Abstract
Neonatal sepsis is considered as alarming medical emergency and becomes the common global reason of neonatal mortality. Non-specific symptoms and limitations of conventional diagnostic methods for neonatal sepsis mandate fast and reliable method to diagnose disease for point of care application. Recently, disease specific biomarkers have gained interest for rapid diagnosis that led to the development of electrochemical biosensor with enhanced specificity, sensitivity, cost-effectiveness and user-friendliness. Other than conventional biomarker C-reactive protein to diagnose neonatal sepsis, several potential biomarkers including Procalcitonin (PCT), Serum amyloid A (SAA) and other candidates are extensively investigated. The present review provides insights on advancements and diagnostic abilities of protein and nucleotide based biomarkers with their incorporation in developing electrochemical biosensors by employing novel fabrication strategies. This review provides an overview of most promising biomarker and its capability for neonatal sepsis diagnosis to fulfil future demand to develop electrochemical biosensor for point-of-care applications.
Introduction
Neonatal sepsis (NS) is considered as a major primacy by World Health Organisation (WHO) due to its disastrous role in global neonatal mortality, affecting an estimated 3 million newborns i.e. 22 per 1000 live births [Citation1]. NS is a microbial infection of preterm and newborn babies, caused due to activated hyper-active inflammatory mediators, such as cytokines, free radicals and other proteins, that ultimately cause failure of vital body organs [Citation2]. The recent reports on global NS distribution have shown increased incidence in developing countries, with 50–70 cases/1000 live births, as compared to lower cases i.e. 1–5 cases/1000 live birth in developed countries, with highest incidence is observed in Asia and Africa, i.e. 25–40 cases/1000 live births [Citation3,Citation4]. Negligence in neonatal care, non-availability of trained health professional, compromised life-saving facilities and unaffordable treatment cost can lead to uncontrolled consequences of NS [Citation5]. Depending upon the emergence onset of NS, it is divided into two major categories, i.e. Early Onset Sepsis (EOS) and Late-Onset Sepsis (LOS). Major differences in EOS and LOS in terms of associated risk factors, pathogens and mode of transmission are summarised in .
Table 1. Major differences in EOS and LOS.
The high rate of neonatal mortality mandates early diagnosis of NS to help physicians for curing it with best treatment modalities. A positive culture from neonatal body fluids such as blood, urine, cerebrospinal fluid, peritoneal fluid etc. is conventional test for clinical detection of NS, out of which blood culture is regarded as the gold standard examination [Citation10]. However, blood culture faces criticism due to its high volume sample requirement (∼1 mL), high testing cost, inability to differentiate pathogens and contaminants, and false negative reports [Citation11]. Other currently used diagnostic tests such as total whites count, absolute neutrophil count, immature-total neutrophil ratio etc. also report unsatisfactory specificity and sensitivity [Citation12]. Recently, disease diagnosis is benefitted by quantifying differential biomarker level in patient’s body that offers early disease detection, better sensitivity, specificity and accuracy [Citation13,Citation14]. In this connection, modern diagnostic techniques such as turbidimetric method [Citation15], immunonephelometry [Citation16], radioimmuno-diffusion, enzyme-linked immunosorbent assay (ELISA) [Citation17] and Polymerase chain reaction (PCR) [Citation18] etc. are widely employed to detect biomarker level but their unsatisfactory accuracy with labour-intensive, time-consuming and complicated detection steps affect the testing efficiency. In recent years, electrochemical biosensors have attracted enormous attention due to their advantages such as high sensitivity, selectivity, accuracy, miniaturisation and portability [Citation19–22]. Recently, studies have reported development of electrochemical biosensors to detect NS by employing various types of nanomaterials, such as nanosheets, nanoparticles, nanotubes, nanowires, nanospheres, and nanoflowers, acting as modifiers to the electrodes, made up of metals such as gold, silver or platinum, semiconductors such as indium tin oxide or carbon materials such as graphite [Citation23]. Further, biomarker specific biomolecules are employed as biorecognition element that are immobilised on nanomaterial modified electrodes, ultimately improving analytical performance of electrochemical sensors by quantifying the biomarker [Citation24]. A schematic representation of fabricating an electrochemical biosensor for diagnosing NS is shown in .
Figure 1. Schematic representation of fabricating electrochemical sensor to detect neonatal sepsis biomarker. [Abbreviations - CV: Cyclic voltammetry, EIS: electrochemical impedance spectroscopy, DPV: differential pulse voltammetry, SWA: square wave voltammetry, CA: chronoamperometry, POC: point of care].
![Figure 1. Schematic representation of fabricating electrochemical sensor to detect neonatal sepsis biomarker. [Abbreviations - CV: Cyclic voltammetry, EIS: electrochemical impedance spectroscopy, DPV: differential pulse voltammetry, SWA: square wave voltammetry, CA: chronoamperometry, POC: point of care].](/cms/asset/a044ee8d-758b-499e-bf09-b52edd292535/ianb_a_2252016_f0001_c.jpg)
Despite extensive advancement in electrochemical biosensing of diseases, there are limited numbers of electrochemical biosensors developed for diagnosing NS [Citation25]. Recent studies have reported a number of biomarker candidates for diagnostic application to detect NS but there is still a debateable conclusion for the most promising biomarker with diagnostic perfection and the establishment of universally accepted diagnsotic biomarker is yet to be achieved [Citation26]. Further, research is mandatory to develop an efficient Point-Of-Care (POC) based electrochemical biosensor that is user-friendly, rapid, cost-effective and can specifically detect the promising NS biomarker with improved sensitivity under wide linear range.
In this connection, the present review intends to provide detailed evaluation of recently investigated diagnostic biomarkers and their further utilisation in developing electrochemical biosensors for NS detection. The present work can contribute in providing direction in establishing a promising biomarker and can contribute in enriching updates for further advancement in fabricating electrochemical biosensors with improved diagnostic performance to detect NS for POC applications.
Diagnostic utility of biomarkers for the diagnosis of NS
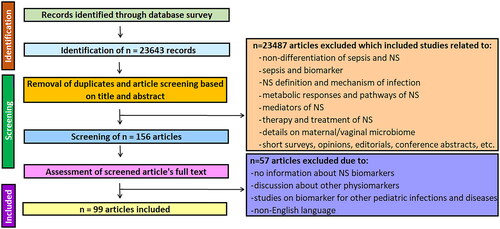
In order to explore articles dealing with recent diagnostic biomarkers of NS, exhaustive literature survey was performed. Briefly, articles search was carried out in the databases including Scopus, PubMed, Science Direct, Google Scholar and Plos. Search keywords for the relevant articles included ‘diagnostic biomarkers’ for ‘neonatal sepsis’ in title/abstract of the articles. For specific search of relevant articles for NS diagnostic biomarker in recent years, articles were screened within publication year range from 2013 to 2023. To exclude irrelevant articles and to focus on recently investigated NS diagnostic biomarkers, exclusion criteria covered articles not related to sepsis, discussing definition, signalling processes, infection mechanisms, therapy, treatment of NS and other neonatal infections and diseases. Articles published in other language than English, articles without details specifically for NS biomarkers were also excluded. The search process for screening the articles discussing diagnostic advantages and applications of recently reported NS biomarkers is represented in .
Based on the database search, record screening and literature review, relevant research studies are included that have reported NS diagnosis by recently investigated diagnostic biomarkers. These biomarkers cover acute phase proteins, cell surface antigens, cytokines, adhesion molecules, and other nucleic acid based biomarkers [Citation27]. The general signalling and expression pathway for the biomarkers in NS has been represented in .
Figure 3. Schematic representation of different diagnostic biomarker expressions due to bacterial infection in neonatal sepsis. [Abbreviations - SAA: serum amyloid A, CRP: C reactive protein, LBP: Lipopolysaccharide Binding Protein, PCT: procalcitonin, IL: interleukin].
![Figure 3. Schematic representation of different diagnostic biomarker expressions due to bacterial infection in neonatal sepsis. [Abbreviations - SAA: serum amyloid A, CRP: C reactive protein, LBP: Lipopolysaccharide Binding Protein, PCT: procalcitonin, IL: interleukin].](/cms/asset/90577550-54be-4897-8594-351e5e6724d2/ianb_a_2252016_f0003_c.jpg)
Protein based biomarkers for diagnosing NS
C-reactive protein
C-reactive protein (CRP), a type of circular, calcium dependent, acute-phase plasma protein, is synthesised in liver, kidney and atherosclerotic tissues [Citation28]. It is the most widely used biomarker for diagnosing NS with normal cut off value of 10 mg/L [Citation29]. In normal neonates, CRP is found be in range of 0–3 mg/L while its value is elevated in NS patients [Citation30]. Various studies have reported the diagnostic role of CRP to detect NS, in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at specific cut-off values, which are summarised in . It is observed that the concentration of CRP may increase upto thousand-fold and elevate within 8–10 h during microbial infection and drops quickly after microbial exclusion, presenting its limitation as a reliable biomarker due to its short half-life, i.e. ∼19 h [Citation49]. The non-specificity, poor sensitivity, false-positive reports and amplified levels in some non-infected neonates are other limitations of CRP, which further lessen its universal acceptance [Citation2, Citation71]. Also, CRP is used as a biomarker in cardio-vascular disorders [Citation72,Citation73], dengue [Citation74], Alzheimer’s disease [Citation75] and solid tumours diagnosis [Citation76]. Recently, CRP level are found to be elevated in COVID-19 patients that may be associated with severity of disease [Citation77,Citation78]. In a latest study, a positive correlation between CRP level and lung lesions were reported, indicating that CRP concentration can indicate disease severity [Citation79]. Another report by Sahu et al. considered CRP as a promising biomarker for evaluating COVID-19 lethality [Citation80], indicating the non-specificity of CRP as a biomarker. Therefore, it is suggested that CRP should be used in combination with other clinical data to make further judgements regarding treatment of NS [Citation81].
Table 2. The diagnostic parameters of biomarkers for the identification of neonatal sepsis.
Recent reports include latest studies suggesting the better diagnostic ability of CRP when combined with other diagnostic biomarkers for rapid and sensitive detection of neonatal sepsis. For instance, a latest study reported by Tessema et al. recommended that the combination of biomarkers Interleukin-6 (IL-6) and CRP showed better diagnostic results in terms if improved sensitivity for early and accurate diagnosis of neonatal sepsis [Citation82].
There are a number of research studies reporting the combined application of CRP and other biomarkers such as CD64 [Citation83], serum amyloid A (SAA) [Citation84], Tumour Necrosis Factor- alpha (TNF-ɑ) [Citation85], procalcitonin and CD11b [Citation59] can enhance diagnostic discriminative power over traditional tests with better diagnostic accuracy. Interestingly, recent studies have suggested that salivary CRP can be employed as an alternative biomarker to serum CRP to diagnose neonatal sepsis with a moderate positive correlation between salivary and serum CRP. However, it is suggested that its widespread application in diagnosis require further research [Citation86]. Another latest study reported by Barekatain etl al provides evidence about the potential ability of salivary CPR combined with serum CRP in the diagnosis of neonatal sepsis. However, these data need to be justified by further research with larger numbers of neonates [Citation87].
Procalcitonin
The pro-hormone of calcitonin called Procalcitonin (PCT), is a 14.5 kD acute-phase protein which is synthesised by liver cells and monocytes. In normal neonates, PCT is found to be in the range of 0.21 ± 0.12 mg/L while its value is elevated, i.e. 56.27 ± 81.89 mg/L in NS patients [Citation30]. After infection, PCT is released in neonatal blood within 4 h, reaches its maximum concentration during 6–8 h, and increase upto 5000-fold in severe infection, maintaining this concentration for next 24 h [Citation88].
As compared to CRP, PCT is regarded as better biomarker for NS diagnosis as per the reports by various studies tabulated in , showing its better diagnostic ability to detect NS. Despite high test price and differential expression under variable factors [Citation42, Citation89], PCT can be considered as a promisng diagnsotic biomarker for its improved sensitivity and accuracy to diagnose NS.
Serum amyloid A
Serum amyloid A (SAA) is an apo-lipoprotein which is synthesised in liver under strict regulation of IL-1, IL-6, and TNF-α during inflammation [Citation89]. In the normal neonates, it is found in the range of 3.2 ± 3.4 mg/dL while its concentration is found elevated, i.e. 44.4 ± 57.3 mg/dL in NS cases [Citation40]. Various studies have recommended SAA as biomarker for EOS as it shows potential diagnostic ability for risk stratification [Citation90]. However, variation in cut-off value of SAA is observed in recent reports, making SAA questionable in diagnosing NS [Citation43]. In addition, no rise in SAA level during early phase of NS is also reported, making the accuracy of SAA doubtful in NS diagnosis [Citation2].
Lipopolysaccharide binding protein
Lipopolysaccharide (LPS) is a major component of outer membrane of Gram negative bacteria [Citation91]. LPS binding protein (LBP), which is secreted by hepatic cells, makes a complex with LPS during infection and directs this complex to monocyte’s cell surface receptor CD14 that leads to activation of signalling pathway and ultimately generating inflammatory response [Citation92]. After infection, LBP level surges within 6–8 h to a detectable window and stays constant for next 48 h, showing diagnostic ability of LBP to detect NS. Despite of good diagnostic role of LBP in the diagnosis of NS [Citation48] as shown in , its inability to differentiate systemic inflammatory response syndrome (SIRS) and sepsis makes it a non-specific biomarker for NS [Citation61].
Interleukin-6
Sepsis development begins after recognition of pathogenic components, such as endotoxins and exotoxins, which stimulate release of inflammatory mediators such as cytokines TNF-α and IL-6 to promote migration and activation of immune cells after infection [Citation93]. There are multiple reports with increased level of IL-6 in NS [Citation94]. Despite of its promising candidate to diagnose NS [Citation95] as represented in , its different cut-off values, less sensitivity and specificity require further investigations [Citation33, Citation49, Citation51]. Although, improved accuracy in NS diagnosis is reported by combining IL-6 with CRP, but false-positive cases and a very short half-life of IL-6 are major disadvantage as a biomarker because increased level of IL-6 returns to its normal level quickly, even before completion of sampling procedure [Citation26, Citation96].
Interleukin-8
Interleukin-8 (IL-8), a pro-inflammatory cytokine, is released by monocytes and endothelial cells that directs neutrophils migration to infection site. Severity of NS and early diagnosis can be examined by observing IL-8 levels in blood serum [Citation61]. The role of IL-8 in NS diagnosis is studied by various research groups, as tabulated in . As per recent reports, IL-8 is found to be more sensitive but less specific than CRP to detect NS [Citation97]. The amalgamation of IL-8 with other biomarkers such as CRP, PCT, IL-6, and presepsin increased accuracy and can help in NS management [Citation52], but short shelf-life of <4 h counts as a major drawback of IL-8 to be utilised for clinical applications [Citation98].
Tumour necrosis factor-alpha
Like other cytokines, tumour necrosis factor-alpha (TNF-α) is secreted in response to infection. In normal neonates, it is found be in the range of 9.3 ± 4.4 pg/mL while its value is found to be elevated, i.e. 161.9 ± 78.4 pg/mL in NS [Citation99]. The elevated level of TNF-α in NS presents its candidature as a biomarker for diagnosing EOS and LOS [Citation100], as shown in recent studies which are summarised in . However, its expression in various other diseases and rapid change in concentration, make it less specific biomarker [Citation101] and therefore is not considered as a practical or cost-effective approach to detect NS [Citation102].
CD64
The cell surface antigen, Cluster of Differentiation-64 (CD64) is found on neutrophils with increased expression during NS [Citation103]. Neutrophils generate CD64 within 1 h after infection that results into rise in neutrophil phagocytic capacity and intracellular killing of pathogen [Citation103]. The diagnostic role of CD64 for detecting NS has been demonstrated by recent studies, represented in . Despite of its good diagnostic performance [Citation104], few recent studies have not recommended CD64 to be used alone for NS diagnosis [Citation104].
CD11b
CD11b belongs to a class of β2 integrin molecules on resting neutrophils [Citation105]. It is utilised in NS diagnosis as its expression rises <5 min of infection [Citation106]. Its diagnostic role for detecting NS has been demonstrated by recent studies, represented in . CD11b alone is not considered as an ideal biomarker for NS and further studies involving its combination with other biomarkers are required for its clinical application [Citation107].
Neopterin
Neopterin is a pyrazino-pyrimidine derivative which is made by catalysis of guanosine triphosphate (GTP). During infection, T cells gets activated and release interferon-ϒ that subsequently results into the activation of macrophages to release neopterin [Citation108] which is found in serum, urine, and cerebro-spinal fluid. The normal serum level of neopterin in healthy cases is reported to be <2.23–2.46 ng/mL with cut-off value 3.04 ng/mL [Citation109]. As per recent studies, elevated serum concentration of neopterin has a positive correlation with severity of NS [Citation62]. Recent studies have reported that noepterin is a better biomarker as compared to CRP with diagnostic ability tabulated in . However, elevated neopterin level is also monitored in Lyme disease, human immunodeficiency virus (HIV) cases, malaria, rheumatoid arthritis, and tumour which make it less specific for NS diagnosis [Citation110].
Presepsin
Bacterial infections result into a complex formation that involves lipopolysaccharides (LPS), LPS binding protein (LPB) and cluster of differentiation 14 i.e. CD14 which are found on the cell-surface membrane of phagocytes such as neutrophils, monocytes and macrophages [Citation111]. This complex results in downstream signalling, thereby releasing cytokines such as tumour necrosis factor-α, IFN-γ, IL-1β, IL-8 and IL-6, and stimulating phagocytes to release more cytokines, which ultimately generate intense inflammatory reactions involved in systemic inflammatory response syndrome, sepsis shock, and multiple organ dysfunction syndrome [Citation112]. After its secretion from phagocytes, presepsin is released to patient’s serum [Citation113]. According to recent reports, serum concentration of presepsin in healthy individuals is very low and almost undetectable and is observed to be sharply elevated within 24 h after infection [Citation114]. As compared to CRP, its better diagnostic ability can be observed in , showing improved performance in terms of 100% sensitivity, specificity and PPV that represent its strong candidature to detect NS [Citation32,Citation67,Citation68]. Presepsin is proven to be advantageous due to its increased level in first 24 h, unaffected by gender, and its ability to predict patient’s prognosis [Citation115]. The exact biological function of presepsin is not well documented and is expected to be a regulatory molecule of adaptive immune system and activator of monocyte phagocytosis [Citation116].
Progranulin
Progranulin is a 593-amino-acid growth factor which is widely expressed in a variety of cell types including neurons, microglia, astrocytes, and endothelial cells, and is demonstrated for its role in regulating cell signalling, function against bacterial infections and inflammatory diseases. Due to its elevated expression in sepsis patients, few studies have reported its use as a diagnostic biomarker as represented in . However, lack of sufficient data requires further research to employ it as a universal biomarker for diagnosing NS. It is also found to be non-specific as it is expressed in other ailments such as Alzheimer’s disease [Citation117], cancer [Citation118], and neurogenerative diseases [Citation119].
Nucleotide-based electrochemical biosensor for NS
The basis of bacterial infection lies in the expression of bacterial virulent factors that are encoded by their genes. Therefore, various gene associated studies and novel genomic approaches such as DNA and RNA profiling have majorly contributed in identifying new diagnostic virulent gene biomarkers to detect NS [Citation120]. Recent research has applied bioinformatics-based studies to identify differentially expressed genes and miRNAs in NS. Due to their potential diagnostic roles, various virulent genes of E. coli such as fimA (fimbriae expression), hylA (hemolysin production), cnf1 (cytotoxic necrotising factor 1), papA, -C, -G, and –EF (P fimbriae expression), sat1 (toxin auto transporter expression), sfaS (S fimbriae expression), focG (F1C fimbriae expression), fyuA (Yersinibactin expression), aer and iucC (aerobactin expression), alX (pathogenicity island marker), ibeA (invasion factor expression), iroN (siderophore receptor expression), and iha (putative adhesion siderophore expression) have gained recent attention [Citation121,Citation122]. Similarly, genes in GBS such as cylE (β-hemolysin formation), cfb (cAMP factor), and neuA (immune invastion) impact virulence for infection [Citation123]. The results from recent interesting studies have shown the elevated expressions of various genes such as ITGAM, TLR8, IL1b, MMP9, MPO, FPR2, ELANE, SPI1, and C3AR1 in patients suffering from NS, which may have an important contribution on patho-physiological mechanism of NS as represented in .
Table 3. Potential genes and miRNA based biomarkers for the diagnosis of NS.
Recent studies have suggested that elevated expression of ITGAM gene is correlated with decreased survival of septic patients and its blocking has significant inhibition of LPS-mediated endotoxin shock and sepsis [Citation124]. Pro-inflammatory cytokine interleukin-1-beta (IL-1𝛽) level has been found to become rapidly increased with up-expression of respective gene during NS [Citation125]. Induction of LPS-induced sepsis stimulates release of activated matrix metalloproteinase-9 (MMP-9) which may activate monocytes infiltration. In NS cases, MMP9 and respective gene expression levels is reported to be increased, which shows their potential candidature to diagnose NS [Citation126]. The expression of gene coding enzyme myeloperoxidase (MPO) is also found to be elevated in NS and is considered as a potential biomarker for NS detection in recent studies. Salmonella pathogenicity island 1 (SPI1) is a major regulator of myeloid lineage specification during haematopoiesis. Since high expression of SPI1 gene has been reported in NS, it is suggested that inhibiting SPI1 may give a new treatment approach for NS [Citation127]. It has been studied that Elastase neutrophil expressed (ELANE) controls LPS-mediated immune response during infection which led to conclude role of ELANE gene in sepsis development [Citation124].miRNAs are non-coding, ∼22 nucleotides long RNA sequences that bind to complementary site of target mRNA, resulting into mRNA degradation and activation of various signalling pathways that make them to be considered as an ideal biomarker [Citation132]. The individual roles and expected functions of miRNAs recently investigated as a biomarker for NS have been represented in . Chromosomal region 9q22.32 generates miR-23b, which is an important regulator of innate immune response in cancer and other inflammatory reactions. Recent studies report that miR-23b is an important contributor to cardiac fibrosis activation that results into development of myocardial dysfunction in sepsis and its inhibition can be an effective approach to control sepsis-related cardiac dysfunction [Citation128]. In another study, miR-34a-5p and miR-199a-3p are shown to play regulatory roles in NS as they are proved to be engaged in regulating TLR signalling and NF-κB-mediated inflammatory response that leads to NS progression. However, the exact functions of miR-34a-5p and miR-199a-3p in NS have yet to be explored [Citation129]. miR-16a is present on chromosome 13q14 and play roles in inhibiting cell proliferation followed by activation of apoptosis. miR-451 gene is situated on 17q11.2 on chromosome 17 and plays role in different pathological and physiological reactions, such as haematopoietic system differentiation. Recent studies show elevated levels of miRNA-16a and miRNA- 451 in NS patients present their biomarker candidature for diagnosing NS [Citation130]. In another study, miR-15a/16 is reported to have potential role in regulating gene expression at post-transcriptional level, which indicates its application for NS diagnosis [Citation131]. It must be noted that above discussed studies are in initial phase of the research and further exhaustive research is required for employing genes and miRNAs to be used as a biomarker to diagnose NS.
Advancements in development of electrochemical biosensor for NS diagnosis
Researchers have been putting efforts to fabricate electrochemical sensors to detect biomarker of NS to achieve sensitivity, accuracy, portability and early response. As discussed, electrochemical biosensors incorporate a biological recognition element, which include whole cells, tissues slices, enzymes, antibodies, nucleic acids etc. and an electrochemical transducer. The development of recently fabricated electrochemical biosensor for NS diagnosis majorly involve the use of antibodies and nucleic acids as recognition elements which selectively recognise their respective differentially expressed biomarker as target analyte. In this connection, recent advancements in developing electrochemical biosensor for diagnosing NS by quantifying biomarkers have been represented in . Recent research utilises high conductivity of metal nanoparticles and excellent specificity of antibodies for fabricating sensitive electrochemical biosensor to diagnose NS, that is shown by a recent study, such as the development of electrochemical impedimetric biosensor to detect CRP by attaching CRP specific polyclonal antibody on gold (Au) electrode, resulting into reusable sensing platform from dilute or whole-blood serum samples [Citation133]. With advancements in nanotechnology, several modifications in nanomaterial synthesis and functionalization are employed by various researchers. Additionally, screen printed electrodes (SPEs) have gained researchers attention as they offer reduced sample volume, easy-use and low cost development of miniaturised electrochemical sensing platforms with the possibility of connecting it to portable instrumentation. A study conducted by Balayan et al. reported development of an electrochemical biosensor by utilising molecularly imprinted polymer (MIP) fabricated on SPE, coated with gold-platinum (Au-Pt) bimetallic nanomaterials, resulted in detection CRP with sensitivity of 0.14 μA/nM. In order to study selectivity of the biosensor, interference study for the developed electrode was performed with glucose, uric acid, acetylcholine, cholesterol, ascorbic acid, SAA, TNF-α, PCT at a concentration of 0.1 nM and the activity of the developed biosensor (%) was examined. The change in the current (loss in biosensor activity) with other antigens was found below 15% when comparing with the response obtained with CRP. The results show that the biosensor shows higher selectivity, In addition, the stability of the electrode was examined while storing it in a dry condition at 4 °C, followed by continuously monitoring every seventh day up to 3 months. The results showed 70% reduction in the electrode response after 6 weeks [Citation135]. The utilisation of SPE for label free CRP detection is reported by Lakshamanakumar by developing of biosensor based on graphene quantum dots and anti-CRP antibodies with sensitivity of 2.45 µA/ng/mL. The selectivity of the fabricated sensor was tested by incubating the fabricated electrode in interfering species like ascorbic acid, bovine serum albumin, and glucose with CRP. The results showed no significant variation in the current response in the presence of interfering biomolecules, indicating high selectivity of the sensor. The stability was also investigated by storing the fabricated electrode at 4 °C, followed by measuring the current response for 4 weeks, after which the sensor showed a 5.21% decline in current response, confirming the stability of the biosensor [Citation136]. Similar study by Guillem et al. reported a portable, reusable and cost effective electrochemical sensor, fabricated by using SPE with immobilised Au nanoparticles and anti-CRP antibodies to detect CRP to be used in wireless mode by using cellphone or laptop [Citation137]. Latest reports have also shown the use of aptamers, which are specific oligonucleic acid sequences (∼ 30 to 100 nucleotides), that recognise specific ligands and specifically bind to various target molecules with high affinity. A recent study showing development of RNA based electrochemical aptasensor based on carbon nanofibres-chitosan nanocomposite immobilised with RNA aptamer probe, resulting into sensitive CRP biosensor. The selectivity of the fabricated biosensor was also studied which showed that no interference was detected for 10-fold quantities of human serum albumin and i mmunoglobulin G in the determination of CRP, indicating the proposed aptasensor has good selectivity. In addition, the stability of fabricated sensor has examined after two weeks. As per the results, no significant change in the signal was observed (∼2.4%), indicating the high stability of the RNA aptasensor [Citation138]. Mahyari et al. developed an electrochemical aptasensor by synthesising a novel structure based on poly deep eutectic solvent modified graphene oxide, immobilised with Au nanoparticles to detect CRP in a label free manner. To investigate the stability of our aptasensor, it was stored at room temperature and measurement was performed in different periods within 10 days. The results showed 96% of the initial response at end of this time, indicating good stability of the aptasensor [Citation139].
Table 4. Recent Advances in developing electrochemical biosensor to diagnose neonatal sepsis.
Recent reports have published studies focussing on development of electrochemical biosensor with better biomarkers than CRP. For example, a sandwich-type electrochemical immunosensor developed by Yang et al. reported using multi-walled carbon nanotubes (MWCNT), functionalised with cobalt phthalocyanine nanoparticles to detect PCT, showing good sensitivity to diagnose NS. In this study, carbohydrate antigen 19-9, BSA and human cytomegalovirus were used as interfering substance to evaluate the selectivity and specificity of the developed immunosensor. As per the reported results, a significant rise induced by the interaction of the immunosensor probe with 10 ng mL−1 PCT was observed compared to 15 U mL−1 carbohydrate antigen 19-9, 200 ng mL−1 BSA and 100 ng mL−1 human cytomegalovirus, indicating good selectivity of the immunosensor towards target antigen [Citation140]. In another study, a chip-based electrochemical magneto-immunosensor, fabricated by employing biotinylated anti-PCT antibodies immobilised on magnetic beads, is developed to detect PCT rapidly with sensitivity of 39.6 ± 7.5 nA/mL/ng by using only 25 µL of sample [Citation141]. Another study reports modification of standard glassy carbon electrode (GCE) by graphitic carbon nitride nanosheets and polypeptide probe for electrochemical detection of PCT with improved sensitivity. In this study, the specificity of the fabricated electrode was measured by DPV method in the Tris-HCl buffer, PCT, CRP and mixed sample of PCT and CRP respectively. The results showed reduced peak current with the PCT and mixed sample of PCT and CRP, and the slight variation for the CRP only, showing outstanding selectivity of electrochemical sensor for the PCT detection [Citation142]. In a work by Jin et al. nanobrushes developed by using poly-glutamic acid (PGA), anti-PCT antibody, fluorescent dye and immune-magnetic beads on polystyrene (PS) nanospheres incorporated with Rhodamine-6G, are reported to fabricate an immunosensor to quantify PCT in human serum albumin in an enzyme-free approach. The selectivity of sensor was measured by employing interference biomarkers including CRP, IL-6 and Immunoglobulin G (IgG) and further examination by fluorescence signal response of the immunosensor. It was observed that the fluorescence signal response for the mixture of the PCT and each interference biomarker was same with that of the PCT, indicating that the interference biomarkers is not affecting the determination of PCT, resulting in high specificity and selectivity [Citation143]. Advancement in developing a sensitive electrochemical sensor using MoS2/NiCo hetero-structures, further functionalised with palladium nanoparticles and loaded with anti-PCT antibodies, is reported by latest study that resulted in label free PCT detection. In this study, Prostate specific antigen (PSA), IgG, alpha fetoprotein (AFP) and carcinoembryonic antigen (CEA) were employed as interfering agents and mixed with PCT to examine selectivity of fabricated immnunosensor. As per the results, no significant variation was observed by these interferants, with RSD of the obtained signals <5%, showing superior specificity of the immunosensor. The acceptable stability of the immunosensor was measured by storing it at 4 °C for variable number of days, followed by their use to detect PCT. The result showed that the current response maintained at 90% after 24 days, indicating the durable stability [Citation144]. Qu et al. developed an electrochemical sensing platform based on Fe3S4-Pd and pineal mesoporous bioactive glass and quantified PCT by immobilising anti-PCT antibodies in a sandwich manner. To examine the stability of the immunosensor, fabricated sensors were stored in a refrigerator at 4 °C, followed by measurement of the current response of the sensor detecting PCT which resulted into <5.0% after one month as compared to the initial current response, suggesting excellent stability of the immunosensor. In addition, the selectivity of the immunosensor was measured by employing eleven electrodes with testing with different interference substances, i.e. prostate-specific antigen (PSA), BSA, carcinoembryonic antigen (CEA), α-fetoprotein (AFP) and ascorbic acid (AA). The relative standard deviation (RSD) in the current response was found <5.0% when the immunosensors with the interferening agents were compared with the control electrode, indicating acceptable selectivity of the immunosensor [Citation145].
The effort for diagnosing NS by detecting SAA was performed by Balayan et al. in which nanomaterials such as MWCNT, manganese oxide nanospheres and cobalt oxide nanoparticles were integrated with molecularly imprinted polymer over SPE for developing an electrochemical biosensor. The stability of the developed biosensor is reported for 3 months by checking the electrode response every seventh day. As per the results, the activity of the electrode was declined to 50% as compared to initial current response after 6 weeks [Citation146]. Another study involving synthesis of MWCNT-1-Butyl-3-methylimidazolium hexafluorophosphate-chitosan based nanocomposite is reported by Xia et al. in which GCE was amended with carboxy-endcapped polypyrrole (PPy-αCOOH) and synthesised nanocomposite was used to immobilise SAA specific antibodies. To further test the selectivity of the fabricated sensor by adsorbing interfering substances, such as CRP, beta-galactoside alpha2, 6 sialytransferase (ST6GAL1), glypican-3 (GPC3), BSA and glucose. The variation in current response of immunosensor was found weaker as compared to response observed by electrode adsorbed with SAA, indicating acceptable selectivity of the immunosensor. Also, the stability of the immunosensor was studied by measuring the current change over a period of 30 days while storing the electrode at 4 °C. The result reported no significant variation in current response and it retained 91.37% of its initial current response, indicating good long-term stability [Citation147]. There are other studies reporting development of electrochemical biosensors for detecting other biomarkers such as detection of LPS by employing a nanoporous nylon membrane integrated on gold microelectrode, immobilised with ant-LPS antibody [Citation148], detection of IL-6 by developing needle shaped microelectrode, based on silicon substrate functionalised with anti IL-6 antibodies [Citation149], by using a sensing surface, functionalised with Dithiobis (succinimidyl propionate) dissolved in DMSO, followed by immobilisation of capture antibodies [Citation150], by utilising molybdenum electrode on nano-porous polyamide substrate coated with gold [Citation151], detection of IL-8 in saliva by using IL-8 surface imprinted polymer nanoparticles, with graphene oxide functionalised with Fe3O4 nanoparticles to develop electrochemical biosensor [Citation152]. Gold electrode immobilised with gold nanoparticles, probes and doxorubicin hydrochloride (DOX) is reported in another study for fabricating a reusable electrochemical sensor for TNF-α detection. The stability of the sensor was also studied after the storage of the sensor at 4 °C for 20 days. The results showed 90.8% of the DPV signal was retained as compared to initial signal which indicated satisfactory stability of the sensor. To check the selectivity of the sensor, the sensor’s responses to six different cell factors, i.e. IL-3, IL-6, Interferon (IFN), colony stimulating factor (CSF), erythropoietin (EPO), and TNF-α, were measured. As per the obtained results, significant signals were observed only with TNF-α. However, other cell factors could not produce significant signals, showing high specificity for TNF-α detection [Citation153]. A recent study reported preparation of MIP film by electrochemical co-polymerization of bithiophene-5-boronic acid and 2-(cytosin-1-yl) ethyl p-bis (2,2′-bithien-5-yl) methylbenzolate as functional monomers with neopterin template with -hexa (thiophene-2-yl)-3,3′-bithiophene as a cross-linking monomer for developing electrochemical sensor with sensitivity of 7.01 ± 0.15 mV mM−1. In the selectivity study of the developed MIP film, it was found that the sensitivity of the fabricated MIP film towards neopterin is found nearly three-and-half times that to interfering agent, i.e. pterin, almost seventy times that to 6-biopterin, and close to seventeen times that to creatinine with no response to other biocompounds such as glucose and xanthine [Citation154]. Another study reported a potentiostatic sensor development to detect neopterin by using poly(ethylene-co-vinyl alcohol) (EVAL) based thin films and ITO glass electrode with sensitivity of 0.041 pg/mL and reference concentration of 35– 55 ng/mL. The selectivity of the fabricated electrodes was also measured with urea and creatinine. As per the reported results, the electrochemical responses for those molecules were <3.0 mA, indicating that the reported selectivity has potential for use as a homecare system [Citation155]. These studies have proved advancements in NS diagnosis by developing electrochemical biosensors. However, the lacking in this arena cannot be ignored including complex fabrication procedures, additional labelling steps and no full elucidation of absolute sensitivity of fabricated devices [Citation149].
The recent studies show that PCT has better diagnostic performance than CRP and therefore, it is a promising NS biomarker. PCT has various advantages such as it can differentiate bacterial/fungal systemic response from other viral infection, ability to differentiate real infections and contaminated blood cultures, its faster rise in 4 h after exposure to bacteria with maxima after 6–8 h, its serum level association with infection severity, its responsiveness to treatment and declines rapidly after antibiotic treatment [Citation156]. PCT is reported to be less deviated by mode of delivery or surgical operations [Citation81]. PCT has got attention as most promising biomarker of NS among several biomarker candidates due to its higher sensitivity, thereby ensuring improved diagnosis, decreased hospitalisation and antibiotic overuse [Citation157].
In addition, recent findings suggested presepsin as a promising biomarker in NS diagnosis with high sensitivity and specificity [Citation158]. The findings show that prespsin plays a significant role in evaluating therapeutic response during clinical follow-ups as its serum level is found to get decreased progressively in course of antibiotic treatment [Citation159]. Reports have also recommended presepsin utilisation with blood culture to diagnose NS because it showed best sensitivity among other biomarker candidates and can detect most of the positive NS cases [Citation65]. It is reported that without significantly affected by different perinatal variables related to non-infectious conditions, presepsin shows its maximum diagnostic efficacy with cut-off value ranged from 650 to 850 pg/ml, and its level is significantly elevated in third and sixth day after neonatal infection [Citation159]. Apart from the fact that it can get influenced by pathophysiological conditions, presepsin presents a strong candidature to be employed as a promising biomarker to diagnose NS [Citation160]. To examine presepsin as a potential biomarker, presepsin ELISA kits are already being employed to detect high presepsin level in patients [Citation161] but development of sensitive and cost-effective electrochemical sensing platform for rapid presepsin detection for NS diagnosis is awaited.
Recently, miniaturised devices named microfluidic based sensors, which are capable of incorporating multiple functionalities on a single sensing platform to develop POC device with low sample volume, have grabbed limelight for their potential applications in disease diagnosis. In a recent study, development of microfluidic based electrochemical magneto-immunosensor is reported by Molinero-Fernandez et al. to detect CRP by utilising whole volume blood, showing excellent analytical performance with LOD of 1.5 ng/mL and linear range of 0.05–1 µg/mL [Citation162]. However, due to limited number of reports, there is an urgent need for employing a reliable biomarker and subsequent development of microfluidic based electrochemical biosensor to diagnose NS by utilising nano-fabrication techniques for smarter healthcare.
Conclusion
Due to drastic consequences of NS, rapid diagnosis holds supreme importance for neonatal survival. Recent studies have reported progression in novel electrochemical biosensor development based on biomarker recognition to detect NS for POC applications. For effective diagnosis, novel biomarker candidates are proposed due to limitations in conventional diagnostic methods and unsatisfactory diagnostic performance by standard biomarker CRP. In the present study, recently investigated biomarkers PCT, presepsin, neopterin, TNF-ɑ and CD11 are observed to play better diagnostic role to detect NS. However, inability of CD11 and neopterin to be utilised alone and non-specificity of TNF-ɑ make them doubtful for diagnosing NS and mandate their combinations with other diagnostic methods for satisfactory performance. Recent reports on identification of novel virulent genes and miRNAs as biomarkers are recently investigated by ongoing research to validate their functions and are supposed to contribute in seeking universally accepted biomarker. In conclusion, PCT and presepsin are found to be satisfactory and promising biomarkers for diagnosing NS due to high sensitivity and specificity as compared to other candidates. Due to promising capabilities of these biomarkers reported recently, their combination with conventional biomarker CRP has also gained interest and has shown potential role in rapid and sensitive diagnosis of neonatal sepsis. Further research is expected to be focussed on utilisation of these biomarkers by employing novel strategies such as microfluidic based electrochemical biosensing platform for rapid and efficient NS detection.
Authors’ contributions
Neha Gopal: Data curation, writing- Original draft preparation, analysis, revision; Nidhi Chauhan: Conception, methodology, reviewing and editing; Utkarsh Jain: Drafting, design, interpretation of data, reviewing and editing; Sujata K Dass: Visualisation, validation and final approval of the version to be published; Hari S Sharma: Conception, design and validation; Ramesh Chandra: Supervision, conceptualisation, methodology and final approval of the version to be published.
Acknowledgements
Authors thank Vice Chancellor, University of Delhi for providing necessary infrastructure and facilities. Authors would like to thank Dr. Suveen Kumar, DST INSPIRE faculty, Department of Chemistry, University of Delhi, for providing his suggestions during writing and drafting the manuscript.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The data that support the findings of this study are openly available in [google scholar, pubmed] at https://www.scopus.com/home.uri, https://scholar.google.com/, https://pubmed.ncbi.nlm.nih.gov/, https://www.researchgate.net/, reference number [1-156].
Additional information
Funding
References
- Molloy EJ, Wynn JL, Bliss J, et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr Res. 2020;88(1):2–4.
- Jyoti A, Kumar S, Srivastava VK, et al. Neonatal sepsis at point of care. Clin Chim Acta. 2021;521:45–58.
- Ko MH-J, Chang H-Y, Li S-T, et al. An 18-year retrospective study on the epidemiology of early-onset neonatal sepsis-emergence of uncommon pathogens. Pediatr Neonatol. 2021;62(5):491–498.
- Glaser MA, Hughes LM, Jnah A, et al. Neonatal sepsis: a review of pathophysiology and current management strategies. Adv Neonatal Care. 2021;21(1):49–60.
- Qazi SA, Stoll BJ. Neonatal sepsis: a major global public health challenge. Pediatr Infect Dis J. 2009;28(1 Suppl):S1–S2.
- Karabulut B, Arcagok BC. New diagnostic possibilities for early onset neonatal sepsis: red cell distribution width to platelet ratio. Fetal Pediatr Pathol. 2020;39(4):297–306.
- Satar M, Arısoy AE, Çelik İH. Turkish neonatal society guideline on neonatal infections-diagnosis and treatment. Turk Pediatri Ars. 2018;53(Suppl 1):S88–S100.
- Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence. 2014;5(1):170–178.
- Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr (Rio J). 2020;96 Suppl 1(Suppl 1):80–86.
- Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–140.
- Bethou A, Bhat BV. Neonatal sepsis—newer insights. Indian J Pediatr. 2022;89(3):267–273.
- Raimondi F, Ferrara T, Maffucci R, et al. Neonatal sepsis: a difficult diagnostic challenge. Clin Biochem. 2011;44(7):463–464.
- Kurul Ş, Simons SH, Ramakers CR, et al. Association of inflammatory biomarkers with subsequent clinical course in suspected late onset sepsis in preterm neonates. Crit Care. 2021;25(1):1–10.
- Martínez-Sagasti F, Velasco-López E, Domingo-Marín S, et al. Usefulness of biomarkers on infection management: with or without them? Rev Esp Quimioter. 2018;31 Suppl 1(Suppl 1):43–46.
- Trivedi R, Amer A, Patel R, et al. Comparison of a rapid Semi-Quantitative latex agglutination slide method against quantitative particle enhanced turbidimetric immunoassay for measurement of C-ReactiveProtein. Int J Med Biomed Sci. 2019;3(5):190–195.
- Ebenebe CU, Hesse F, Blohm ME, et al. Diagnostic accuracy of interleukin-6 for early-onset sepsis in preterm neonates. J Matern-Fetal Neonatal Med. 2021;34(2):253–258.
- Verma MS, Tsaloglou M-N, Sisley T, et al. Sliding-strip microfluidic device enables ELISA on paper. Biosens Bioelectron. 2018;99:77–84.
- Oeser C, Pond M, Butcher P, et al. PCR for the detection of pathogens in neonatal early onset sepsis. PLoS One. 2020;15(1):e0226817.
- Yoon J, Cho H-Y, Shin M, et al. Flexible electrochemical biosensors for healthcare monitoring. J Mater Chem B. 2020;8(33):7303–7318.
- Kaya HO, Cetin AE, Azimzadeh M, et al. Pathogen detection with electrochemical biosensors: advantages, challenges and future perspectives. J Electroanal Chem (Lausanne). 2021;882:114989.
- Huang X, Xu D, Chen J, et al. Smartphone-based analytical biosensors. Analyst. 2018;143(22):5339–5351.
- Sun AC, Hall DA. Point‐of‐care smartphone‐based electrochemical biosensing. Electroanalysis. 2019;31(1):2–16.
- La M. Electrochemical, electrochemiluminescent and photoelectrochemical immunosensors for procalcitonin detection: a review. Int J Electrochem Sci. 2020;15(7):6436–6447.
- Tsounidi D, Petrou PS, Raptis I. Current progress on biosensors and point-of-Care devices for sepsis diagnosis. IEEE Sens J. 2021;21(11):12840–12855.
- Murković I, Steinberg MD, Murković B. Sensors in neonatal monitoring: current practice and future trends. THC. 2003;11(6):399–412.
- Iroh Tam P-Y, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82(4):574–583.
- Hedegaard SS, Wisborg K, Hvas A-M. Diagnostic utility of biomarkers for neonatal sepsis–a systematic review. Infect Dis (Lond). 2015;47(3):117–124.
- Balayan S, Chauhan N, Chandra R, et al. Recent advances in developing biosensing based platforms for neonatal sepsis. Biosens Bioelectron. 2020;169:112552.
- Yochpaz S, Friedman N, Zirkin S, et al. C-reactive protein in early-onset neonatal sepsis–a cutoff point for CRP value as a predictor of early-onset neonatal sepsis in term and late preterm infants early after birth? J Maternal-Fetal Neonatal Med. 2022;35(23):4552–4557.
- Park IH, Lee SH, Yu ST, et al. Serum procalcitonin as a diagnostic marker of neonatal sepsis. Korean J Pediatr. 2014;57(10):451–456.
- Boseila S, Seoud I, Samy G, et al. Serum neopterin level in early onset neonatal sepsis. J Am Sci. 2011;7(7):343–352.
- El-Masry HM, Hassan A-EA, Amin HH, et al. Evaluation of serum presepsin concentrations as a biomarker of sepsis in high-risk neonates. Al-Azhar Assiut Med J. 2021;19(1):160.
- Ganesan P, Shanmugam P, Sattar SBA, et al. Evaluation of IL-6, CRP and hs-CRP as early markers of neonatal sepsis. J Clin Diagn Res. 2016;10(5):DC13–DC17.
- Bharti AK, Verma MK, Gupta A, et al. Role of procalcitonin in diagnosis of neonatal sepsis and procalcitonin guided duration of antibiotic therapy. 2020.
- Ahmed AM, Mohammed AT, Bastawy S, et al. Serum biomarkers for the early detection of the early-onset neonatal sepsis: a single-center prospective study. Adv Neonatal Care. 2019;19(5):E26–E32.
- Edmond K, Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010;7(3):e1000213.
- Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1–13.
- Rajeev D, Honnumurthy J, Avinash S. Predictability of biomarkers in diagnosis of neonatal sepsis: a cross sectional study. Int J Contemp Pediatr. 2016;3(2):485.
- Tejaswi Nandan D, Vijay M. Assessment of the expenses and benefits of procalcitonin testing in the diagnosis of early onset neonatal sepsis. Eur J Mol Clin Med. 2021;7(9):2798–2805.
- Cetinkaya M, Özkan H, Köksal N, et al. Comparison of serum amyloid a concentrations with those of C-reactive protein and procalcitonin in diagnosis and follow-up of neonatal sepsis in premature infants. J Perinatol. 2009;29(3):225–231.
- Al-Azaowi OA, El-Kaream A, Samir A, et al. Procalcitonin as a diagnostic marker for neonatal sepsis. J Biosci Appl Res. 2018;4(4):432–443.
- Aloisio E, Dolci A, Panteghini M. Procalcitonin: between evidence and critical issues. Clin Chim Acta. 2019;496:7–12.
- Abd Elkhalek HM, Abed N, Abdel Haie O, et al. Role of serum amyloid a protein in the early detection of late onset sepsis in neonate. Benha Med J. 2020;0(0):0–0.
- Wu F, Hou X, Sun R, et al. The predictive value of joint detection of serum amyloid protein A, PCT, and Hs-CRP in the diagnosis and efficacy of neonatal septicemia. Eur Rev Med Pharmacol Sci. 2019;23(13):5904–5911.
- Dessì A, Pravettoni C, Ottonello G, et al. Neonatal sepsis. J Pediatr Neonatal Individual Med (JPNIM). 2014;3(2):e030273–e.
- Leante-Castellanos JL, de Guadiana-Romualdo LG, Fuentes-Gutiérrez C, et al. The value of lipopolysaccharide binding protein for diagnosis of late-onset neonatal sepsis in very low birth weight infants. J Perinat Med. 2015;43(2):253–257.
- Pavcnik-Arnol M, Hojker S, Derganc M. Lipopolysaccharide-binding protein in critically ill neonates and children with suspected infection: comparison with procalcitonin, interleukin-6, and C-reactive protein. Intensive Care Med. 2004;30(7):1454–1460.
- Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, et al. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009;35(11):1950–1958.
- Sharma A, Thakur A, Bhardwaj C, et al. Potential biomarkers for diagnosing neonatal sepsis. Curr Med Res Pract. 2020;10(1):12–17.
- Bhowmik A, Samanta M, Hazra A, et al. Study to evaluate the role of TNFa, IL1ß, IL6 in diagnosis and severity assessment of neonatal sepsis among term, appropriate for gestational age newborns. Perinatal J. 2021;29(3):179–185.
- Prashant A, Vishwanath P, Kulkarni P, et al. Comparative assessment of cytokines and other inflammatory markers for the early diagnosis of neonatal sepsis–a case control study. PLoS One. 2013;8(7):e68426.
- Boskabadi H, Zakerihamidi M. Evaluate the diagnosis of neonatal sepsis by measuring interleukins: a systematic review. Pediatr Neonatol. 2018;59(4):329–338.
- Mussap M, Noto A, Cibecchini F, et al. Emerging biomarkers in neonatal sepsis. Drugs Fut. 2012;37(5):353.
- Kocabaş E, Sarikçioğlu A, Aksaray N, et al. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49(1):7–20.
- Gilfillan M, Bhandari V. Neonatal sepsis biomarkers: where are we now. RRN. 2019;ume 9:9–20.
- Mally P, Xu J, Hendricks-Munoz KD. Biomarkers for neonatal sepsis: recent developments. Res Rep Neonatol. 2014;4:157–168.
- El-Madbouly AA, El Sehemawy AA, Eldesoky NA, et al. Utility of presepsin, soluble triggering receptor expressed on myeloid cells-1, and neutrophil CD64 for early detection of neonatal sepsis. Infect Drug Resist. 2019;12:311–319.
- Wang K, Bhandari V, Chepustanova S, et al. Which biomarkers reveal neonatal sepsis? PLoS One. 2013;8(12):e82700.
- Fouad NA, Fouad MA, Assar EH, et al. Combination of procalcitonin, CRP and CD11b biomarkers in early detection of neonatal sepsis. Egypt J Immunol. 2020;27(1):77–86.
- Hashem HE, Ibrahim ZH, Ahmed WO. Diagnostic, prognostic, predictive, and monitoring role of neutrophil CD11b and monocyte CD14 in neonatal sepsis. Dis Markers. 2021;2021:4537760–4537712.
- Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451(Pt A):46–64.
- Awad H-M, Hawary M-A, Kholef EFM, et al. Serum neopterin level in early onset neonatal sepsis. Egypt J Hosp Med. 2020;81(1):1193–1203.
- Singh Laishram R, Khuraijam D. R. Hematological and biological markers of neonatal sepsis. Iran J Pathol. 2013;8(3):137–146.
- Hincu M-A, Zonda G-I, Stanciu GD, et al. Relevance of biomarkers currently in use or research for practical diagnosis approach of neonatal early-onset sepsis. Children. 2020;7(12):309.
- Kumar N, Dayal R, Singh P, et al. A comparative evaluation of presepsin with procalcitonin and CRP in diagnosing neonatal sepsis. Indian J Pediatr. 2019;86(2):177–179.
- Rowisha MA, Ibrahim AM, Saad MA. Presepsin as an early diagnostic marker of neonatal sepsis in preterm neonate.
- Poggi C, Bianconi T, Gozzini E, et al. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics. 2015;135(1):68–75.
- Montaldo P, Rosso R, Santantonio A, et al. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2017;81(2):329–334.
- Rao L, Song Z, Yu X, et al. Progranulin as a novel biomarker in diagnosis of early-onset neonatal sepsis. Cytokine. 2020;128:155000.
- Yang K-D, He Y, Xiao S, et al. Identification of progranulin as a novel diagnostic biomarker for early-onset sepsis in neonates. Eur J Clin Microbiol Infect Dis. 2020;39(12):2405–2414.
- Cantey JB, Bultmann CR. C-reactive protein testing in late-onset neonatal sepsis: hazardous waste. JAMA Pediatr. 2020;174(3):235–236.
- Avan A, Tavakoly Sany SB, Ghayour‐Mobarhan M, et al. Serum C‐reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol. 2018;233(11):8508–8525.
- Totan M, Antonescu E, Catana MG, et al. C-reactive protein-A predictable biomarker in ischemic stroke. Rev Chim. 2019;70(6):2290–2293.
- Vuong NL, Le Duyen HT, Lam PK, et al. C-reactive protein as a potential biomarker for disease progression in dengue: a multi-country observational study. BMC Med. 2020;18(1):35.
- Karaboğa MNS, Sezgintürk MK. A novel silanization agent based single used biosensing system: detection of C-reactive protein as a potential alzheimer’s disease blood biomarker. J Pharm Biomed Anal. 2018;154:227–235.
- Shrotriya S, Walsh D, Nowacki AS, et al. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS One. 2018;13(8):e0202555.
- Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92(7):856–862.
- Sharifpour M, Rangaraju S, Liu M, et al. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One. 2020;15(11):e0242400.
- Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332–334.
- Sahu BR, Kampa RK, Padhi A, et al. C-reactive protein: a promising biomarker for poor prognosis in COVID-19 infection. Clin Chim Acta. 2020;509:91–94.
- Eschborn S, Weitkamp J-H. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol. 2019;39(7):893–903.
- Tessema B, Lippmann N, Willenberg A, et al. The diagnostic performance of interleukin-6 and C-reactive protein for early identification of neonatal sepsis. Diagnostics. 2020;10(11):978.
- Song Y, Chen Y, Dong X, et al. Diagnostic value of neutrophil CD64 combined with CRP for neonatal sepsis: a meta-analysis. Am J Emerg Med. 2019;37(8):1571–1576.
- Edgar JDM, Gabriel V, Gallimore JR, et al. A prospective study of the sensitivity, specificity and diagnostic performance of soluble intercellular adhesion molecule 1, highly sensitive C-reactive protein, soluble E-selectin and serum amyloid a in the diagnosis of neonatal infection. BMC Pediatr. 2010;10(1):22.
- Lam HS, Ng PC. Biochemical markers of neonatal sepsis. Pathology. 2008;40(2):141–148.
- Datla S, Kitchanan S, Sethuraman G. Diagnostic reliability of salivary C-reactive protein as an alternative noninvasive biomarker of neonatal sepsis. Indian Pediatr. 2021;58(8):745–748.
- Barekatain B, HasanGhalyaei N, Mohammadizadeh M, et al. Investigation of salivary C-reactive protein and interleukin-18 for the diagnosis of neonatal sepsis. J Res Med Sci. 2021;26(1):131.
- Tanak AS, Jagannath B, Tamrakar Y, et al. Non-faradaic electrochemical impedimetric profiling of procalcitonin and C-reactive protein as a dual marker biosensor for early sepsis detection. Anal Chim Acta X. 2019;3:100029.
- Liu C, Fang C, Xie L. Diagnostic utility of procalcitonin as a biomarker for late-onset neonatal sepsis. Transl Pediatr. 2020;9(3):237–242.
- Mithal LB, Palac HL, Yogev R, et al. Cord blood acute phase reactants predict early onset neonatal sepsis in preterm infants. PLoS One. 2017;12(1):e0168677.
- Zhang J, Oueslati R, Cheng C, et al. Rapid, highly sensitive detection of gram-negative bacteria with lipopolysaccharide based disposable aptasensor. Biosens Bioelectron. 2018;112:48–53.
- Wu Z, Zhang Z, Lei Z, et al. CD14: biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019;48:24–31.
- Ye Q, Du L-Z, Shao W-X, et al. Utility of cytokines to predict neonatal sepsis. Pediatr Res. 2017;81(4):616–621.
- Leal YA, Álvarez-Nemegyei J, Lavadores-May AI, et al. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern-Fetal Neonatal Med. 2019;32(17):2830–2836.
- Celik İH, Demirel FG, Uras N, et al. What are the cut‐off levels for IL‐6 and CRP in neonatal sepsis? J Clin Lab Anal. 2010;24(6):407–412.
- Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin. 2013;60(2):367–389.
- Deleon C, Shattuck K, Jain SK. Biomarkers of neonatal sepsis. NeoReviews. 2015;16(5):e297–e308.
- Zhou M, Cheng S, Yu J, et al. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One. 2015;10(5):e0127170.
- Ucar B, Yildiz B, Aksit MA, et al. Serum amyloid A, procalcitonin, tumor necrosis factor-, and interleukin-1 levels in neonatal Late-Onset sepsis. Mediators Inflamm. 2008;2008:737141–737147.
- Lv B, Huang J, Yuan H, et al. Tumor necrosis factor-α as a diagnostic marker for neonatal sepsis: a meta-analysis. Sci World J. 2014;2014:1–14.
- Fattah M, Al Fadhil AO, Asaif S, et al. Utility of cytokine, adhesion molecule and acute phase proteins in early diagnosis of neonatal sepsis. J Nat Sci Biol Med. 2017;8(1):32–39.
- Arunachalam AR, Pammi M. Biomarkers in early-onset neonatal sepsis: an update. Ann Clin Med Microbio. 2015;1(2):1007.
- El Shimi MS, Abou Shady NM, Hamed GM, et al. Significance of neutrophilic CD64 as an early marker for detection of neonatal sepsis and prediction of disease outcome. J Maternal-Fetal Neonatal Med. 2017;30(14):1709–1714.
- Abd Elkareem RM, Ahmed HM, Meabed MH, et al. Diagnostic value of CD64 in early detection of neonatal sepsis. Comp Clin Pathol. 2020;29(3):639–643.
- ELMeneza S, Mohamed W, Elbagoury I, et al. Role of neutrophil CD11b expression in diagnosis of earlyonset neonatal sepsis in full-term infant. Clin Exp Pediatr. 2021;64(1):44–45.
- Qiu X, Li J, Yang X, et al. Is neutrophil CD11b a special marker for the early diagnosis of sepsis in neonates? A systematic review and meta-analysis. BMJ Open. 2019;9(4):e025222.
- Heo JS. Neutrophil CD11b as a promising marker for early detection of neonatal sepsis. Clin Exp Pediatr. 2021;64(1):28–30.
- Saeed OG, Zamzam SM, Elnga AMA, et al. Sepsis markers at PICU and the utility of serum neopterin. Eur J Mol Clin Med. 2021;8(3):3813–3823.
- Centi S, Tombelli S, Puntoni M, et al. Detection of biomarkers for inflammatory diseases by an electrochemical immunoassay: the case of neopterin. Talanta. 2015;134:48–53.
- Selvolini G, Marrazza G. MIP-based sensors: promising new tools for cancer biomarker determination. Sensors. 2017;17(4):718.
- Kojic D, Siegler BH, Uhle F, et al. Are there new approaches for diagnosis, therapy guidance and outcome prediction of sepsis? World J Exp Med. 2015;5(2):50–63.
- Zou Q, Wen W, Zhang X. Presepsin as a novel sepsis biomarker. World J Emerg Med. 2014;5(1):16–19.
- Parri N, Trippella G, Lisi C, et al. Accuracy of presepsin in neonatal sepsis: systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2019;17(4):223–232.
- Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376.
- Mahmoud Zayed K, Abd ELmoez Ali Saad A, Mohamed Amin W, et al. Diagnostic value of presepsin in detection of early-onset neonatal sepsis. Al-Azhar J Pediatr. 2020;23(2):825–851.
- Memar MY, Baghi HB. Presepsin: a promising biomarker for the detection of bacterial infections. Biomed Pharmacother. 2019;111:649–656.
- Sleegers K, Brouwers N, Van Broeckhoven C. Role of progranulin as a biomarker for alzheimer’s disease. Biomark Med. 2010;4(1):37–50.
- Edelman MJ, Feliciano J, Yue B, et al. GP88 (progranulin): a novel tissue and circulating biomarker for non–small cell lung carcinoma. Hum Pathol. 2014;45(9):1893–1899.
- Ghidoni R, Paterlini A, Benussi L. Circulating progranulin as a biomarker for neurodegenerative diseases. Am J Neurodegen Dis. 2012;1(2):180.
- Z Oikonomakou M, Gkentzi D, Gogos C, et al. Biomarkers in pediatric sepsis: a review of recent literature. Biomark Med. 2020;14(10):895–917.
- Chauhan N, Tiwari S, Jain U. Potential biomarkers for effective screening of neonatal sepsis infections: an overview. Microb Pathog. 2017;107:234–242.
- Soto S, Bosch J, Jimenez de Anta M, et al. Comparative study of virulence traits of Escherichia coli clinical isolates causing early and late neonatal sepsis. J Clin Microbiol. 2008;46(3):1123–1125.
- Rajagopal L. Understanding the regulation of group B streptococcal virulence factors. 2009.
- Xie K, Kong S, Li F, et al. Bioinformatics-Based study to investigate potential differentially expressed genes and miRNAs in pediatric sepsis. Med Sci Monit. 2020;26:e923881-1.
- Khaertynov KS, Boichuk S, Khaiboullina S, et al. Comparative assessment of cytokine pattern in early and late onset of neonatal sepsis. J Immunol Res. 2017;2017:8601063–8601068.
- Cardoso FL, Herz J, Fernandes A, et al. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflamm. 2015;12(1):82.
- Smith CL, Dickinson P, Forster T, et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat Commun. 2014;5(1):4649.
- Fatmi A, Rebiahi SA, Chabni N, et al. miRNA-23b as a biomarker of culture-positive neonatal sepsis. Mol Med. 2020;26(1):1–9.
- Abdelaleem OO, Mohammed SR, El Sayed HS, et al. Serum miR-34a-5p and miR-199a-3p as new biomarkers of neonatal sepsis. PLoS One. 2022;17(1):e0262339.
- El-Hefnawy SM, Mostafa RG, Elfeshawy EM, et al. Biochemical and molecular study on serum miRNA-16a and miRNA-451 as neonatal sepsis biomarkers. Biochem Biophys Rep. 2021;25:100915.
- Huang L, Qiao L, Zhu H, et al. Genomics of neonatal sepsis: has-miR-150 targeting BCL11B functions in disease progression. Ital J Pediatr. 2018;44(1):145.
- Ahmad S, Ahmed MM, Hasan P, et al. Identification and validation of potential miRNAs, as biomarkers for sepsis and associated lung injury: a network-based approach. Genes (Basel). 2020;11(11):1327.
- Bryan T, Luo X, Bueno PR, et al. An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood. Biosens Bioelectron. 2013;39(1):94–98.
- Molinero-Fernández Á, Moreno-Guzmán M, López MÁ, et al. An array-based electrochemical magneto-immunosensor for early neonatal sepsis diagnostic: fast and accurate determination of C-reactive protein in whole blood and plasma samples. Microchem J. 2020;157:104913.
- Balayan S, Chauhan N, Chandra R, et al. Electrochemical based C-Reactive protein (CRP) sensing through molecularly imprinted polymer (MIP) pore structure coupled with Bi-Metallic tuned Screen-Printed electrode. Biointerface Res Appl Chem. 2022;6:38.
- Lakshmanakumar M, Nesakumar N, Sethuraman S, et al. Fabrication of GQD-Electrodeposited Screen-Printed carbon electrodes for the detection of the CRP biomarker. ACS Omega. 2021;6(48):32528–32536.
- Guillem P, Bustos R-H, Garzon V, et al. A low-cost electrochemical biosensor platform for C-reactive protein detection. Sens Bio-Sens Res. 2021;31:100402.
- Amouzadeh Tabrizi M, Acedo P. Highly sensitive RNA-Based electrochemical aptasensor for the determination of C-Reactive protein using carbon Nanofiber-Chitosan modified Screen-Printed electrode. Nanomaterials. 2022;12(3):415.
- Mahyari M, Hooshmand SE, Sepahvand H, et al. Gold nanoparticles anchored onto covalent poly deep eutectic solvent functionalized graphene: an electrochemical aptasensor for the detection of C-reactive protein. Mater Chem Phys. 2021;269:124730.
- Yang Z-H, Zhuo Y, Yuan R, et al. Electrochemical activity and electrocatalytic property of cobalt phthalocyanine nanoparticles-based immunosensor for sensitive detection of procalcitonin. Sens Actuators, B. 2016;227:212–219.
- Molinero-Fernández Á, López MÁ, Escarpa A. An on-chip microfluidic-based electrochemical magneto-immunoassay for the determination of procalcitonin in plasma obtained from sepsis diagnosed preterm neonates. Analyst. 2020;145(14):5004–5010.
- Liu J, Liu Y, Bao L, et al. Electrochemical sensitive determination of sepsis biomarker procalcitonin at graphitic carbon nitride nanosheets modified electrodes. Available at SSRN 3950172. 2021.
- Jin Y, Wu J, Hu D, et al. Development of enzyme-free immunosensor based on nanobrush and fluorescence dye for sensitive detection of procalcitonin. Dyes Pigm. 2021;193:109548.
- Ding H, Yang L, Jia H, et al. Label-free electrochemical immunosensor with palladium nanoparticles functionalized MoS2/NiCo heterostructures for sensitive procalcitonin detection. Sens Actuators, B. 2020;312:127980.
- Qu L, Yang L, Ren Y, et al. A signal-off electrochemical sensing platform based on Fe3S4-Pd and pineal mesoporous bioactive glass for procalcitonin detection. Sens Actuators, B. 2020;320:128324.
- Balayan S, Chauhan N, Chandra R, et al. Molecular imprinting based electrochemical biosensor for identification of serum amyloid A (SAA), a neonatal sepsis biomarker. Int J Biol Macromol. 2022;195:589–597.
- Xia C, Li Y, Yuan G, et al. Immunoassay for serum amyloid a using a glassy carbon electrode modified with carboxy-polypyrrole, multiwalled carbon nanotubes, ionic liquid and chitosan. Microchim Acta. 2015;182(7-8):1395–1402.
- Panneer Selvam A, Prasad S. Companion and point-of-care sensor system for rapid multiplexed detection of a panel of infectious disease markers. SLAS Technol. 2017;22(3):338–347.
- Russell C, Ward AC, Vezza V, et al. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin-6 (IL-6) in real time. Biosens Bioelectron. 2019;126:806–814.
- Tanak AS, Muthukumar S, Krishnan S, et al. Multiplexed cytokine detection using electrochemical point-of-care sensing device towards rapid sepsis endotyping. Biosens Bioelectron. 2021;171:112726.
- Kamakoti V, Kinnamon D, Choi KH, et al. Fully electronic urine dipstick probe for combinatorial detection of inflammatory biomarkers. Future Sci OA. 2018;4(5):FSO301.
- Tang P, Zhang H, Huo J, et al. An electrochemical sensor based on iron (II, III)@ graphene oxide@ molecularly imprinted polymer nanoparticles for interleukin-8 detection in saliva. Anal Methods. 2015;7(18):7784–7791.
- Li L, Li M, Wang W, et al. High sensitivity determination of TNF-α for early diagnosis of neonatal infections with a novel and reusable electrochemical sensor. Sensors. 2017;17(5):992.
- Sharma PS, Wojnarowicz A, Sosnowska M, et al. Potentiometric chemosensor for neopterin, a cancer biomarker, using an electrochemically synthesized molecularly imprinted polymer as the recognition unit. Biosens Bioelectron. 2016;77:565–572.
- Huang C-Y, Hsieh C-H, Chen Y-L, et al. Portable potentiostatic sensor integrated with neopterin-imprinted poly (ethylene-co-vinyl alcohol)-based electrode. IET Nanobiotechnol. 2011;5(4):126–131.
- Morad EA, Rabie RA, Almalky MA, et al. Evaluation of procalcitonin, C-reactive protein, and interleukin-6 as early markers for diagnosis of neonatal sepsis. Int J Microbiol. 2020;2020:8889086–8889089.
- Anugu NR, Khan S. Comparing the diagnostic accuracy of procalcitonin and C-Reactive protein in neonatal sepsis: a systematic review. Cureus. 2021;13(11):e19485.
- Kamel MM, Abd-Ullah Hf E, Sayed M, et al. Presepsin as an early predictor of neonatal sepsis. Int J Pediatr. 2021;9:13359–13369.
- Bellos I, Fitrou G, Pergialiotis V, et al. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. 2018;177(5):625–632.
- Iskandar A, Arthamin MZ, Indriana K, et al. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J Maternal-Fetal Neonatal Med. 2019;32(23):3903–3908.
- Li S, Renick P, Senkowsky J, et al. Diagnostics for wound infections. Adv Wound Care (New Rochelle). 2021;10(6):317–327.
- Molinero-Fernández Á, Moreno-Guzmán M, López MÁ, et al. Magnetic bead-based electrochemical immunoassays on-drop and on-chip for procalcitonin determination: disposable tools for clinical sepsis diagnosis. Biosensors (Basel). 2020;10(6):66.