the catalyst nanostructure was 2.33 electron volts, respectively. The reason for the decrease in the bandgap is the increase in particle size, which is consistent with TEM images.
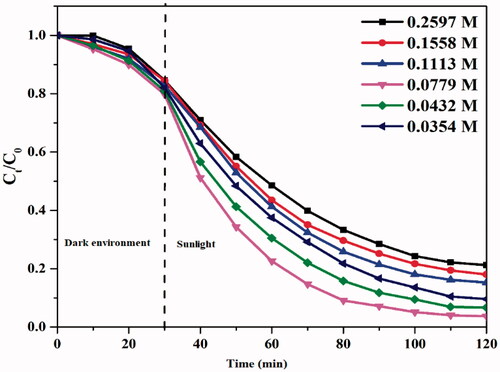
In photocatalytic activity, by receiving the necessary energy from ultraviolet light, an electron-hole pair is formed in ZnO nanoparticles. The formed cavity reacts with the water present in the medium to produce OH radicals. The OH radical produced attacks on the coloured organic compounds and breaks down their bonds and breaks down the composition into lighter organic compounds. This phenomenon is known as photocatalytic degradation. The electrons produced in ZnO are reduced in the graphene oxide conduction band and from there to the conduction band of Fe3O4 nanoparticles. Iron III in Fe3O4 structure by electron conversion to iron II and iron II reacts with oxygenated oxygen in the environment, producing ion and OH radicals and again iron III. The created OH radical destroys the organic compound. This phenomenon is called the Fenton process. As the concentration of oxygenated water increases, the rate of production of OH radicals from the Fenton canal increases, and there is an increase in degradation. After a certain concentration, the degradation process will be reduced.
The reason for this is that after a certain concentration with increasing oxygenated water, the OH radicals produced with H2O2 present in the environment not yet reacted with Fe II react, and the rate of the Fenton process decreases with the conversion of H2O2 to ordinary water (). The optimum conditions for the degradation of methylene blue with a concentration of 1.61 g L−1 catalyst and 0.0779 mol L−1 of H2O2 was obtained and, under the above conditions, methylene blue was degraded by 97% in 120 min.
Based on this mechanism, at first, 2-hydroxynaphthalene-1,4-dione 1 tautomerizes to intermediate 6. The primary condensation of 4-hydroxy-1,2-naphthoquinone 6 with benzene-1,2-diamine 2 obtains benzo[a]phenazin-5-ol 3. A reaction pathway involves the formation of an intermediate (II) medium when reacted with the indole group after being added to the carbonyl group or upon the replacement of the side-chain hydroxy group. Based on the second route, arylglyoxal and indole developed the open shaft (III) or the OH group electron pair. This, then forms intermediate III informing the C–C and C-–N bonds through the intermolecular [3 + 2] ring reaction, which is made after reacting with the intermediate middle (I) benzo[a] phenazine-5-ol, caused by the loss of water during the ring formation process before the desired product is obtained ().
Conclusions
In conclusion, the reaction of 2-hydroxynaphthalene-1,4-dione, arylglyoxal, indoles in the presence H3PW12O40@Fe3O4-ZnO MCNPs afforded benzo[a]furo[2, 3-c]phenazine derivatives in excellent yields. The present procedure has the advantage that the reaction is performed under solvent-free conditions using microwave irradiation and the starting material can be used without any activation or modification. In conclusion, a simple and efficient method for the synthesis of phenazines derivatives has been explored. Mild reaction conditions, shorter reaction time, easy and quick isolation of the products, low power consumption, and excellent yields are the main advantages of this method, which makes it an attractive and useful contribution to the present methodologies. The morphology and particle size were investigated using XRD, FESEM, TEM, AFM and VSM analysis reveals the functional compounds present in the nanoparticles. Photocatalytic degradation results show 97% methylene blue degradation under conditions has the best.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang G, Chen D, Li N, et al. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl Catal B Environ. 2019;250:313–324.
- Thangavel S, Krishnamoorthy K, Kim SJ, et al. Designing ZnS decorated reduced graphene-oxide nanohybrid via microwave route and their application in photocatalysis. J Alloy Comp. 2016;683:456–462.
- Moussa H, Girot E, Mozet K, et al. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl Catal B Environ. 2016;185:11–21.
- Alberti S, Caratto V, Peddis D, et al. Synthesis and characterization of a new photocatalyst based on TiO2 nanoparticles supported on a magnetic zeolite obtained from iron and steel industrial waste. J Alloy Comp. 2019;797:820–825.
- Deng C, Hong R, Jing M, et al. Photocatalytic performance of TiO2 thin film decorated with Cu2O nanoparticles by laser ablation. Opt Mater. 2019;94:130–137.
- Chiarello GL, Dozzi MV, Selli E. TiO2-based materials for photocatalytic hydrogen production. J Energy Chem. 2017;26(2):250–258.
- Zheng K, Liu H, Nie C, et al. Controllable synthesis of honeycomb-structured ZnO nanomaterials for photocatalytic degradation of methylene blue. Mater Lett. 2019;253:30–33.
- Ye Y, Bruning H, Li X, et al. Significant enhancement of micropollutant photocatalytic degradation using a TiO2 nanotube array photoanode based photocatalytic fuel cell. Chem Eng J. 2018;354:553–562.
- Yu JG, Yu XX. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ Sci Technol. 2008;42(13):4902–4907.
- Ma SS, Xue JJ, Zhou YM, et al. Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity. J Mater Chem. 2014;2(20):7272–7280.
- Niknam K, Saberi D, Sadegheyan M, et al. Silica-bonded S-sulfonic acid: an efficient and recyclable solid acid catalyst for the synthesis of 4,40-(arylmethylene)bis(1H-pyrazol-5-ols). Tetrahed Lett. 2010;51(4):692–694.
- Shirini F, Zolfigol MA, Mohammadi K. Silica sulfuric acid as a mild and efficient reagent for the acetylation of alcohols in solution and under solvent free conditions. Korean Chem Soc. 2004;25:325–327.
- Wu H, Shen Y, Fan L-y, et al. Stereoselective synthesis of β-amino ketones via direct Mannich-type reaction catalyzed with silica sulfuric acid. Tetrahedron. 2007;63(11):2404–2408.
- Bloxham J, Dell CP, Smith C. Preparation of some new benzylidenemalononitriles by an SNAr reaction: application to naphtho[1,2-b]pyran synthesis. Heterocycles. 1994;38(2):399–308.
- Pan L, Olson DH, Ciemnolonski LR, et al. Separation of hydrocarbons with a microporous metal-organic framework. Angew Chem. 2006;118(4):632–635.
- Jhung SH, Lee J-H, Yoon JW, et al. Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability. Adv Mater. 2007;19(1):121–124.
- Tong M, Liu D, Yang Q, et al. Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. J Mater Chem A. 2013;1(30):8534–8537.
- Zhou M, Wu YN, Qiao J, et al. The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al)). J Colloid Interface Sci. 2013;405:157–163.
- Jung BK, Hasan Z, Jhung SH. Adsorptive removal of 2,4- dichlorophenoxyacetic acid (2,4-D) from water with a metal-organic framework. Chem Eng J. 2013; 234:99–105.
- Maes M, Schouteden S, Alaerts L, et al. Extracting organic contaminants from water using the metal-organic framework CrIII(OH)·{O2C-C6H4-CO2}. Phys Chem Chem Phys. 2011;13(13):5587–5589.
- Veisi V, Ozturk T, Karmakar B, et al. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2–NH2 as magnetic catalyst in Suzuki–Miyaura coupling and 4-nitrophenol reduction. Carbohydr Polym. 2020;235:115966–115974.
- Zhang W, Veisi H, Sharifi R, et al. Fabrication of Pd NPs on pectin-modified Fe3O4 NPs: a magnetically retrievable nanocatalyst for efficient C-C and C-N cross coupling reactions and an investigation of its cardiovascular protective effects. Int J Biol Macromol. 2020;160:1252–1256.
- Tamoradi T, Veisi H, Karmakar B, et al. A competent green methodology for the synthesis of aryl thioethers and 1H-tetrazole over magnetically retrievable novel CoFe2O4@l-asparagine anchored Cu, Ni nanocatalyst. Mater Sci Eng C Mater Biol Appl. 2020;107:110260–110268.
- Veisi H, Mohammadi L, Hemmati S, et al. In situ immobilized silver nanoparticles on rubia tinctorum extractcoated ultrasmall iron oxide nanoparticles: an efficient nanocatalyst with magnetic recyclability for synthesis of propargylamines by A3 coupling reaction. ACS Omega. 2019; 4(9):13991–14003
- Veisi H, Tamoradi T, Rashtiani A, et al. Palladium nanoparticles anchored polydopamine-coated graphene oxide/Fe3O4 nanoparticles (GO/Fe3O4@PDA/Pd) as a novel recyclable heterogeneous catalyst in the facile cyanation of haloarenes using K4[Fe(CN)6] as cyanide source. J Ind Eng Chem. 2020; 90:379–388.
- Khurana JM, Chaudhary A, Lumb A, et al. An expedient four-component domino protocol for the synthesis of novel benzo[a]phenazine annulated heterocycles and their photophysical studies. Green Chem. 2012;14(8):2321–2327.
- Saluja P, Chaudhary A, Khurana JM. Synthesis of novel fluorescent benzo[a]pyrano[2,3-c] phenazine and benzo[a]chromeno[2,3-c]phenazine derivatives via facile four-component domino protocol. Tetra Lett. 2014;55(23):3431–3435.
- Rajeswari M, Khanna G, Chaudhary A, et al. Multicomponent domino process for the synthesis of some novel benzo[a]chromenophenazine fused ring systems using H2SO4, phosphotungstic acid, and[NMP]H2PO4. Synth Commun. 2015;45(12):1426–1432.


![Figure 13. Proposed mechanism for the synthesis of benzo[a]furo[2, 3-c]phenazine derivatives.](/cms/asset/1a5f56f3-28c4-407d-81f4-ac5d8e782871/ianb_a_1894163_f0013_c.jpg)