?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recently green nanotechnology has gained great interest as a promising tool for drug discovery. In the present study, we synthesized and characterized silver nanoparticles (AgNPs) using Azadirachta indica (AI) and evaluated their hemocompatibility and effect against Plasmodium falciparum strains. AI leaves and barks were used for aqueous extracts (AIL and AIB) and AgNPs synthesis. AgNPs were characterized using spectroscopic, diffraction, electron microscopic and electrostatic techniques. Anti-plasmodial and haemolytic activity were assessed following the SYBR Green I fluorescence assay and Miki et al. protocol, respectively. The normalized fluorescence counts were plotted against the log-transformed drug concentration and half-maximal inhibitory concentration (IC50) determined by analyzing the dose–response curves. AgNPs were stored for 120 days at room temperature-RT, +4 °C and −20 °C and subsequently their stability was evaluated by spectroscopy. Both NPs were predominantly spheroidal, crystalline in nature, stable, well dispersed with mean size of 13.01 nm for AIL-NPs and 19.30 nm for AIB-NPs and exhibited good antiplasmodial activity against 3D7 and RKL9 P. falciparum strains with IC50 of 9.27 µg/mL and 11.14 µg/mL for AIL-NPs, 8.10 µg/mL and 7.87 µg/mL for AIB-NPs, respectively. A. indica contain bioactive phyto-compounds indicating great potential for anti-malarial drug development through green nanotechnology. The AgNPs were structurally stable after 120 days but antiplasmodial activity was considerably affected. A significant haemolytic activity (>25%) was observed with AIL- and AIB-AgNPs at concentrations ≥125 µg/mL.
Introduction
Malaria is an endemic disease of the intertropical regions caused by a protozoan belonging to the genus Plasmodium and transmitted to human through infected female Anopheles mosquito. According to World Health Organization (WHO), 241 million malaria cases were recorded worldwide in 2020, with 82.2% of cases in the sub-Saharan African (sSA) region [Citation1]. The fight against this disease includes prophylaxis, regular screening and early treatment mainly in children under five years of age and pregnant women, the main vulnerable targets. The coverage use of insecticide-treated mosquito nets ∼65% in sSA is constantly increasing with a satisfactory impact [Citation1]. Artemisinin-based combination therapies (ACT) recommended by WHO remain the most effective antimalarial till date even though resistance has been reported from some South Asian and African regions [Citation2,Citation3]. No effective vaccine for malaria is available currently, though clinical trial results of RTS, S/AS01 vaccine are promising and new generation malaria vaccines (e.g.: R21/Matrix-M) are already in clinical phases [Citation4]. In sSA countries, the low standards of living and the lack of good quality anti-malarial drugs would be the limiting factors for malaria elimination programs [Citation1–3,Citation5].
Nanobiotechnology is a multidisciplinary field that has revolutionized the biological world through synthesis and manipulation of nanomaterials with diverse applications [Citation6]. In the fight against malaria, several approaches have been developed based on nanomaterials of different nature including liposomes, polymers, dendrimers and inorganic nanoparticles (NPs) [Citation7]. The synthesis of NPs based on plant extracts with universal solvent such as water has gained momentum due to its simple, cost-effective, fast and eco-friendly character [Citation6,Citation8–10]. Phyto-nanotechnology allows today the synthesis of biocompatible NPs from noble metals (e.g., silver and gold) and phyto-materials (e.g, leaves, root, fruit, stem) available and non-toxic, justifying their use in biomedical field [Citation11,Citation12]. This biosynthesis approach has many advantages related to its physicochemical properties including stability and size/shape modulation [Citation6,Citation7,Citation11]. The charge, morphology and nanometric size of NPs have been proven to enhance their adhesion and absorption with the effect of increasing biological activities justifying their popularization [Citation13,Citation14].
Among medicinal plants, Azadirachta indica – neem is well known for its healing property in more than 40 different health issues/diseases such as malaria, intestinal disorders, diabetes etc. in Africa, Asia and South America [Citation15,Citation16]. In almost all African countries, populations take A. indica decoction for malaria fever owing to several factors like: (a) its ready availability, (b) aversion to synthetic drugs, (c) the drug cost and (d) unavailability of antimalarial drugs [Citation16].
Anti-plasmodial activity of silver nanoparticles (AgNPs) synthesized from A. indica leaves and seed kernel have been reported in very few studies from India [Citation17–19] and Malaysia [Citation9]. Genetic, edaphic and climatic factors can qualitatively and/or quantitatively affect the metabolic composition of plants and consequently modulate the synthesis and biological activity of NPs [Citation20,Citation21]. Also, to the best of our knowledge, no study on in vitro antiplasmodial activity of AgNPs synthesized from A. indica bark has been reported from Africa. In this work, we conducted a comparative study with A. indica leaves and bark originating from Far North region of Cameroon (Central Africa). This study aimed to determine the chemical composition of A. indica leaves and bark aqueous extracts from Far North region of Cameroon. The AgNPs using A. indica aqueous extract was synthesized, characterized and also evaluated for their anti-plasmodial activity, hemocompatibility and stability.
Material and methods
Biological material
Azadirachta indica leaves and bark have been collected from MOKOLO town in the Far North region of Cameroon (10°44′32″ N, 13°48′08″ E, altitude: 813 m) and identified by Victor Nana, a taxonomist at the National Herbarium of Cameroon by comparison to the specimens deposited under the voucher number 4447SRFK. Leaves and bark were washed with distilled water and dried in shade at room temperature (25-30 °C) for 7 and 15 days respectively and finally crushed. The obtained powders were kept at room temperature (RT).
Ethic consideration and permission
This study has received permission and clearance from the Indian Ministry through the Centre for International Co-operation in Science (CICS) for the entry of plant material into Indian territory and its use for in vitro research purposes.
Preparation of crude extract and characterization
The aqueous extracts were prepared for NPs synthesis and anti-plasmodial activity assay. Briefly, 10 grams of plant powder (leaves or bark) was added to 100 ml of Mili-Q water and boiled for 10 min, cool downed and then filtered through Whatman paper grade I. The filtrate solution was stored at 4 °C for 14 days maximum due to the gradual denaturation of secondary phytometabolites.
Silver nanoparticles synthesis and optimization
AgNPs were synthesized as previously described using silver nitrate (AgNO3) as the metal source and plant aqueous extracts as reducing and capping agents [Citation10]. Briefly, AgNO3 solution was mixed with a certain amount of plant extract and incubated at a given temperature for some time.
To optimize the synthesis of AgNPs, gradients of four main parameters influencing NPs synthesis were set: (a) incubation time (15 min − 4 h), (b) AgNO3 concentration (0.5–6 mM), (c) crude extract amount (10–100 μL) and (d) temperature (25–75 °C). The effect of a given parameter were assessed by keeping the other parameters constant. The solutions were monitored as a function of each parameter to determine the optimal production of AgNPs by checking the absorbance peak using a double beam UV-Visible spectrophotometer (BR BIOCHEM BI-2700) at a resolution of 1 nm over the range of 200–1000 nm.
After synthesis, the colloidal solution of AgNPs was centrifuged at 12000 rpm for 20 min and the supernatant discarded. The pellet was suspended in Mili-Q solution and thoroughly washed twice with Mili-Q and once with absolute ethanol by centrifugation in the same condition. The purified AgNPs pellet thus obtained was dried and kept at room temperature for physico-chemical characterization and biological assays.
Characterization of silver nanoparticles
Characteristics of surface plasmon resonance (SPR) of AgNPs were carried out by recording and analyzing UV–visible spectra with a precision of 1 nm over the range of 200–1000 nm using UV spectrophotometer (BR BIOCHEM BI-2700). The crystalline structures of green synthesized AgNPs were analysed on Panalitycal Make Xpert Pro Model X-ray diffraction (XRD) instrument by interpreting the XRD spectra recorded over the scanning range 2θ:10–80° (Voltage = 45 KV, current = 40 mA, Cu Kα1 = 1.5405 Å, Cu Kα2 = 1.5444 Å, Cu Kβ = 1.3922 Å, Scan speed = 0.2°/min). The potential functional groups of biomolecules associated with synthesized AgNPs were examined using a Fourier-transform infra-red spectroscope (FTIR) Perkin Elmer Make, Frontier Model. The FTIR spectra of purified AgNPs were recorded in the diffuse reflectance mode in potassium bromide (KBr) pellets at a resolution of 4 cm−1 over the range of 4000–400 cm−1. The Zeta potential and hydrodynamic size distribution of AgNPs, were determined by dynamic light scattering (DLS) using Malvern zetasizer Nano (Malvern Panalytical). The surface morphology, particle size and size distribution were analysed using transmission electron microscopy (TEM), operated at an accelerated voltage of 200 kV on TECNAI TF20 (Fei, Electron Optics). The particles size were also estimated using Debye–Scherrer equation: D = Kλ/βcosθ, where D is the mean diameter size of nanoparticles, K: the Scherrer constant, λ: the X-ray radiation source wavelength, β: the full width at half the maximum value of the XRD peak, and θ the Bragg’s angle [Citation22]. Their particles size distribution were obtained considering about 200 particles, measured using ImageJ software version 1.8.0 (https://imagej.nih.gov/ij/download.html). Scanning electron microscopy (SEM) images and energy-dispersive X-ray (EDX) spectroscopy measurements were recorded on a Zeiss EVO40 microscope at an accelerating voltage of 15 keV for 250 s. AgNPs were mounted on metal stubs using double-sided carbon adhesive and chemically sputter-coated with gold.
In vitro anti-plasmodial assay
Plasmodium falciparum chloroquine-sensitive (CQs) strain 3D7 and chloroquine-resistant (CQr) strain RKL9 were used for in vitro assay to evaluate the anti-plasmodial efficacy of A. indica leaves and bark aqueous extract and their corresponding AgNPs. The culture was maintained as per methods described by Trager and Jensen [Citation23]. P. falciparum cultures were maintained in fresh human blood erythrocytes suspended at 2% haematocrit in RPMI 1640 supplemented [25 mM HEPES buffer, 0.2% glucose, 10 µg/mL gentamycin, 25 mM Na2HCO3] and incubated at 37 °C under a gas mixture of 15% O2 and 5% CO2.
Anti-plasmodial activity of different compounds were assessed following the SYBR Green I fluorescence assay described by Smilkstein et al. [Citation24]. Briefly, 10 μL of synchronized culture (0.5-0.8% parasitaemia) were added to each well 96 wells culture plates containing 90 µL of complete medium with a serial dilution of aqueous extract or AgNPs (0 to 500 μg/mL) and incubated at the same condition as described above. Chloroquine (CQ) (0 to 125 μg/mL) and DMSO (0.4% v/v) were used as positive and negative controls, respectively. After 48 h, 100 µL of SYBR Green I lysis buffer [Tris (20 mM), EDTA (5 mM), Triton X-100 (0.08% v/v), saponin (0.008%wt/v) and SYBR Green I (0.02% v/v)] was added to each well, mixed and incubated in the dark at room temperature for an hour. Fluorescence was measured on cytofluorometer Synergy HTX Multi-Mode Microplate Reader (Serial number 1708152) coupled with Gen5 software (Version 3.03). The excitation and emission wavelength bands were centred at 485/20 and 528/20 respectively and the gain set at 50. Results were validated microscopically by examination of JSB-stained slides for each treatment and control. The normalized fluorescence counts were plotted against the log-transformed drug concentration and half-maximal inhibitory concentration (IC50) were determined by analyzing the dose–response curves. The interpretation of the IC50 ( μg/mL) values was done following the classification proposed by Gathirwa et al.: high activity (IC50<10), moderate activity (10 ≤ IC50<50), low activity (50 ≤ IC50≤100) and inactive (IC50 >100) [Citation16].
Hemocompatibility
The haemolytic activity test was performed against the nanoparticles following the protocol adapted from that described by Miki et al. [Citation25]. Freshly collected human blood from a healthy subject in a tube with an anti-coagulant was centrifuged at 3000 rpm for 5 min and the pellet was resuspended in phosphate-buffered saline (PBS) (v/v; pH 7.2). The process was repeated twice to remove the buffy coat and the cells were finally suspended in PBS.
Treatments of increasing concentrations of NPs up to 1 mg/mL were applied. 1 ml of erythrocyte suspension was added to 1 ml of each solution, mixed gently and incubated at 37 °C for 2 h. Triton X-100 (10%) and PBS (normal) were used as positive and negative controls respectively. After incubation, the samples were centrifuged as described before. The supernatant was then collected and absorbance read at 540 nm using a UV-vis spectrophotometer (SPECTROstar Nano, BMG LABTECH) against a blank PBS solution. The percentage of the haemolysis index (%) was calculated according to the formula below:
Quenching effect
As fluorescence based techniques are subject to the quenching effect, we assessed this potential artefact for all tested compounds. To perform that, cultures with 2% haematocrit and 8-10% parasitaemia were incubated at 37 °C with (100 μg/mL of crude extract or AgNPs) or without a drug for an hour. Then, 100 µL of SYBR Green I lysis buffer was added to each well and incubated for another hour [Citation26]. Fluorescence was read in the same condition as earlier mentioned.
Temporal stability of nanoparticles
The stability of AgNPs was spectrophotometrically evaluated under different storage conditions: room temperature (RT), +4 °C and −20 °C. The absorption spectrum of each sample was recorded every 30 days over a period of 120 days under the same conditions as mentioned above. The antiplasmodial activity of conserved aliquots were evaluated.
Statistical analysis
Data analysis and graph plotting were performed using OriginPro 2021 b and GraphPad 9.03 software. All continuous variable results were expressed as mean ± standard deviation and statistical differences between groups were considered to be significant at p value < .05. Means were compared using one-way analysis of variance and Tukey’s multiple comparison post hoc tests were used to assessed significant differences.
Results and discussion
Optimization of synthesis parameters
The stoichiometric optimization of AgNPs biosynthesis, including incubation time, temperature, AgNO3 concentration and crude aqueous extract proportion volume has been monitored by a series of UV-visible spectroscopy, an indirect but rapid and efficient method (). The appearance of the absorption peak is a typical physical process that occurs when the oscillations of free electrons on the surface of metal NPs align in resonance with the wavelength of the irradiated plane-polarized light under total internal reflection conditions [Citation27].
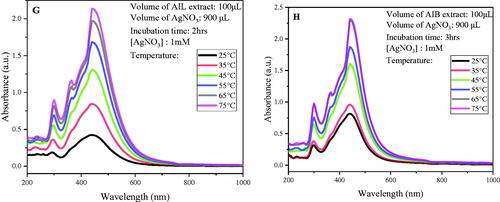
Figure 1. Optimization of biosynthesis AgNPs using A. indica leaves and bark aqueous extract by monitoring UV–Visible absorption spectra: Effects of incubation time (A: leaves; B: bark); AgNO3 concentration (C: leaves; D: bark); Extract concentration (E: leaves; F: bark) and Temperature (G: leaves; H: bark).
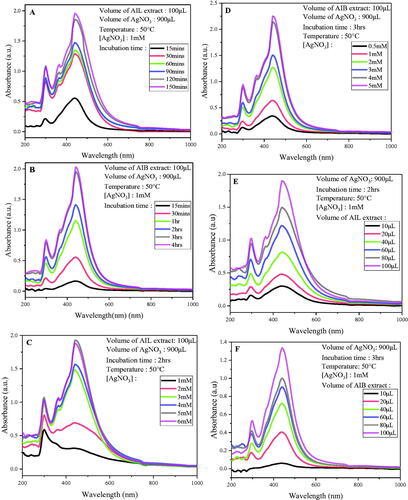
From the first 15 min after incubation, surface plasmon resonance (SPR) band has been observed in the range of 436–446nm characteristic of AgNPs followed by a time-dependent increase of absorbance () as reported by Ahmed et al. [Citation28]. A slight quick reactivity has been observed with A. indica leaves (AIL) extract compared to A. indica bark (AIB). The synthesis reaction was almost complete after 2 and 3 h of incubation for AIL and AIB aqueous extracts respectively.
Optimal synthesis has been obtained at a concentration of 5 and 4 mM for AIL and AIB extracts respectively at 50 °C, with a 1: 9 ratio (extract: AgNO3). The synthesis yield was not significantly different with 5 and 6 mM of AgNPs using AIL leave extract. (). The UV-vis spectra represented in show an optimal synthesis with a volume of 100 ml for both extracts with increase in absorbance with extract volume.
Similarly, an increasing SPR as function of extract temperature was observed. The optimal synthesis for AIL was optimized at 75 °C while AIB optimized at 65 °C. (). Comparable results have been reported by other authors, who found good NPs synthesis at higher temperature [Citation29,Citation30]. Actually, high temperature accelerate the nucleation process, thus improving the synthesis yield, which result in the increase of SPR band intensity [Citation31].
Overall SPR band on surface increases with each parameter evaluated as seen in other plants such as Syzygium jambos [Citation29], Olea europaea [Citation32] and Megaphrynium macrostachyum [Citation33]. The quality of synthesis is reflected by the intensity, sharpness and position of the SPR peak, which typically occur between 380 and 450 nm [Citation34]. In this study, an intense and sharp peak without shifting has been observed which is indicative of good quality of NPs synthesis.
Finally, for further analysis the following stoichiometric conditions were retained for NPs synthesis: 100 µL of crude aqueous extract, 900 mL AgNO3 of 5 mM concentration, incubated at 75 °C for 2 and 3 h for AIL-AgNPs and AIB-AgNPs, respectively.
Characteristics of silver nanoparticles
UV visible spectroscopy
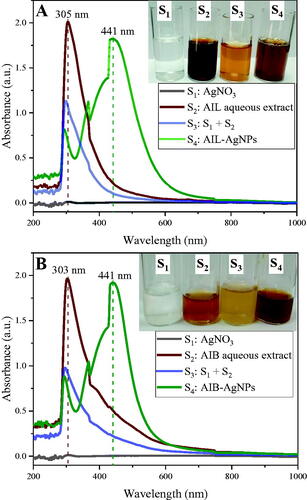
AgNPs have been synthesized by mixing AIL and AIB aqueous extract with AgNO3 (5 mM) for 2 and 3 h, respectively, at 75 °C. The formation of AgNPs is indicated by the colour change of reaction mixture from pale yellow to dark brown caused by the reduction of Ag+ to Ag0. These atoms then form clusters and are stabilized by the biomolecules present in plant extracts [Citation8]. UV-visible spectra of different solutions are shown in . Freshly prepared AgNO3 solution alone did not show any absorption peak between 200 and 1000 nm. A clear absorption peaks have been recorded at 303 and 305 nm for plant extract + AgNO3 solutions just after their mixing, corresponding to the absorption of AIL and AIB crude aqueous extracts, respectively. These peaks faded progressively for AgNPs peacks at longer wavelength. The absorption spectra of both AgNPs solutions show a single SPR band in the range of 436–446 nm with a peak at λmax 441 nm (), which is consistent with previous studies [Citation10,Citation28,Citation34–36]. Referring to Mie’s theory and Martínez-Castañón’s et al. results, this observation suggests presence of spheroidal shaped NPs with a size below 50 nm and further confirmed by TEM experiments [Citation37,Citation38].
XRD patterns
The crystalline nature of AgNP was assessed by X-ray crystallography. The XRD analysis of AIL-AgNPs and AIB-AgNPs showed similar pattern with various Bragg’s peaks, sets of lattice planes (). Peaks located at 2θ of 38.10°, 44.20°, 64.41° and 77.39°, corresponding to the (111), (200), (220) and (311) planes respectively, matched well with the facets of face-centred cubic (fcc) pattern of crystal structure of AgNPs in the Joint Committee on Powder Diffraction Standards reference library (cards 4-0783).
Figure 3. X-ray diffraction pattern of silver nanoparticles from A. indica leaves (AIL) and bark (AIB). ● represents silver nanocrystallites and ![]()
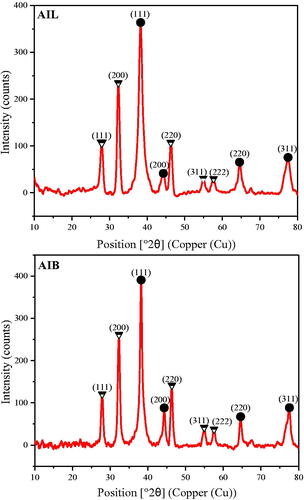
Similarly, peaks located at 28°, 32.6°, 46.6°, 55.2° and 57.8° corresponding to the (111), (200), (220), (311) and (222) planes, respectively, could be assigned to the cubic structure of silver chloride nanocrystallites. The presence of chloride has been confirmed by the EDX experiment.
Crystallinity index of AIL-AgNPs and AIB-AgNPs were 0.759 and 0.764, respectively. The XRD pattern thus clearly shows that AgNPs synthesized by the reduction of Ag + ions by A. indica aqueous extract are crystalline in nature. These results are in agreement with previous studies [Citation35,Citation36]. The average crystallite size using Debye–Scherrer formula were found to be 13.50 ± 1.83 nm for AIL-AgNPs and 18.23 ± 2.74 nm for AIB-AgNPs.
SEM and EDX experiments
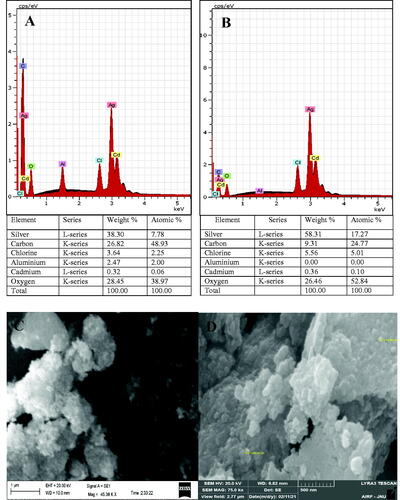
SEM and EDX experiments have been used to observe the surface morphology and to identify and quantify the chemical elements of AgNPs respectively. EDX spectra of both AgNPs have showed the presence of strong signals of silver atom (Ag) and other peaks less important corresponding to carbon (C,) chlorine (Cl), aluminium (Al), cadmium (Cd), and oxygen (O) elements (). C, O, and Cl belong to the plant extracts that cap and stabilize biogenic NPs and that has been confirmed by GC-MS and FTIR results. Al and Cd elements not found in aqueous extracts would come from the sample preparation material. SEM images revealed an agglomerate state of spherical grains AgNPs, even though FTIR results demonstrate the presence of proteins on the NPs surface that can protect them from agglomeration (). This observation, probably due to the high surface energy per unit volume ratio has been reported by other studies too [Citation39,Citation40].
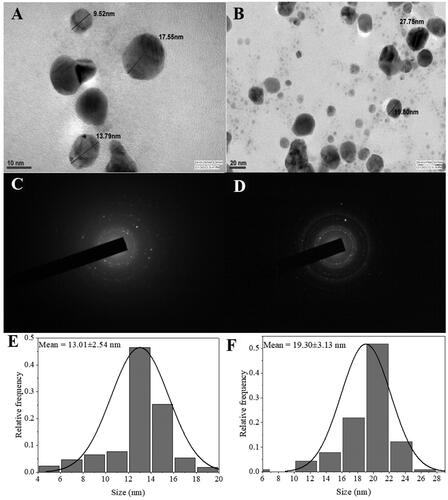
TEM imaging
The morphology and size of AgNPs were analysed by TEM imaging. Contrary to the SEM results TEM imaging showed well dispersed NPs, predominantly spheroidal in shape (). It could be assumed that NPs aggregation occurred during the low vacuum drying in SEM analysis [Citation41]. representing the selected area electron diffraction (SAED) pattern show multiple diffraction rings, indicating their polycrystalline nature which is consistent with the XRD results. The NPs size ranged from 4 to 28 nm with an average size of 13.01 ± 2.54 and 19.30 ± 3.13 nm for AIL-AgNPs and AIB-AgNPs, respectively (). These values were close to that calculated above with Debye–Scherrer formula from XRD results and conform to the shape of SPR band in the UV visible spectrum.
Figure 5. Transmission electron microscopy images of biosynthesized silver nanoparticles (AgNPs) from A. indica leaves (A) and bark (B). Selected area electron diffraction pattern showing multiple diffraction rings indicating polycrystalline nature of AgNPs leaves (C) and bark (D). Particles size distribution of the AgNPs leaves (E) and bark (F).
DLS
The zeta potentials and hydrodynamic particles’ size distribution were obtained from the DLS experiment (Supplementary Figure 1). Both AIL-AgNP and AIB-AgNP showed a negative surface charge with zeta potential values of −16.90 and −9.03 mV respectively which avoid particles agglomeration as visible on TEM images (). The average particle size of AIL-AgNP and AIB-AgNP were found to be 110.2 and 282 nm respectively very high as compared to values obtained from TEM viewing and XRD experiments. The hydrodynamic size seen by DLS comprises of core and ligand layer which significantly increases the real size of NPs [Citation42].
FTIR
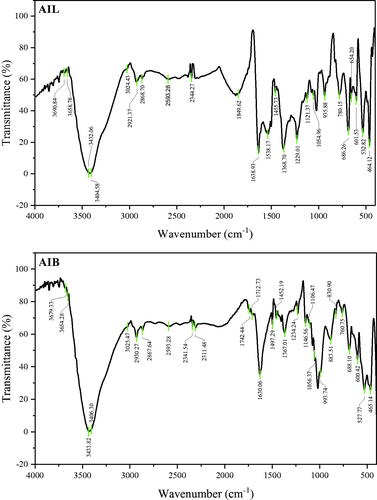
The FTIR spectra of the AIL-AgNPs and AIB-AgNPs were recorded to identify functional groups involved in the synthesis of AgNPs. Analysis of FTIR spectra revealed the presence of a couple of strong prominent peaks in the range of 3750–500 cm−1 reflecting their complex nature (). Numerous peaks were particularly found between 1500 and 500 cm−1 which coincide to the fingerprint region, corresponding to stretching vibration of many functional groups (). These results suggest the presence of polyphenol, protein/amino acid, carbohydrate, phytosterol, terpenoids and flavonoids on the surface of AgNPs responsible for the reduction of silver ions during the synthesis of NPs and act as capping and stabilizing agents. The presence of some of these compounds in plant extracts were further confirmed by GC-MS results (Supplementary Tables 1 and 2). This finding is consistent with other studies that identified the presence of various active phytomolecules belonging to these groups of molecules [Citation34,Citation36].
Table 1. FTIR profile of silver nanoparticles synthesized from A. indica leaves and bark.
Table 2. Comparison of some characteristics with previous studies that reported antiplasmodial activity of silver nanoparticles synthesised from A. indica.
Anti-plasmodial activity
The in vitro antimalarial potentials of aqueous extract and synthesized AgNPs of A. indica leaves and bark have been assessed against 3D7 (CQs) and RKL9 (CQr) P. falciparum strains. In vitro assays revealed the activity of AIL and AIB aqueous extract appear to be lower as compared to their corresponding AgNPs (). AIL-AgNPs and AIB-AgNPs showed an antiplasmodial activity with IC50 values of 9.27 and 8.10 μg/mL against 3D7; 11.14 and 7.87 μg/mL against RKL9 respectively whereas, IC50 values of leaves and bark aqueous extract were 33.97 and 49.64 μg/mL against 3D7; 46.82 and 54.10 μg/mL against RKL9 strain respectively (). Overall, A. Indica leaves aqueous extract as well as the corresponding NPs showed a better antiplasmodial activity than the bark against both tested strains except for AIB-AgNPs against RKL9 (Supplementary Table 3). The more frequent use of leaves than bark both in local population and reported scientific studies could be attributed to effective antiplasmodial property of AI leaves. In vitro growth inhibition potential of AgNPs was validated by light microscopy ().
Figure 7. Anti-plasmodial activity of Chloroquine (CQ), A. indica leaves and Bark aqueous extract and their corresponding silver nanoparticle against CQ-sensitive strain (3D7) and CQ-Resistant strain (RKL9). AIL-AgNP: A. indica leaves silver nanoparticles; AIB-AgNP: A. indica bark silver nanoparticles; AIL-CE: A. indica leaves crude aqueous extract; AIB-CE: A. indica bark crude aqueous extract.
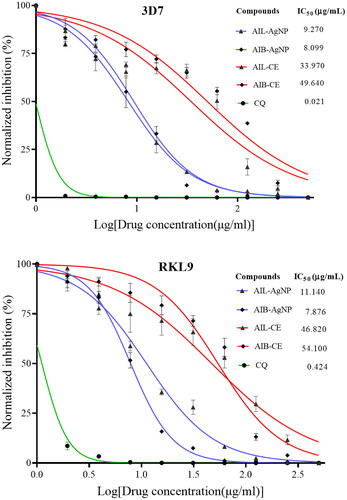
Figure 8. (A) Aspect of culture after synchronization showing healthy parasite at ring stage. Same synchronized culture was used for all tested compounds. (B) Aspect of culture after 24 and 48 h of incubation with different drugs. CQ: Chloroquine; AIL-AgNPs: A. indica leaves silver nanoparticles, AIB-AgNPs: A. indica bark silver nanoparticles.
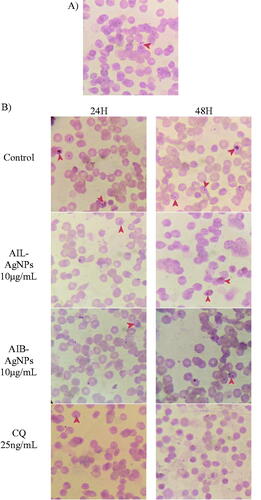
Table 3. Antiplasmodial activity of aqueous extract and A. indica AgNPs after 120 days of storage.
Very few studies from India and Malaysia reported antiplasmodial potential of A. indica AgNPs, summarized in [Citation9,Citation17–19]. No significant difference was observed between our results and recent data published by Ghazali and collaborators from Malaysia for both AIL-AgNPs and AIB-AgNPs (Supplementary Table 4) [Citation9]. In contrast, compared to studies from India which have used same Plasmodium strain (3D7) and same antiplasmodial assessment assay (SYBR Green I fluorescence-based assay), results of the present study show a better antiplasmodial activity of synthesized A. indica AgNPs [Citation17,Citation18]. Actually, growing in different geographical region may significantly affect the metabolic composition of plants and consequently their activity [Citation20,Citation21]. On the other hand, none of these studies optimized the NPs synthesis conditions, which could have affected the quality of synthesis and therefore their efficacy. While the resistance index (RI) of RKL9 against chloroquine has shown a value of 20.19, no resistance against the synthesized AgNPs was observed with RI of 1.20 and 0.97 for AIL-AgNPs and AIB-AgNPs respectively. Murugan et al. have reported the same observation with A. indica seed kernel AgNPs that has shown a RI of 1.04 [Citation18].
It is known that plants synthesize a large arsenal of secondary metabolites mainly for their defence mechanism against insects and microorganisms which also are source for drug discovery [Citation43,Citation44]. To date, the isolated phytometabolites from plants are estimated to be less than 10% of the total number present in medicinal plants [Citation45]. A couple of bioactive compounds including polyphenol, protein/amino acid, cardiac glycosides, anthocyanins, phytosterol, terpenoids, flavonoids and steroids were identified in A. indica crude extracts and NPs as also seen elsewhere which could be responsible for observed antiplasmodial activity [Citation15,Citation44,Citation45]. Most of these secondary metabolites have been reported to exhibit antiplasmodial activity in many other plants [Citation41,Citation44–46]. For instance, quinine, the first phyto-antimalarial compound isolated and artemisinin the most effective antimalarial molecule currently, are both isolated from plants and belong to alkaloids and terpenes groups respectively.
Phenolic compounds play an important function in the synthesis and stabilization of AgNPs [Citation41]. Bio-compounds containing two or more phenolic hydroxyl groups can reduce Ag+ ions to Ag0 by being oxidized into quinones [Citation41,Citation47,Citation48], which serve as a source of stable free radicals and can bind irreversibly with protein resulting in loss of their biological functions [Citation43]. Alkaloids and flavonoids act as iron chelators and inhibitors of nucleic acid base pairing indispensable for parasites survival and proliferation [Citation45,Citation49]. Similarly, the terpenes molecules group seen to be involved in the production of toxic plasmodial free radicals by the cleavage of their peroxide bridge and/or acting as protein inhibitors [Citation50,Citation51]. Additionally, NPs employ various biological mechanisms against multiple molecules target to exert their antimicrobial activity that could be helpful to overcome parasite drug resistance [Citation52]. All these evidences support the fact that medicinal plants are a “reservoir” of potential molecules for antimalarial drug discovery and their activity could be improved by nanotechnology.
Hemocompatibility of AgNPs
Regardless of the drug administration route, molecules reach their targets via blood leading to an inevitable interaction between drug and blood components. Therefore, the biocompatibility of the synthesized AgNPs was carried out by using haemolysis assay. Both AI-AgNPs induced haemolysis as a result of membrane damage at different pH values. A significant haemolytic activity (>25%) was observed with AIL-AgNPs and AIL-AgNPs at concentrations ≥125µg/mL in basic and acid medium (). Among the four-pH evaluated (6.6, 6.8, 7.2 and 7.4) NPs caused significantly higher haemolysis at pH = 6.6 exceeding 50% at 125 μg/mL. Previous studies on the hemocompatibility of nanoparticles have shown that small particles size (<50 nm) have a high haemolytic activity [Citation53–55]. Chen et al. have reported ∼60% haemolysis with AgNPs size of 15 nm and only ∼20% haemolysis with AgNPs of 50 and 100 nm at a concentration of 20 μg/mL [Citation53]. In contrast, relatively low haemolysis rates of ∼8 and ∼10% have been observed with zinc oxide NPs of sizes 40-50 and 70-90 nm respectively, at a concentration of 5 mg/mL [Citation56,Citation57]. In addition, the negative charge on NPs surface favours interaction with the organic cations of erythrocyte membrane [Citation55]. We can assume that the high haemolytic activity observed is related to the small particle size and negative charge of synthesized AgNPs [Citation53].
Figure 9. Haemolysis potential of human red blood cells of synthesized A. indica silver nanoparticles leaves (A–D) and bark (E–H). Triton X (10%) was used as control. Results are plotted as normalized mean ± standard deviation. The green dash line represents a 5% cut off line according to the ASTM criteria (E2524-08 card). Fluorescence quenching effects of aqueous extracts and silver nanoparticles using 3D7 (I) and RKL9 (J) strains. AIL-AgNPs: A. indica leaves silver nanoparticles; AIB-AgNPs: A. indica bark silver nanoparticles; AIL-CE: A. indica leaves crude extract; AIB-CE: A. indica bark crude extract.
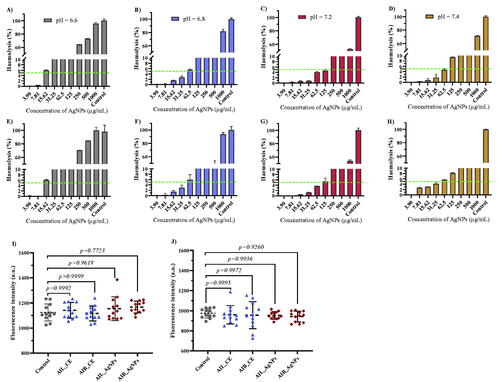
According to the American society for testing and materials criteria (ASTM: E2524-08 card), NPs that can induce a haemolysis percentage >5% are considered damaging to red blood cells [Citation58]. This threshold has been reached at concentrations of 125 and 62.5 µg/mL at pH = 7.2 (the nearest tested value to the physiological pH of human blood) for AIL-AgNPs and AIL-AgNPs respectively. These values are high compared to the IC90 values (39.72 and 37.53 µg/mL with 3D7, 41.77 and 20.81 µg/mL with RKL9 using AIL-AgNPs and AIL-AgNPs respectively) obtained with both tested strains demonstrated the promising potential of these NPs. Thus, we observed that synthesized AgNPs using A. indica leaves and bark are biocompatible in nature at lower concentration (<50 μg/mL).
Quenching effect
Besides, the fluorescence quenching effect artefact potentially generated by crude aqueous extracts and synthesized AgNPs were assessed. Neither aqueous extract nor AgNPs showed any significant quenching effects at 100 μg/mL with 10% parasitemia of 3D7 and RKL9 culture supporting the validity of SYBR Green I assay based results described in this study (). Similarly, Kaushik et al. reported no quenching effect using 25 Indian medicinal plants using the same method [Citation26].
Temporal stability of AgNPs
Very few studies focus on temporal stability of green synthesis NPs [Citation59]. The stability of AI-AgNPs under different storage conditions were evaluated over a period of 120 days. SPR spectrum recorded at regular intervals of 30 days revealed a constant absorption peak at λmax = 441 nm without shifting or apparition of new peak regardless of storage conditions (). AI-AgNPs appear to be very stable at low temperature. The SPR of aliquots stored at +4 °C and −20 °C gare quasi-identical over time. However, the absorption intensity of aliquots stored at RT decreased over the time particularly for AIB-AgNPs. Similar result has been reported by Yildiztekin et al. after 45 days with Crocus mathewii mediated AgNPs [Citation59]. Tejamaya reported a loss of colour, a SPR change and shifting of λmax with citrate coated AgNPs depending on the storage medium and the caping agent in 21 days monitoring [Citation60]. None of these changes were observed in our study up to 120 days. The same study also reported that the presence of sulphate or nitrate in a diluted medium conferred better stability to NPs. The presence of these two minerals confirmed by GC-MS are probably responsible for this conserve SPR supporting their structural stability. We report here that capping agents present in A. indica extract involve in NPs synthesis are able to ensure a good stability for the synthesized AgNPs.
Figure 10. Surface plasma resonance stability of silver nanoparticles in different storage condition. AIL-AgNPs: A. indica leaves silver nanoparticles; AIB-AgNPs: A. indica bark silver nanoparticles; RT: room temperature.
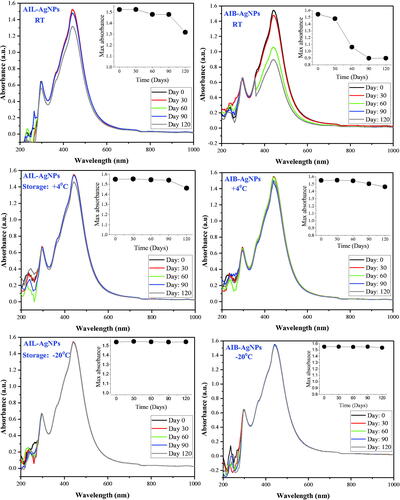
The preservation of antiplasmodial activity of NPs was also assessed for each storage condition after 120 days. A significant loss of antiplasmodial activity of both aqueous extracts and NPs has been observed for aliquots stored at +4 degrees and RT (). This observation is not surprising for aqueous extract since partial/complete degradation of secondary metabolites is a well-known phenomenon in plants that occurs notably by auto-oxidation affecting their biological activity [Citation61,Citation62]. As for NPs, stability of the SPR bands observed in spectroscopy has not been linked to conservation of their biological activity. A significant reduction of anti-plasmodial activity of NPs stored at +4 °C was observed though their SPR band showed very little variation. The structural stability of NPs that can be monitored by spectroscopy does not necessarily reflect their functional stability. Thus, UV-vis spectroscopy would not be a suitable technique applicable for monitoring the biological activity of NPs. Conversely, a 4-months storage at −20 °C did not generate any significant modification of the NPs anti-plasmodial activity (), reflecting their structural and functional stability at low temperature.
Conclusions
In summary, colloidal phyto-functionalized AgNPs were successfully synthesized from A. indica (leaves and bark) aqueous extract. Both AgNPs were found to be spheroidal in shape, crystalline in nature, negatively charged with a diameter less than 20 nm. Polyphenol, flavonoids and alkaloids could be majorly involved in the synthesis with the contribution of other compound containing amide/amine and sulphur groups notably proteins. AIB-AgNPs and AIB-AgNPs exhibited a good antiplasmodial activity against P. falciparum sensitive (3D7) and resistant (RKL9) strains thereby justifying the traditional use of the plant by African and Asian populations. A. indica contain bioactive phyto-compounds that have a great potential for anti-malarial drug development. AI-AgNPs synthesized are heat-labile structurally though functional stable at −20 °C. Due to their small size, both AgNPs exhibited higher haemolysis effect against fresh human RBCs. To overcome this issue, further work has to be carried out to understand the AgNPs-RBC’s complex interactions that lead to toxicity in order to improve the hemocompatibility of AgNPs.
Consent for publication
All authors have read and provided consent for the manuscript to be published.
Author contributions
Conception and design: JH and VS
Data Analysis and interpretation: JH, LPKF and VS
Drafting of the paper: JH, LPKF
Revision and intellectual critic: VP and VS
Supervision: VS
Supplemental Material
Download MS Word (135.3 KB)Acknowledgements
The authors are grateful to the Department of Biotechnology (DBT), Government of India and The World Academy of Sciences (TWAS) for awarding Post Graduate Fellowship to JH and LPKF at the ICMR-National Institute of Malaria Research, Delhi, India. The authors are also thankful to Advanced Instrumentation Research Facility (JNU, New Delhi) and Sophisticated Analytical Instrumentation Facility (AllMS, New Delhi) for providing technical platform for plant extracts and nanoparticles characterization.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and in supplementary files.
Additional information
Funding
References
- World Malaria Report. 2021. https://www.who.int/publications-detail-redirect/9789240040496
- Abuaku B, Duah-Quashie NO, Quashie N, et al. Trends and predictive factors for treatment failure following artemisinin-based combination therapy among children with uncomplicated malaria in Ghana: 2005-2018. BMC Infect Dis. 2021;21(1):1255.
- Nhama A, Nhamússua L, Macete E, et al. In vivo efficacy and safety of artemether-lumefantrine and amodiaquine-artesunate for uncomplicated Plasmodium falciparum malaria in Mozambique, 2018. Malar J. 2021;20(1):390.
- Takashima E, Tachibana M, Morita M, et al. Identification of novel malaria transmission-blocking vaccine candidates. Front Cell Infect Microbiol. 2021;11:805482.
- Gansané A, Candrinho B, Mbituyumuremyi A, et al. Design and methods for a quasi-experimental pilot study to evaluate the impact of dual active ingredient insecticide-treated nets on malaria burden in five regions in Sub-Saharan Africa. Malar J. 2022;21(1):19.
- Ratan ZA, Haidere MF, Nurunnabi M, et al. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers (Basel). 2020;12(4):855.
- Borgheti-Cardoso LN, Anselmo MS, Lantero E, et al. Promising nanomaterials in the fight against malaria. J Mater Chem B. 2020;8(41):9428–9448.
- Jebril S, Fdhila A, Dridi C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci Rep. 2021;11(1):22060.
- Ghazali SZ, Mohamed Noor NR, Mustaffa KMF. Anti-plasmodial activity of aqueous neem leaf extract mediated green synthesis-based silver nitrate nanoparticles. Prep Biochem Biotechnol. 2022;52(1):99–107.
- Poopathi S, De Britto LJ, Praba VL, et al. Synthesis of silver nanoparticles from azadirachta indica—a most effective method for mosquito control. Environ Sci Pollut Res Int. 2015;22(4):2956–2963.
- Mousavi SM, Hashemi SA, Ghasemi Y, et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study, Artificial Cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S855–S872.
- Dos Santos CA, Seckler MM, Ingle AP, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103(7):1931–1944.
- Yaqoob AA, Ahmad H, Parveen T, et al. Recent advances in metal decorated nanomaterials and their various biological applications: a review. Front Chem. 2020;8:341.
- Oves M, Ahmar Rauf M, Aslam M, et al. Green synthesis of silver nanoparticles by conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J Biol Sci. 2022;29(1):460–471.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (azadirachta indica): an indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20.
- Gathirwa JW, Rukunga GM, Mwitari PG, et al. Traditional herbal antimalarial therapy in kilifi district, Kenya. J Ethnopharmacol. 2011;134(2):434–442.
- Mishra A, Kaushik NK, Sardar M, et al. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf B Biointerfaces. 2013;111:713–718.
- Murugan K, Panneerselvam C, Samidoss CM, et al. In vivo and in vitro effectiveness of azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res Vet Sci. 2016;106:14–22.
- Sardana M, Agarwal V, Pant A, et al. Antiplasmodial activity of silver nanoparticles: a novel green synthesis approach. Asian Pac J Trop Biomed. 2018;8(5):268.
- Sampaio BL, Edrada-Ebel R, Da Costa FB. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci Rep. 2016;6:29265.
- Pérez de la Vega M. Plant genetic adaptedness to climatic and edaphic environment. Euphytica. 1996;92(1-2):27–38.
- He S, Yao J, Xie S, et al. Superlattices of silver nanoparticles passivated by mercaptan. J. Phys. D: Appl. Phys. 2001;34(24):3425–3429.
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675.
- Smilkstein M, Sriwilaijaroen N, Kelly JX, et al. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48(5):1803–1806.
- Miki M, Tamai H, Mino M, et al. Free-radical chain oxidation of rat red blood cells by molecular oxygen and its inhibition by alpha-tocopherol. Arch Biochem Biophys. 1987;258(2):373–380.
- Kaushik NK, Bagavan A, Rahuman AA, et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar J. 2015;14:65.
- Wiley BJ, Im SH, Li Z-Y, et al. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B. 2006;110(32):15666–15675.
- Ahmed S, Ahmad M, Swami BL, et al. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9(1):1–7.
- Dutta PP, Bordoloi M, Gogoi K, et al. Antimalarial silver and gold nanoparticles: green synthesis, characterization and in vitro study. Biomed Pharmacother. 2017;91:567–580.
- Busari ZA, Dauda KA, Morenikeji OA, et al. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front Pharmacol. 2017;8:622.
- Smitha SL, Nissamudeen KM, Philip D, et al. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2008;71(1):186–190.
- Khalil MMH, Ismail EH, El-Baghdady KZ, et al. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem. 2014;7(6):1131–1139.
- Eya’ane Meva F, Segnou ML, Ebongue CO, et al. Spectroscopic synthetic optimizations monitoring of silver nanoparticles formation from Megaphrynium macrostachyum leaf extract. Rev Bras Farmacogn. 2016;26(5):640–646.
- Asimuddin M, Shaik MR, Adil SF, et al. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J King Saud Univ Sci. 2020;32(1):648–656.
- Al Aboody MS. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis, artificial cells. Artif Cells Nanomed Biotechnol. 2019;47(1):2107–2113.
- Shankar SS, Rai A, Ahmad A, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496–502.
- Mie G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann Phys. 1908;330(3):377–445.
- Martínez-Castañón GA, Niño-Martínez N, Martínez-Gutierrez F, et al. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res. 2008;10(8):1343–1348.
- Talabani RF, Hamad SM, Barzinjy AA, et al. Biosynthesis of silver nanoparticles and their applications in harvesting sunlight for solar thermal generation. Nanomaterials (Basel). 2021;11(9):2421.
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2019;12(7):908–931.
- Macovei I, Luca SV, Skalicka-Woźniak K, et al. Phyto-functionalized silver nanoparticles derived from conifer bark extracts and evaluation of their antimicrobial and cytogenotoxic effects. Molecules. 2021;27(1):217.
- Pabisch S, Feichtenschlager B, Kickelbick G, et al. Effect of interparticle interactions on size determination of zirconia and silica based systems – a comparison of SAXS, DLS, BET, XRD and Chem Phys Lett. 2012;521(C):91–97.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582.
- Bekono BD, Ntie-Kang F, Onguéné PA, et al. The potential of anti-malarial compounds derived from African medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malar J. 2020;19(1):183.
- Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front Microbiol. 2019;10:911.
- Phillipson JD, Wright CW. Antiprotozoal agents from plant sources. Planta Med. 1991;57(7 Suppl):S53–S59.
- Benedec D, Oniga I, Cuibus F, et al. Origanum vulgare mediated green synthesis of biocompatible gold nanoparticles simultaneously possessing plasmonic, antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:1041–1058.
- Zuorro A, Iannone A, Natali S, et al. Green synthesis of silver nanoparticles using bilberry and red currant waste extracts. Processes. 2019;7(4):193.
- Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26(5):343–356.
- Liao LL, Kupchan SM, Horwitz SB. Mode of action of the antitumor compound bruceantin, an inhibitor of protein synthesis. Mol Pharmacol. 1976;12(1):167–176.
- Guimarães AC, Meireles LM, Lemos MF, et al. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24(13):2471.
- Pandey RP, Mukherjee R, Priyadarshini A, et al. Potential of nanoparticles encapsulated drugs for possible inhibition of the antimicrobial resistance development. Biomed Pharmacother. 2021;141:111943.
- Chen LQ, Fang L, Ling J, et al. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 2015;28(3):501–509.
- de la Harpe KM, Kondiah PPD, Choonara YE, et al. The hemocompatibility of nanoparticles: a review of cell–nanoparticle interactions and hemostasis. Cells. 2019;8(10):1209.
- Han Y, Wang X, Dai H, et al. Nanosize and surface charge effects of hydroxyapatite nanoparticles on red blood cell suspensions. ACS Appl Mater Interfaces. 2012;4(9):4616–4622.
- Das D, Nath BC, Phukon P, et al. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf B Biointerfaces. 2013;111:556–560.
- Najoom S, Fozia F, Ahmad I, et al. Effective antiplasmodial and cytotoxic activities of synthesized zinc oxide nanoparticles using rhazya stricta leaf extract. Evid Based Complement Alternat Med. 2021;2021:5586740.
- ASTM E2524 - 08(2013) | Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles | ASTM | STATNANO, (n.d.). Available from: https://statnano.com/standard/astm/50/ASTM-E2524-08. (2013) (accessed 15 January 2022).
- Yildiztekin M, Nadeem S, Yildiztekin F, et al. Green synthesis and characterization of silver nanoparticles from crocus mathewii; a disremembered turkish flowering plant. Indian J Pharm Sci. 2017;79(4):536–543.
- Tejamaya M, Römer I, Merrifield RC, et al. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46(13):7011–7017.
- Thakur L, Ghodasra U, Patel N, et al. Novel approaches for stability improvement in natural medicines. Pharmacogn Rev. 2011;5(9):48–54.
- Ali A, Chong CH, Mah SH, et al. Impact of storage conditions on the stability of predominant phenolic constituents and antioxidant activity of dried piper betle extracts. Molecules. 2018;23(2):484.