Abstract
The mammary gland is a dynamic organ with various physiological processes like cellular proliferation, differentiation, and apoptosis during the pregnancy-lactation-involution cycle. It is essential to understand the molecular changes during the lactogenic differentiation of mammary epithelial cells (MECs, the milk-synthesizing cells). The MECs are organized as luminal milk-secreting cells and basal myoepithelial cells (responsible for milk ejection by contraction) that form the alveoli. The branching morphogenesis and lactogenic differentiation of the MECs prepare the gland for lactation. This process is governed by many molecular mediators including hormones, growth factors, cytokines, miRNAs, regulatory proteins, etc. Interestingly, various signalling pathways guide lactation and understanding these molecular transitions from pregnancy to lactation will help researchers design further research. Manipulation of genes responsible for milk synthesis and secretion will promote augmentation of milk yield in dairy animals. Identifying protein signatures of lactation will help develop strategies for persistent lactation and shortening the dry period in farm animals. The present review article discusses in details the physiological and molecular changes occurring during lactogenic differentiation of MECs and the associated hormones, regulatory proteins, miRNAs, and signalling pathways. An in-depth knowledge of the molecular events will aid in developing engineered cellular models for studies related to mammary gland diseases of humans and animals.
Introduction
Mammary gland comprises various types of cells such as epithelial cells, fibroblast cells, adipocytes, endothelial cells, and immune cells with the mammary epithelial cells (MECs) serving as the functional unit of milk production. The development of this gland starts from the embryonic stage and most of its development occurs after birth. This is a dynamic gland with cellular proliferation, differentiation and apoptosis occurring in a cyclic manner in the process of pregnancy, lactation, and involution cycle [Citation1]. These physiological processes have made this gland a suitable model for studying organogenesis, epithelial-mesenchymal transition, lactation physiology, and cancer progression. Cellular proliferation occurs when a cell grows and divides into two daughter cells and in this process the tissue growth occurs with increase in cell number exponentially [Citation2]. Cellular differentiation occurs when a proliferative cell undergoes molecular and morphological changes to undergo a special function. The mammary gland after puberty undergoes major development by the hormonal stimulation and epithelial cell proliferation undergoes to form the ductal network. Once the pregnancy is initiated, intensive cell proliferation occurs forming the side branches, lobules, and alveoli [Citation3]. The alveolar epithelial cells further undergo differentiation to perform the milk synthesis and secretion. These differentiated luminal epithelial cells are responsible to maintain the lactation in mammary gland [Citation4,Citation5]. Various hormones such as oestrogen, progesterone, prolactin, and growth factors like epidermal growth factor (EGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) regulate the development of mammary gland. There are six developmental stages of the mammary gland occurs such as embryonic, prepubertal, pubertal (the linear phase), pregnancy, lactation, and involution stage (the cyclic phase) [Citation6,Citation7]. The cyclic phase of the mammary gland bears wide plasticity under the influence of various hormones, growth factors, and regulatory proteins [Citation8].
The MECs comprise two layers: luminal cells (synthesize milk) and basal myoepithelial cells (help in milk ejection). Specific markers characterize these two types of cells: myoepithelial cells express the keratin 5 and 14, and α-smooth muscle actin (α-SMA); luminal cells express keratin 8 and 18. Besides, a subset of luminal cells bears the receptors for oestrogen, progesterone, and prolactin. These cells mediate the systemic hormonal signals to local signals like direct, juxtacrine signalling or paracrine signalling [Citation9]. The secretion of milk mainly follows apocrine secretion, although few species show merocrine secretion. In merocrine secretion the cells release their products by exocytosis and no part of the cell is damaged, whereas in apocrine secretion the secretory products are accumulated at the apical end of the polarized epithelial cells (e.g. differentiated MECs) and the contents bud off in the form of extracellular vesicles. Hence, there is loss of some cytoplasm in the apocrine secretions. The molecular signalling and morphological changes vary when an epithelial cell is differentiated to show either merocrine or apocrine mode of secretion [Citation10–12]. A study by Rios et al. showed that there exists binucleated MECs during lactation as revealed in five species such as humans, mice, wallabies, cows, and seals [Citation13]. These polyploid binucleate cells have more potential for milk synthesis to feed the offspring. It is concluded that the existence of polyploid binucleate cells (lacking cytokinesis during cell division) in all mammals is a conserved process for successful lactation. Two enzymes such as Aurora kinase A and Polo-like kinase 1 (overexpressed during lactogenic switch) seem to promote the binucleate cell formation through signals from prolactin and epidermal growth factor. Various regulatory molecules and signalling pathways are involved in the mammary gland development and lactogenic differentiation of cells for successful lactation and many intriguing mechanisms are yet to be elucidated.
The present review article discusses the latest advancementsin the molecular events in MEC differentiation, lactogenesis and persistent lactation in livestock animals with more focus on cow species, in relevance to the regulatory proteins, extracellular matrix (ECM) and associated signalling pathways.
Role of ECM in MEC differentiation
ECM plays a crucial role in promoting differentiation of MECs and rendering the cells responsive towards the lactogenic hormones. The ECM components such as laminin, integrins (transmembrane receptors), tenascin, matrix metalloproteinases (MMPs), connective tissue growth factor (CTGF, also known as CCN2), and clusterin are involved in mammary gland development and MEC differentiation, as evidenced from various studies [Citation14–20].
Laminins are the basement membrane’s major components, and act as ligands for the heterodimeric transmembrane receptors such as integrins. Integrins form heterodimers with various isoforms of α and β subunits and execute their functions with varied activity [Citation21]. The MECs connect to the basement membrane by integrins which mediate the lactogenic differentiation signal from ECM to the luminal cells [Citation22,Citation23]. It was observed that absence of β1-integrin function impeded the translocation of signal transducer and activator of transcription 5 (STAT5) to the nucleus and thus affected the casein gene expression [Citation24,Citation25]. The laminin-binding integrins play a crucial role in maintaining the functional lactating MECs [Citation14]. The laminin-binding integrin dimers such as α3/β1, α6/β1 and α6/β4 are present in the MECs during development [Citation15]. The study using mutant mice (lacking α3 and α6 integrins), showed impeded lobulo-alveolar development and myoepithelial cell morphology was changed. Moreover, lactation was not maintained in mutant females and precocious involution of the mammary glands was observed due to disturbance in luminal cell baso-apical polarization and early cell death [Citation14]. Interestingly, genetic suppression of p53 revived the growth but not the differentiation status of MECs which showed that p53 – mediated pathway is crucial for MEC proliferation and survival downstream of the laminin-binding integrin signalling. A study by Romagnoli et al. revealed that basal mammary cells lacking laminin-binding integrins exhibit inhibition of clonogenic activity mediated by p53 activation [Citation26].
Tenascins are ECM glycoproteins, abundantly present in the growing embryos and there are 4 members (tenascin-C, -R, -X, and -W) exist in the tenascin gene family [Citation27,Citation28]. A study performed on the HC11 mouse MEC cell line showed laminin and tenascin to modulate the MEC differentiation [Citation16]. Plating of HC11 cells on E8 laminin fragments made the cells competent towards lactogenic hormone response; whereas competent cells plated on tenascin – coated dishes showed inhibition of lactogenic hormone induction. This study showed that tenascin assembly and β-casein production are opposing effects in the differentiation of HC11 cells. Tenascin-C (a member of tenascin family) is associated with cellular proliferation and motility in organogenesis and tumorigenesis in the mammary gland. It is observed that expression of this protein is higher in the growth phase and lower during differentiation, as studied in mice MECs [Citation17]. It was found that EGF regulates the expression of tenascin-C and downregulation of tenascin-C makes the MECs responsive to lactogenic hormones for differentiation and milk protein expression. Conversely, β-casein expression in MECs was reduced in the cultural condition enriched with tenascin-C substrate. It was seen that transforming growth factor-β (TGF-β) which is involved in regulating growth and differentiation in mammary glands, induces the tenascin-C expression [Citation17].
Morrison and Cutler discussed the role of ECM components in lactogenesis and highlighted many proteins regulating the MEC differentiation [Citation18]. The matrix metalloproteinases (MMPs) breakdown the ECM proteins to facilitate the branching morphogenesis and MMP-3 plays a crucial role in the side branching of the terminal end buds (TEBs) [Citation29,Citation30]. The milk components after their synthesis need to be secreted to the lumen through a polarized secretion process. This is ensured by the tight junction proteins such as occludin and zona occludens-1 (ZO-1) [Citation31].
Connective tissue growth factor (CTGF) is an ECM protein found to upregulate during late pregnancy and early lactation in mice [Citation19]. To understand the role of this protein in MEC differentiation, tetracycline-regulated expression of CTGF in the HC11 MECs revealed many interesting findings [Citation32]. It was observed that CTGF enhanced the expression of many markers of lactogenic differentiation and increased β-casein expression and mammosphere formation (morphologic marker of MEC differentiation). In addition, the upregulated CTGF in HC11 MECs reduced the requirement of ECM proteins in the β-casein expression, indicating its role in MEC differentiation by regulation of matrix-dependent cell adhesion. The lactogenic differentiation was promoted by CTGF through binding to integrin complexes and enhancing the expression of matrix proteins that culminate in enhanced integrin functions [Citation32].
A study by Itahana et al. performed on the clusterin in mice mammary glands, revealed that it plays an important role in MEC differentiation [Citation20]. The upregulation was found at two stages such as at the end of pregnancy, and subsequently at the initiation of involution. The first upregulation is induced by laminin, prolactin, and hydrocortisone, whereas TGF-β1 induces the second upregulation. Clusterin plays a vital role in milk protein expression which was proved using siRNA gene silencing. Additionally, clusterin has shown its role in mammary gland apoptosis which needs further study.
Study with the bovine MEC cell line BME-UV1 grown on ECM components (matrigel) revealed the role of ECM in lactogenesis and stimulated the β-casein expression which is not observed in 2D culture system. However, prolactin is required to efficiently produce milk proteins [Citation33]. Similarly, non-eutherian lactation model (Tammar wallaby) showed the importance of ECM in regulating the caloric content of milk, proliferation, differentiation, and apoptosis of MECs [Citation34]. This study involved the transcriptome analysis of MECs, treated with ECM of mid and late lactating stage tammar wallaby mammary gland. Differential gene expressions (in MECs) related to sugar and lipid metabolism were observed. The important pathways that were significantly responsive to ECM were galactose metabolic pathway, cell adhesion molecules (CAM), p53 signalling, and Nod-like receptor signalling pathways [Citation34]. The MEC culture on the basement membrane has a better lactogenesis process as compared to the MECs grown on a plastic substratum [Citation35]. The cells adhered to the basement membrane show insulin-induced tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) and association of PI3K for further signalling. However, the insulin signalling is affected in the culture done on the plastic substratum (lacks basement membrane) due to the higher expression of RhoA and Rho-associated kinase (ROCK), where the RhoA and ROCK undergo serine phosphorylation of IRS-1. This study concluded that RhoA/ROCK pathway impedes the insulin signalling in the cells lacking proper adhesion to the basement membrane, as is the case in wounding and mammary gland involution [Citation35]. The study by Barcellos-Hoff et al. on primary mammary epithelium isolated from mid-pregnant (11–14 day) CD-1 mice and their culture on a reconstituted basement membrane, revealed tissue-specific vectorial secretion and formation of functional alveoli-like structures [Citation36]. The MECs established polarity and showed various polarity features such as vectorial secretion, apical junctions, a sequestered compartment, and basal lamina formation. Mouse MECs cultured on a reconstituted basement membrane, showed differentiation features with cytoplasmic polarization initiated at 3rd day of culture and enough casein and lipid synthesis and secretion by 4th day of culture with the development of lumina [Citation37]. The localization of β-casein was found to be in the luminal MECs of in vitro cultured cells. Besides, the stromal-epithelial interaction has a crucial role in the differentiation of MECs which is very well supported by the study of Darcy et al. [Citation38]. This research group showed in a coculture system of mammary fibroblasts and epithelial cells that fibroblasts promote the MEC differentiation with casein accumulation, although ductal morphogenesis was not significantly visible. In addition to the above functions of ECM components, it was also revealed that ECM molecules facilitate the induction of histone acetylation and transcription factor binding to the β-casein promoter by cooperating with the prolactin molecules, and enhancing the expression of β-casein genes [Citation39].
Role of hormones in branching morphogenesis and lactation
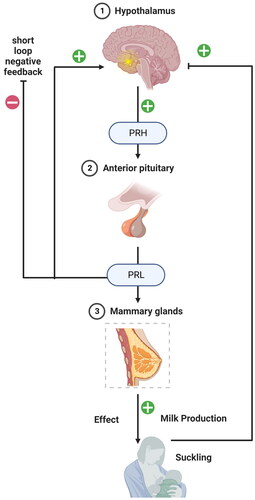
The hypothalamus-anterior pituitary gland-mammary gland axis is a complex system that is critical in regulating lactation and other reproductive functions () [Citation40]. The axis involves a series of hormonal signals originating in the hypothalamus, travelling to the anterior pituitary gland, and stimulating the mammary gland to produce and secrete milk. The hypothalamus is a region of the brain that plays a critical role in regulating homeostasis, including regulating reproductive functions. The hypothalamus produces and releases several hormones, including gonadotropin-releasing hormone (GnRH) and prolactin-releasing hormone (PRH) [Citation40]. GnRH stimulates the release of luteinizing hormone (LH) and follicle- stimulating hormone (FSH) from the anterior pituitary gland, which are critical for regulating reproductive functions, including ovulation and spermatogenesis. PRH stimulates the release of prolactin (PRL) from the anterior pituitary gland, which is critical for lactation [Citation41]. The anterior pituitary gland is a small gland located at the base of the brain that secretes several hormones in response to signals from the hypothalamus. In addition to LH and FSH, the anterior pituitary gland also secretes PRL in response to PRH stimulation [Citation41]. PRL acts on the mammary gland to stimulate milk production and secretion. In addition, PRL also plays a role in regulating reproductive functions, including ovulation and sexual behaviour [Citation42]. During pregnancy, the mammary gland undergoes extensive development, preparing for the production and secretion of milk. PRL stimulates the mammary gland to produce and secrete milk, which is critical for nourishing the newborn infant. The hypothalamus-anterior pituitary gland-mammary gland axis is a tightly regulated system critical for maintaining reproductive function and lactation. Disruptions in any part of the axis can lead to a range of reproductive and lactation disorders, including infertility, menstrual irregularities, and lactation failure [Citation43]. Hormonal therapies targeting the axis, such as GnRH agonists or PRL antagonists, can treat these disorders and restore normal reproductive and lactation function [Citation44].
Figure 1. The hypothalamus-anterior pituitary gland-mammary gland axis showing regulation of prolactin secretion and milk production. The prolactin-releasing hormone (PRH) from the hypothalamus signals the anterior pituitary for prolactin (PRL) secretion, which further binds to PRLR in MECs to signal the milk protein synthesis.
Hormones play a crucial role in branching mammary gland’s morphogenesis and initiating the lactogenesis process [Citation45]. The events in the MECs before parturition is divided into two phases such as secretory initiation phase (stage I, where initiation of milk component synthesis occurs) and activation phase (stage II, where massive synthesis and secretion of milk occurs) [Citation46]. The functional events that occur in these two stages are mainly governed by hormonal stimulation, structural rearrangements, and signalling from the ECM. It is observed that besides prolactin being the primary hormone inducing the lactogenic changes, mechanical stimulation also acts synergistically to enhance the lactogenesis in stage I [Citation47].
Prolactin is the major hormone in lactation initiation, which binds to the prolactin receptor and induces the JAK-STAT signalling pathway to carry the message for casein synthesis (). In a review article, Pang and Hartmann have thoroughly discussed the biochemical and endocrinological processes that are involved in the initiation of lactation [Citation48]. Lactation initiation has two stages: secretory differentiation and secretory activation in humans. The secretory differentiation stage involves the differentiation of MECs during pregnancy to synthesize milk constituents under the influence of lactogenic hormone complex. The secretory activation stage involves the copious secretion of milk with change in milk composition. Progesterone withdrawal induces the activation stage with the involvement of prolactin, insulin and cortisol hormones. A study by Mida et al. on lactogenesis in HC11 MECs, showed that of the three lactogenic hormones (insulin, prolactin and hydrocortisol) insulin plays a vital role in the compositional changes in the membrane lipids of MECs [Citation49]. Insulin enhanced the phosphatidylethanolamine and phosphatidylinositol and reduced sphingomyelin and cholesterol. Insulin addition to the culture medium enhanced the total fatty acid content by 50% and monounsaturated fatty acids by 12%, reducing saturated fatty acids by 35%. All three hormones combinedly induced the β-casein synthesis, indicating the coordinated function of the lactogenic hormones in the differentiation and lactogenesis in MECs. Lactogenic hormones stimulate the expression of lipogenic genes and milk protein genes but do not induce the expression of GLUT genes (GLUT1, GLUT8, and GLUT12) in the bovine mammary gland during the onset of lactation [Citation50]. The in vitro study with mouse HC11 MECs and bovine primary MEC cultures showed similar results. Moreover, lysosomal biogenesis is also activated upon lactogenic hormone treatment (observed in HC11 cells) and there is upregulation of lysosomal-associated membrane glycoprotein 1 (LAMP1) during the lactogenesis which can act as a marker of MEC differentiation [Citation51]. The increased lysosomal vesicle number and their content might have intracellular and extracellular remodelling functions, an important feature of cellular differentiation. The overexpression of insulin-induced genes INSIG1 and INSIG2 using adenoviral transfection method in goat MECs, resulted in decrease of triacyl glycerol content, total cholesterol content, and lipid droplet accumulation, along with downregulation of genes such as SREBF1, ACACA, FASN, SCD1, GPAM, DGAT2, ATGL, and HSL genes [Citation52]. It was found that triacyl glycerol synthesis is primarily regulated by INSIG2, whereas, both INSIG1 and INSIG2 regulate the total cholesterol synthesis. The INSIG1 and INSIG2 were found to regulate the lipid metabolism in goat MECs by synergistic action through modulation of expression of genes involved in lipid metabolism. However, more studies are required to understand the mechanism, which can help in manipulation of fat content in milk [Citation52]. A study on human MECs cultured on mouse ECM components (to provide a 3D model for the growth of cells) showed that insulin is inducing the mammosphere formation which is the typical morphologic marker of MEC differentiation [Citation53]. Microarray analysis of the mammospheres revealed upregulation of genes associated with cell-cell junction, differentiation, and cytoskeletal organization. It is suggested that mammosphere formation is induced through the integrin-linked kinase (ILK) signalling by which integrin interacts with the ECM proteins to induce MEC differentiation and mammosphere formation. Besides, the comparative study on the role of insulin and insulin-like growth factor-I (IGF-I) on the mammosphere formation, revealed that IGF-I induced the spherical structure formation. Still, MECs were not polarized appropriately and induction of milk protein genes was also lacking. In contrast, insulin played crucial role in mammary acinar development for secretory function. It is indicated that women with obesity and gestational diabetes mellitus might have decreased lactational potential due to inappropriate insulin signalling [Citation53].
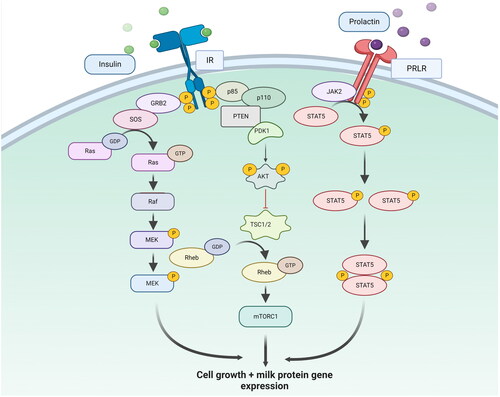
Figure 2. STAT5 and Ras signalling pathway regulating the expression of milk protein genes and cell growth. Prolactin binds to the prolactin receptor (PRLR) present on the cell surface of MECs and induces the STAT5 pathway for milk protein gene expression. The RAS signalling pathway is activated by growth factors and cytokines that bind to their receptors on the cell surface and activates a cascade of protein kinases, including the RAF-MEK-ERK pathway. The activated ERK protein kinase induces the transcription of target genes involved in cell proliferation, differentiation, and survival.
A study by Gallego et al. on the role of prolactin, growth hormone (GH) and EGF in mouse mammary gland development and differentiation revealed that prolactin receptors exert their function in MECs [Citation54]. In contrast, GH receptors and EGF receptors exert their function through the mesenchymal compartment of the mammary gland. A study on the role of autocrine GH in MEC differentiation in mice, showed that GH prevents the lactogenic differentiation of MECs. Maximum expression of GH occurred during puberty and the level was reduced in late pregnancy and lactation, indicating its role in cellular proliferation rather than cellular differentiation. In an in vitro model (HC11 cells), similar findings were observed where the forced differentiation of MECs resulted in reduced GH expression and conversely, forced expression of GH abrogated the lactogenic differentiation as evidenced by decreased β-casein expression and loss of lateral epithelial localization of E-cadherin [Citation55].
The steroidal hormones are found to have significant role in proliferation and differentiation of MECs. A study on rat MECs about the effect of hydrocortisone and progesterone on proliferation, morphogenesis, and differentiation, revealed that hydrocortisone induced alveolar and multi-lobular branching morphogenesis and enhanced casein accumulation. However, the lobulo-ductal branching morphogenesis was suppressed by hydrocortisone [Citation56]. Progesterone is necessary for the induction of proliferation, multi-lobular branching and lobulo-ductal branching but suppresses casein accumulation. The study with the Saanen goats on the effects of cortisol on milk yield and expression pattern of crucial genes (with role in lactation) in MECs, revealed many interesting findings [Citation57]. In vivo administration of ACTH enhanced the cortisol release (acute release) which subsequently increased the milk yield through overexpression of prolactin receptor (PRLR) and growth hormone receptor (GHR) genes. Higher cortisol level in the MECs (derived from mammary gland of goats) in the in vitro study (chronic treatment) showed increased expression of glucocorticoid receptor (NR3C1), GHR, PRLR, and BAX (pro-apoptotic) genes and decreased expression of IGF1 and insulin receptors. The in vitro study revealed that chronic treatment of cortisol induces apoptosis of MECs thereby decreasing the number of MECs [Citation57]. The HC11 cells showed that androgens promote cell proliferation and differentiation through the androgen receptors. It needs further study to unravel the proliferative and differentiating properties of androgens [Citation58]. The retinoid RE80 enhanced the casein expression in rat MECs cultured in vitro within a reconstituted basement membrane [Citation59]. Moreover, RE80 acted as an anti-progestin by removing the inhibitory effect of progesterone on casein accumulation and showed anti-proliferative effects on the MECs in the appropriate culture condition. This study showed that retinoids could facilitate the transcription of casein genes through direct activation of the binding of retinoid receptors to the casein promoter or indirectly by regulating the effect of other hormones.
The serotonin (5-hydroxy tryptamine) derived from the mammary gland appears to maintain the body’s calcium homeostasis by communicating to bone through the expression of parathyroid hormone-related protein (PTHrP) in the mammary gland during lactation. The PTHrP secreted from the MECs reaches the bones, releasing calcium for the synthesis of milk (rich in calcium) [Citation60,Citation61]. A study by Sheftel and Hernandez showed that serotonin stimulated the PTHrP production in the MECs by transglutaminase-dependent serotonylation, with RhoA being the potential serotonylation target protein [Citation62].
Signalling pathways involved in lactogenic differentiation
It is well established that prolactin pathway is the major differentiation pathway to induce lactogenesis in MECs (). Two important signalling pathways in the prolactin pathway are the STAT5 and RAS signalling pathways. The STAT5 signalling pathway is activated by prolactin binding to its receptor on the surface of target cells [Citation63]. Once activated, the receptor undergoes a conformational change, activating the Janus kinase (JAK) family of kinases. The activated JAKs then phosphorylate the STAT5 proteins, leading to their dimerization and translocation to the nucleus. In the nucleus, the STAT5 dimer binds to specific DNA sequences, known as STAT5 response elements (SREs). It activates the transcription of target genes involved in cell proliferation, differentiation, and survival [Citation63]. The RAS signalling pathway, on the other hand, is activated by growth factors and cytokines that bind to their receptors on the cell surface [Citation64]. Once activated, the receptor recruits and activates a series of downstream proteins, including the RAS GTPase, which activates a cascade of protein kinases, including the RAF-MEK-ERK pathway. The activated ERK protein kinase then translocates to the nucleus, activating the transcription of target genes involved in cell proliferation, differentiation, and survival [Citation64]. In the prolactin pathway, the RAS signalling pathway is activated by prolactin in a JAK2-independent manner. Prolactin stimulates the activation of the RAS pathway through the recruitment and activation of the adaptor protein SHC, which activates the RAS-RAF-MEK-ERK pathway [Citation65]. The activation of the RAS pathway, in turn, leads to the phosphorylation of STAT5, enhancing its transcriptional activity and leading to the upregulation of target genes involved in lactation and other reproductive functions [Citation65].
In summary, both STAT5 and RAS signalling pathways play critical roles in the regulation of prolactin secretion and the maintenance of reproductive function. While the STAT5 pathway is directly activated by prolactin binding to its receptor, the RAS pathway is indirectly activated by prolactin through the recruitment and activation of SHC, leading to the activation of the RAS-RAF-MEK-ERK pathway and the phosphorylation of STAT5. The PTEN and AKT signalling pathways are also important regulators of the prolactin pathway and play important roles in regulating lactation and other reproductive functions [Citation66]. The PTEN pathway acts as a negative regulator of the AKT pathway, preventing its activation and promoting cell death. In contrast, the AKT pathway is activated downstream of the RAS pathway and plays a role in regulating cell growth and survival. The interplay between these signalling pathways is complex and can have important implications for developing and progressing breast cancer and other diseases [Citation66].
The JAK-STAT pathway, activated by prolactin for milk component synthesis involves the molecular axis such as prolactin receptor – mediated activation of Janus kinase 2 (tyrosine kinase) which further phosphorylates the STAT5A transcription factor. The activated STAT5A reaches the nucleus and induces transcription of milk protein genes including the β-casein gene [Citation67–69]. Moreover, the STAT5A is also activated by another receptor tyrosine kinase (RTK) ERbB4 (a member of the epidermal growth factor receptor RTK family) for lactogenesis in MECs. The ErbB family of RTKs regulates mammary gland development in various ways [Citation70]. There are four family members of ErbB RTKs, namely ErbB1, ErbB2, ErbB3, and ErbB4. ErbB4 mediates a crucial role in MEC differentiation; whereas other isoforms are involved in MEC proliferation [Citation70,Citation71]. MEC differentiation requires signalling from both prolactin and ErbB4 dependent pathways and both these pathways ultimately activate STAT5A for further transcription of milk protein genes. The study by Muraoka-Cook et al. on the role of ErbB4 receptor ligand heparin-binding epidermal growth factor (HB-EGF) on HC11 cultured cells differentiation in 3D culture system, revealed that both prolactin and HB-EGF could induce the STAT5A activation and lumen formation with enhanced expression of differentiation markers [Citation72]. The intracellular domain of ErbB4 seemed to mediate the differentiation signal of MECs [Citation72]. It is observed that the activity of ErbB4 is increased during pregnancy and lactation in the alveolar MECs as that of STAT5A. Knock out studies of ErbB4 showed the impaired function of STAT5A, decreased terminal differentiation of MECs and defective lactation, which indicates the importance of ErbB4 in lactogenesis [Citation73–75]. Two splice variants of ErbB4 such as Cyt1 and Cyt2 show opposing effects on the growth and differentiation of MECs. The expression of s80Cyt1 and s80Cyt2 in HC11 MECs and their growth on three-dimensional matrices revealed distinct effects. The s80Cyt1 decreased growth of MECs but increased the lumen formation, whereas s80Cyt2 increased cellular proliferation but did not promote lumen formation. These two isoforms vary by a 16-aminoacid intra-cellular sequence (present in Cyt1 but absent in Cyt2) [Citation76]. The ErbB3 protein promotes MEC survival and differentiation during pregnancy and lactation [Citation77]. The cell survival ability is mediated by PI3K/AKT pathway and ErbB3 knockout mouse study revealed impaired Akt phosphorylation and impaired activation of STAT5A, suggesting the involvement of ErbB3 in cell survival and lactogenesis [Citation77].
It is found that loss of PRLR expression in hormone response-positive MCF-7 cells and HER2-enriched SKBR-3 breast cancer cells promote cellular viability, migration and invasiveness [Citation78]. The loss of PRLR increased the HER2-driven tumorigenesis, cellular invasion leading to metastasis, and cancer cells developing resistance to therapeutics. Conversely, PRL/PRLR pathway being major differentiating pathway can drive differentiation in breast cancer cells and can be adopted as a therapeutic strategy in the future with more in-depth studies in this regard. A study using the pathway-specific inhibitors, optical imaging technique and HC11-Lux cell model (a subclone of COMMA-1D cells) found that JAK2/STAT5A pathway and PI3K/Akt pathway primarily promote the lactogenic differentiation; whereas Raf1/MEK/MAPK pathway is responsible for the cellular proliferation. In this study, the β-casein promoter activity was detected by the light emitted and real – time imaging through the optical imaging system [Citation79]. AKT is a serine-threonine protein kinase that is activated by various signals through the PI3K-dependent pathway and regulates many functions such as growth, proliferation, cellular migration etc. [Citation80]. The three isoforms of AKT1, AKT2, and AKT3 play prominent roles in the mammary gland development during pregnancy, lactation and involution, as observed in mice [Citation81]. The ablation of AKT1 showed a delay in the differentiation of MECs during pregnancy and lactation and promoted cellular apoptosis leading to involution, whereas ablation of AKT2 showed the opposite effect with inhibition of apoptosis and delay in the involution process. Ablation of AKT3 showed minor defects in the MEC differentiation and lactation. The study revealed that AKT1 ablation hampers the phosphorylation of STAT5a and thus impedes the prolactin signalling through the JAK-STAT pathway for milk protein synthesis. Taken together, AKT1 plays a crucial role out of the three isoforms, for functional differentiation of MECs during pregnancy and lactation [Citation81].
It has been found that EGF block the lactogenic differentiation through RAS/MEK/ERK pathway and phosphatidylinositol-3-kinase (PI-3-kinase) pathway [Citation82–83]. It is observed that EGF activates AKT and subsequent downstream proteins such as p70S6 kinase, RPS6, eIF4E and 4E-BP1 through the PI3 kinase/Akt signalling pathway. Moreover, inhibition of PI3 kinase, AKT, or mammalian target of rapamycin (mTOR) reverts the EGF-induced differentiation block in HC11 cells [Citation83]. The mTOR is a downstream effector of PI3/Akt pathway and regulates both proliferation and differentiation of MECs through the induction of helix-loop-helix Id1 and Id2 transcriptional regulatory proteins [Citation84]. The proliferative action by mTOR is induced by Id1 and differentiating action is induced by Id2 protein. A study by Shan et al. on Id4 expression in rat mammary gland and in the HC11 cells reveal that this protein might contribute to carcinogenesis through inhibition of MEC differentiation and stimulation of MEC proliferation [Citation85]. The Id1 protein seemed to negatively affect the differentiation of MECs as studied in murine MEC cell line SCp2 [Citation86]. This protein was observed to be refractory to differentiation signals but receptive to proliferative signals, possibly due to the inactivation of one or many basic helix-loop-helix proteins that regulate growth and differentiation in MECs [Citation86].
Inhibition of mTOR by rapamycin in the HC11 MECs and mice mammary glands reduced β-casein expression even in the presence of lactogenic hormones. A study by Wang et al. with the bovine MECs upon lactogenic differentiation revealed that prolactin/STAT5 and AKT1/mTOR pathways play significant role in milk protein gene transcription and translation, respectively [Citation87]. There was higher expression of phosphorylated proteins such as p-STAT5, P-AKT1, and p-mTOR proteins in the differentiated cells compared to the control cells. Moreover, there was a synchronized upregulation or downregulation of these three proteins as observed through RNA silencing technique of STAT5 or AKT1 protein. These findings revealed these two pathways to synchronize to regulate transcription and translation of milk proteins in bovine MECs [Citation87].
A study by Jeon et al. on the effect of D-methionine (D-Met) and methionine precursor 2-hydroxy-4-methylthiobutanoic acid I (HMBi) on milk protein synthesis in the immortalized bovine MECs (MAC-T), found that both these components stimulated protein synthesis through induction of various metabolic pathways [Citation88]. The HMBi supplementation enhanced the milk protein synthesis by inducing eukaryotic translation initiation factor 3 subunit protein required for the translation initiation process. Pathway analysis revealed that D-Met stimulates fructose–galactose metabolism, glycolysis pathway, phosphoinositide 3 kinase, and pyruvate metabolism; whereas, HMBi stimulates pentose phosphate pathway and glycolysis pathway. Analysis of metabolite production showed that D-Met enhanced the level of seven metabolites and reduced uridine monophosphate (UMP) level and HMBi enhanced the level of 3 metabolites and reduced UMP and N-acetyl-L-glutamate levels. The upregulated metabolites contributed to the increased milk protein synthesis in the MAC-T cells compared to the control ones.
The expression pattern of caveolin-1 (a 22-kDa integral membrane protein) in various stages of mouse mammary gland development, showed that its level is decreased during late pregnancy and lactation. Conversely, its level is increased during involution [Citation89]. Recombinant expression of caveolin-1 in HC-11 MECs (treated with lactogenic hormones) revealed that it suppresses the β-casein promoter activity and thus impedes the milk protein synthesis. Similarly, treatment of human MECs (hTERT-HME1) with lactogenic hormones showed prolactin primarily mediating the downregulation of caveolin-1 protein. This study unravelled the mechanism of caveolin-1 expression regulation and showed that prolactin undergoes the negative regulation of caveolin-1 during lactation through the ras-dependent signalling pathway [Citation89].
WNT1 is a mammary oncogene that induces transcription through the beta-catenin/TCF complexes. Twist is a transcription factor of basic helix-loop-helix class and it is observed that Twist expression is regulated by the WNT/beta-catenin signalling and both the Twist and WNT1 do not promote the lactogenic differentiation of MECs which might promote the cells towards tumorigenesis [Citation90].
Bone morphogenic proteins (BMPs) are the members of the TGF-β superfamily and the corresponding receptors after activation, phosphorylating the SMAD proteins (SMAD1/5/8), the DNA-binding proteins. Each of these SMAD proteins upon heterodimerization with SMAD4, translocate into the nucleus and regulate the transcription of downstream genes [Citation91]. It was observed that the BMP receptor-1A (BMPR-1A) pathway plays a vital role in the lactogenic differentiation of the MECs as studied in HC11 cells [Citation92]. The BMPR1A-SMAD1/5/8 pathway is found to be active in undifferentiated MECs as compared to the differentiated one. The knockdown of BMPR-1A resulted in decreased β-casein expression during lactogenic differentiation by hormonal treatment. Moreover, addition of BMP antagonist noggin also prevented the β-casein expression. These findings showed BMP-BMPR1A-SMAD1/5/8 signalling pathway is essential for β-casein expression which is the marker of MEC differentiation [Citation92].
Proteins upregulated during lactation
There are many proteins found to be upregulated during the lactation process with direct or indirect influence on lactogenesis. Various researchers have deciphered the mechanism of action of many upregulated proteins. However, in-depth studies are required for many such proteins that facilitate lactation. The heparan sulphate interacting protein (HIP) is identical to ribosomal protein L29 (RPL29) and found to be highly expressed in MECs during pregnancy and lactation [Citation93]. The HIP/RPL29 levels are significantly increased during lactation, although their levels are low in virgin mammary gland and during involution. Their levels were more in the cytoplasm of lactating MECs. In vitro study with COMMA-D cells (cell line derived from mid-pregnant mouse mammary gland) showed no alteration in HIP/RPL29 level upon differentiation with lactogenic hormones, which indicates that its expression is not induced by lactogenic stimulation; however, it needs further investigation.
The neuronal guidance molecule netrin-1 is expressed in mouse mammary glands and has a crucial role in promoting lactogenic differentiation [Citation94]. It promotes cellular adhesion between the cap cells and body epithelial cells (nonneuronal cells) in the growing TEBs by interacting with neogenic and thus stabilizes the multipotent progenitor cap cells during mammary gland morphogenesis and provides an adhesive role (rather than the guidance role) in the nonneuronal organogenesis [Citation95]. Besides, netrin-1 is observed to antagonize the action of Cripto-1 protein (EGF-like protein and is a target gene for Oct-4 and Nanog pluripotent genes, and responsible for epithelial-mesenchymal transition) that induces the invasion and migration of MECs in vitro [Citation96]. Strizzi et al. studied the mammary glands of virgin CR-1 transgenic mice and early pregnant FVB/N mice to unravel the role of netrin-1 in mammary gland development and morphogenesis [Citation94]. It was observed that netrin-1 expression was higher; whereas Crypto-1 and Nanog expression were lower during lactation in the FVB/N mice. Moreover, a study with HC-11 MECs showed that netrin-1 increased the effect of lactogenic hormones significantly.
The expression pattern of connexin30 (a gap junction protein) at different developmental stages of the mouse mammary gland revealed its upregulation during pregnancy and lactation with maximum expression at the onset of lactation. These data were supported by experiments with cultured MECs showing connexin30 expression in MECs upon induction by lactogenic hormones [Citation97]. These findings reveal a higher level of connexin30 in mammary glands during lactation. However, its role in lactogenesis is underexplored and needs further study.
Mist1 is a transcription factor of helix-loop-helix/basic helix-loop-helix (HLH/bHLH) protein family, associated with MEC differentiation and lactogenesis, as studied in mouse and human mammary glands [Citation98]. Mist1 knockout lactating mammary gland showed impaired lobulo-alveolar organization, shedding of cells to the alveolar lumen and premature activation of STAT3 transcription factor (STAT3 mediates mammary gland involution).
Chemerin (a chemokine, produced primarily by hepatocytes and adipocytes) shows its function by regulating insulin sensitivity and adipocyte differentiation. The study on the effect of chemerin on MAC-T cells differentiation and lactogenesis, showed upregulation of chemerin receptors such as chemokine like-receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2) along with the upregulation of genes involved in fatty acid synthesis, glucose absorption, insulin signalling and casein synthesis [Citation99]. However, there was a downregulation of CMKLR1 in the MAC-T cells by the lactogenic hormones, adiponectin and TNF-α which needs further investigation.
The protein sterol regulatory element binding protein 1 (SREBP1) plays vital role in promoting de novo synthesis of fatty acid and accumulation of triacyl glycerol in goat MECs, and notably the liver X receptor (LXR) protein regulates the transcription of genes which are involved in milk fat synthesis in goat MECs, through transactivation of the SREBP1 transcription factor [Citation100–101]. The protein peroxisome proliferator- activated receptor gamma (PPAR-γ) was found to be the central molecule in regulating the lipid metabolism (enhances lipid accumulation) in goat MECs and it mediates its function by controlling the expression of adipocyte differentiation-related protein (ADRP) gene [Citation102]. Shi et al. studied on the role of ADRP on synthesis of matured cytosolic lipid droplet synthesis in goat MECs and found that enhanced expression of ADRP resulted in increased lipid droplet accumulation and triacyl glycerol content [Citation103]. Moreover, ADRP was found to stabilize the triacyl glycerol in the cytosolic lipid droplets by preventing the access to lipase enzyme action. Thus, ADRP plays crucial role in synthesis and stabilization of cytosolic lipid droplets in goat MECs [Citation103].
Taxreb107 is a leucine zipper DNA-binding protein (transcription factor) the expression of which was upregulated in mouse MECs (SCp2 and HC11 cells) while induced for differentiation by the lactogenic hormones [Citation104]. Similarly, a study with the mouse mammary gland revealed higher expression of Taxreb107 in the mid and late pregnancy. The expression level was persistently high during lactation, indicating that taxreb107 is a hormone-responsive gene involved in MEC differentiation and lactogenesis [Citation104].
Haematopoietic PBX-interacting protein (HPIP, also known as PBXIP1) is an oestrogen receptor (ER) interacting protein that primarily regulates the oestrogen – mediated cancerous cell proliferation and tumorigenesis. A study by Dwivedi et al. revealed that HPIP is essential for lactogenic differentiation induced by prolactin in the in vitro system. It is observed that prolactin induces the expression of HPIP through the STAT5a in the mouse mammary gland [Citation105]. HPIP promotes the acini development, β-casein expression, and lipid droplets formation through the prolactin-induced lactogenesis process and its function is mediated through the activation of PI3K/AKT pathway. Besides, HPIP also regulates the autocrine prolactin signalling in MECs and its function is inhibited by the miR-148a.
A study performed on primary cultures of mouse MECs by Lochrie et al. revealed that IGFBP-5 is over-expressed in both the differentiated (lactogenic hormone-induced cells) as well as apoptotic MECs (apoptosis induced by TGF-β3 treatment) [Citation106]. However, in the HC11 mouse MEC cell line there was no up regulation of IGFBP-5 when induced by TGF-β3 either under serum-free culture or with the dexamethasone, insulin, and prolactin (DIP-lactogenic hormones also represented as DIP) supplemented culture, although there was decreased β-casein synthesis. These findings indicate further studies are needed to confirm the role of IGFBP-5 in lactogenic differentiation and during the involution stage of the mammary glands.
The macrophage colony-stimulating factor (CSF-1) and its receptor (CSF-1R) expression level was found to be regulated by the lactogenic hormones. Study with the human breast biopsy, in vitro organ culture and MEC cell line culture revealed that CSF-1 and CSF-1R expression is enhanced during lactogenic differentiation, showing their involvement in the lactation initiation, which needs further study [Citation107].
The nascent proteins after their synthesis, need to be folded properly in the endoplasmic reticulum (ER) to undergo their function. This folding process can be disrupted by ischaemic conditions or due to the overproduction of abnormal proteins from mutated genes and ER stress [Citation108]. Unfolded protein response (UPR) is a defense process against the ER stress to maintain homeostasis in the cell and this process is highly conserved in eukaryotes [Citation109]. The knockdown of the UPR-related gene ATF4 (transcription factor) resulted in the suppression of lactogenic hormone receptor (glucocorticoid and insulin receptors) expression in MAC-T cells [Citation110]. A study with the mouse MECs also revealed the lowered expression of lactogenic hormone receptors in the ATF4 knockdown which indicates the involvement of this protein in lactation [Citation111]. Expression pattern study of the UPR-related genes (spliced XBP1, ATF4, and C/EBP homologous protein (CHOP)) in bovine mammary gland tissue during pregnancy and lactation revealed the increased expression of these 3 proteins immediately after parturition as compared to that of 50 days before parturition [Citation110]. These findings revealed that the MECs might be under ER stress due to increased production of milk proteins and cells undergo homeostasis through expression of UPR-response related genes. The CHOP protein is a transcription factor and induces apoptosis of the cells where severe ER stress persists and UPR genes not able to revive the homeostasis. In this condition, ATF4 induces the CHOP that promotes apoptosis [Citation112]. An in-depth study is required to delineate the function of UPR genes in molecular details during lactogenic differentiation.
The enzyme isocitrate dehydrogenase (IDH1, cytosolic NADP+ dependent) is responsible for the production of NADPH in the cytosol which is further used for fatty acid synthesis in the mammary gland. Its expression is increased dramatically during early lactation in the bovine mammary gland. In parallel, the BME-UV MECs (in vitro study) also showed enhanced IDH1 expression under the influence of ECM and lactogenic hormone. However, insulin and foetal calf serum decreased its expression, which was further reversed by prolactin treatment. The prolactin-induced IDH1 expression was mediated by MAPK and JAK-STAT pathway and the metabolic effectors also regulated the IDH1 expression in bovine MECs [Citation113].
The CCAAT/enhancer-binding proteins (C/EBPs) bind to the sites in the promoter region of β-casein and their expression level varies during different stages of mammary glands development. It is observed that C/EBPβ plays a vital role in the functional differentiation of mouse mammary glands with ductal morphogenesis, lobular and alveolar development, and lactogenic differentiation of MECs in mice, whereas C/EBPα had no significant effect on the differentiation characteristics [Citation114].
Proteins that downregulate the lactogenic differentiation
Many proteins are found to downregulate the lactogenic differentiation of MECs, or conversely, some proteins are downregulated during this process, thus making the cellular transition as highly regulated event. A study by Anand et al. on mammary gland protein − 40 (MGP-40) in buffalo mammary epithelial cell line (BuMEC), showed its inhibitory effect on the MEC differentiation and induction of epithelial to mesenchymal transition (EMT) – like features [Citation115]. Overexpression of this protein in BuMECs reduced dome formation, acinar polarization and casein synthesis. In addition, this protein seemed to have a protective role on the cells in serum-free conditions by inducing cell proliferation and protecting from apoptosis.
It is found that β-casein expression (induced by lactogenic hormones) is blocked by the addition of EGF to the lactogenic hormone – containing medium [Citation116]. Besides, in the HC11 MECs that are transformed by oncogene and display autocrine activation of EGF receptor, the prolactin – induced differentiation is blocked [Citation117,Citation118]. The study by Horsch et al. on the mechanism behind effect of EGF on prolactin – induced MEC differentiation, revealed that EGF drastically reduces the JAK2 kinase activation. This effect of EGF is mediated by a cytosolic protein tyrosine phosphatase – PEST (proline glutamine serine threonine-rich sequences) which is upregulated in HC11 cells treated with EGF. Reduction of phosphorylated JAK2 ultimately impedes the JAK2-STAT5 signalling and thus inhibits the differentiation process [Citation119].
The AP-2 family transcription factors involve cellular proliferation, apoptosis and differentiation It is observed that during lactation the transcription factors AP-2alpha and AP-2gamma are downregulated and their overproduction in transgenic mice hampered lactation. A study by Jäger et al. with HC11 cell line to understand the relevance of the downregulation of these transcription factors during lactogenic differentiation revealed that the MEC differentiation is not necessarily dependent on the downregulation of AP2 transcription factors. However, this phenomenon in the in vitro study may not be reflected in the in vivo condition which needs further investigation [Citation120].
The enzyme AMP-activated protein kinase (AMPK) senses the cellular energy level to maintain a balance between energy demand and supply by controlling cell metabolic processes [Citation121]. A study on the role of AMPK in lipid and lactose synthesis in the bovine MECs revealed that allosteric activation of AMPK (by glucose deprivation) induces a decrease in lipid synthesis accompanied by enhanced phosphorylation of ACCα and expression of FABP3. Moreover, AMPK activation decreased the lactose synthesis and SLC2A1 expression in the bovine MECS, indicating a tight regulation of milk component synthesis as per the availability of precursors to maintain cellular homeostasis [Citation122]. Another study revealed that AMPK activation inhibits protein synthesis in mammary cells through mTOR complex 1 in MAC-T cells (immortalized bovine mammary epithelial cells) [Citation123]. Study revealed that goat mammary gland adapts to high glucose level by inducing de novo fatty acid synthesis and enhancing milk fat content [Citation124,Citation125]. Both the in vitro (goat MECs isolated from lactating Saanen goats) and in vivo (lactating Guanzhong dairy goats) study showed that the de novo fatty acid synthesis by glucose, is mediated by AMPK-ChREBP axis. The AMPK in mammals is activated by low glucose concentration through AMP/ATP and ADP/ATP ratios [Citation126]. Similarly, the carbohydrate-response element binding protein (ChREBP) is a glucose sensor which gets activated and binds to target genes (fatty acid synthase and acetyl CoA carboxylase) to promote lipogenesis [Citation127].p130Cas is an adaptor molecule that acts as a scaffold for many interacting proteins and mediates various cellular processes. It is overexpressed in many human cancer diseases [Citation128]. The overexpression of p130Cas in murine mammary epithelial cells showed impaired lactogenic differentiation and hyperactivation of the tyrosine kinase C-kit [Citation129]. The C-kit activity is deregulated by p130Cas leading to impaired differentiation of MECs. Interestingly, the downregulation of C-kit reinstated the differentiation process in the MECs overexpressed with p130Cas. The overexpression of p130Cas may lead to the plasticity of luminal cells and priming these cells to develop into cancerous cells [Citation129].
The leptin (a protein hormone produced by adipose cells) was found to regulate the mammary gland development and study by Li et al. in Chinese Guan Zhong dairy goat mammary gland showed that its expression (along with its long form receptor) is lower during lactation but higher during involution and pregnancy [Citation130]. Leptin was seen to promote ductal epithelial cell proliferation and differentiation during pregnancy by JAK-MAPK signalling, modulate β-casein expression in cultured MECs by regulating JAK-STAT5 signalling, and induce apoptosis during involution through JAK-STAT3 signalling pathway. The complex interaction of leptin and its long form receptor regulates the functional mammary gland development in different stages, which needs further study [Citation130]. Besides its expression in adipocytes, leptin is also found in MECs and its expression is reduced in bovine MECs under the influence of GH and lactogenic hormones [Citation131].
The AU-rich elements (AREs) – binding proteins (AUBPs) promote the rapid degradation of mRNAs which have AU-rich elements and thus mediate the post-transcriptional regulation [Citation132]. ARE/poly(U)-binding/degradation factor 1 (AUF1) is one of the well -studied AUBPs that showed an important role during MEC differentiation [Citation133]. The HC11 MECs induced by lactogenic hormones revealed downregulation of AUF1 protein and enhanced β-casein expression; whereas overexpression of AUF1 inhibited the effects of lactogenic hormone stimulation in these MECs. Moreover, AUF1 protein localization was in the nucleus and not in the cytoplasm, during the lactogenic switch of MECs and ound to the c-myc RNA, not the β-casein RNA; thus milk protein synthesis is enhanced [Citation133].
Comparative genomics and proteomics studies highlighting molecular events involved in lactation
Various studies on comparative genome and proteome analysis have identified gene or protein signatures of lactation. Comparative proteomic analysis of proliferative and differentiated mouse HC11 MECs showed 60 differentially expressed proteins between these two stages. Bioinformatic analysis of the findings showed differential expression of cytoskeletal components, chaperones, regulating proteins in protein folding and stability, calcium – binding proteins, and proteins of RNA processing pathways [Citation134]. A study by Jaswal et al. revealed novel protein signatures involved in lactogenic differentiation in buffalo MECs. TMT-based mass spectrometry analysis of the differentiated MECs at day 3, 6, 12, and 15 identified 4934 proteins in total and 681 differentially expressed proteins [Citation135]. Out of them, approximately 307 DEPs attained the highest expression on day 12 of differentiation. Bioinformatic analysis revealed the differentially expressed proteins associated with many signalling pathways that induce the MEC differentiation.
The comparative transcriptome profiling of HC11 MECs at three different stages such as undifferentiated, primed (treated with glucocorticoids), and differentiated (treated with glucocorticoid and prolactin) and their comparison with the murine embryonic stem cells by next-generation mRNA sequencing revealed many stage-specific genes and signalling pathways involved in lactation [Citation136]. There is a downregulation of Pou5f1 and Sox2 genes (pluripotent genes) in the differentiated MECs and an upregulation of the epigenetic regulator Cited4 gene which is required in the lactation stage. Terminal differentiation of the MECs occurred upon treatment with both glucocorticoid and prolactin and there was upregulation of Xbp1 and Cbp genes required for the cellular growth and differentiation. Moreover, this study revealed the expression of chromatin modulators such as Dnmt3l and Chd9 which play a vital role in the lactogenic differentiation of MECs.
Microarray analysis of differentially expressed genes between differentiated HC11 MECs and the normal control cells revealed 998 genes upregulated with more than two-fold expression than the control one [Citation137]. Many signalling pathways are involved in differentiation by undergoing transcriptional regulation. The genes like CTGF/CCN2 and osteopontin were highly expressed in differentiated HC11 cells in vitro. Similar results were found in vivo in the pregnant and lactating mouse mammary gland and decreased during involution, which suggests their role during lactogenic differentiation [Citation137].
Proteins facilitating JAK-STAT pathway
Studies revealed many proteins facilitating the JAK-STAT pathway and enhancing the milk component synthesis. The GTPases of the RAB family are critical regulators, controlling the transport of various biomolecules through vesicles and membrane trafficking in eukaryotic cells. These GTPases recruit the effector molecules and undergo molecular transport among the vesicles by intracellular localization [Citation138]. The RAB6 proteins such as RAB6A and RAB6A’ are abundantly found GTPases involved in Golgi apparatus transport [Citation139]. A study on the function of these two proteins in the mammary gland revealed their vital role in secretory function in the MECs. This study showed that lactogenic differentiation, maturation and secretory function of MECs are affected in the absence of RAB6A GTPase, which ultimately impaired the lactation process [Citation140]. The RAB6A protein seems to promote the trafficking of prolactin receptors in the luminal MEC lineage membrane, thus facilitating the prolactin – induced signalling mechanism to activate STAT5 (transcription factors which induce expression of casein genes) proteins.
Kitayama et al. found a novel cell adhesion apparatus (distinct from desmosomes and gap junctions) at the boundary between luminal alveolar epithelial cell and basal myoepithelial cell [Citation141]. Nectin-1 protein in the basal cells forms the apparatus through trans-interaction with the nectin-4 protein in the luminal cell. The nectin-4 further undergoes cis-interaction with prolactin receptor of luminal cell and promote the JAK-STAT signalling for alveolar development and lactogenic differentiation [Citation141].
The compound 2-Amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) was found to enhance the β-casein expression in the HC11 MECs that have been treated with lactogenic hormones [Citation142]. Moreover, this study showed that PhIP mediates its lactogenic function through the enhanced phosphorylation of STAT5A and bears antiapoptotic properties as evidenced by the enhanced expression of Bcl-2 gene and reduced expression of Bax gene.
Study performed in a rat model with the fruit Hordei Fructus Germinatus (HFG, the raw and processed fruit water decoctions of Hordeum vulgare L. used in the experiment) demonstrate that raw HFG increases the milk yield by regulating the prolactin-mediated JAK-STAT signalling pathway [Citation143].
The homeobox gene family proteins have sequences called homeodomain (DNA-binding domain), and many of these proteins act as transcription factors that regulate normal body development and tumorigenesis [Citation144]. Many homeobox genes are found to play crucial role in mammary gland development during lobulo-alveolar growth [Citation145,Citation146]. Zinc finger homeobox 3 (ZFHX3) is one of the homeobox gene family member having 23 zinc fingers and 4 homeodomains and has dynamic biological functions [Citation147]. A study by Zhao et al. revealed that ZFHX3 protein is crucial for lactogenic differentiation through the maintenance of prolactin signalling pathway [Citation8]. It maintains the prolactin receptor expression and thus promotes the JAK2-STAT5 signalling pathway induced by prolactin signalling. The JAK2-STAT5 pathway is primarily responsible for the synthesis of milk components by regulating transcription of corresponding genes. Studies show that Zfhx3 expression level varies in different mammary glands’ developmental stages, with the highest level during the lactation stage [Citation148]. Moreover, it is found that oestrogen and progesterone induce the expression of ZFHX3 in human and mice mammary epithelial cells [Citation149,Citation150].
Circadian clocks seem to regulate the body physiology by setting a daily rhythm of gene expression and maintaining the cellular homeostasis. The mammary epithelial circadian clock also plays a crucial role in mammary gland development and lactation. A study by Casey et al. showed that the core circadian clock transcription factor BMAL1 regulates MEC growth, differentiation, and milk component synthesis [Citation151]. Identification of transcriptional targets of BMAL1 through ChIP-Seq method of undifferentiated and differentiated HC11 MECs showed 2773 genes unique to the differentiated cells. The experiment with BMAL1-knockout cultured HC11 cells showed lowered cell density and decreased expression of prolactin receptors and beta casein gene expression in the knocked-out cells, which suggests that BMAL1 is essential for MEC differentiation and lactogenesis by mediating the action of prolactin (through enhancing the prolactin receptors) through the JAK-STAT pathway for milk protein synthesis [Citation151].
The transcription factor Elf5 is very much involved in the development of the mammary gland as evidenced in the study with mammary-glands specific Elf5 conditional knockout mice [Citation152]. The MECs lacking Elf5 showed reduced STAT5 level and failure of STAT5 activation, leading to retardation in functional differentiation. This study showed that Elf5 is crucial in regulating the expression of genes that mediate the PrlR/Jak2/Stat5 signalling pathway.
Lee et al. studied the effect of vitamin A derivative retinoic acid on the lactogenesis of MAC-T cells and found that a combination of retinoic acid and prolactin had a synergistic effect on the expression of the casein gene than that of prolactin alone [Citation153]. Interestingly, treatment with only retinoic acid showed an inhibitory effect on the proliferation of MAC-T cells, but the combination with prolactin increased lactogenic differentiation. Expression of α-s1 casein (3.9 fold), α-s2 casein (4.5 fold) and β-casein (4.4 fold) were enhanced significantly than the control with the combinatorial treatment.
Role of miRNAs and non-coding RNAs in MEC differentiation and lactation
The miRNAs are also found to be involved in regulating gene expression of the lactogenic differentiation process. A study with the cultured BMECs that are differentiated by lactogenic hormones (dexamethasone, insulin, and prolactin) revealed that miRs like miR-21–5p, miR-26a, and miR-320a have decreased expression in the differentiated cells [Citation154]. The miR-148a level was increased in the cell culture medium derived from differentiated cells indicating its involvement in lactogenic differentiation and synthesis of milk components [Citation154]. The ratio (culture medium: cells) of expression was higher for miR-103, miR-148a, and miR-223 in the differentiated BMECs, suggesting the lactogenic differentiation-induced secretion of these miRNAs.
The miR-200a promotes epithelial cell differentiation in the mammary gland as evidenced by the study in mice [Citation155]. The level of miR-200a is increased gradually from mid-pregnancy till lactation. The in vitro study using EpH4 mouse MECs revealed that lactogenic hormones (used for differentiation of MECs) stimulated the expression of miR-200a along with the induction of β-casein gene (differentiation marker) and E-cadherin gene (epithelial cell marker) expression. Interestingly, the knockdown of miR-200a showed decreased β-casein and E-cadherin expression and increased ZEB1 (marker of epithelial-mesenchymal transition) expression, indicating and increasing ZEB1 (marker of epithelial-mesenchymal transition) expression, indicating that this miR positively regulates the lactogenic differentiation of MECs and promotes lactation. The miR chi-miR-3031 was found to activate the PI3K/AKT-mTOR pathway and enhance the expression of β-casein by downregulating the expression of IGFBP5 gene in goat MECs [Citation156]. Studies with goat MECs showed that miR-27a suppresses triacyl glycerol accumulation by suppressing PPAR-γ protein levels [Citation157], whereas miR-103 increases milk fat synthesis, triacyl glycerol accumulation, and lipid droplet synthesis [Citation158]. The miR-24 found to enhance the unsaturated fatty acid concentration in milk by targeting the fatty acid synthase gene in goat MECs [Citation159]. The miR-24 also accumulates milk fat droplets and enhances triacyl glycerol level in goat MECs. The expression of miR-27a, and miR-103 were found to be highest during the mid lactation period, and that of miR-24 was highest during peak lactation in dairy goats [Citation157–159].
Noncoding RNAs (ncRNAs) play a crucial role in regulating metabolic processes during development. More specifically, the long ncRNAs (lncRNAs) have shown their regulatory function in many developmental processes and disease conditions [Citation160–162]. The pregnancy-induced ncRNA (PINC) is found to be associated with polycomb repressive complex 2 (PRC2) and regulates the differentiation of MECs [Citation163]. This study revealed that in the mouse mammary gland after puberty, the PINC lncRNA is enriched in luminal and alveolar progenitor cells. Moreover, its level increases throughout pregnancy but decreases in early lactation when the terminal differentiation of alveolar cells occurs. In vitro study with a mouse HC11 cell line (MEC cell line) showed that overexpression of mouse PINC RNA blocks lactogenic differentiation. Furthermore, its level is decreased when the HC11 cells are induced to differentiate and undergo lactogenesis [Citation163]. It is deduced from these findings that PINC lncRNAs inhibit terminal differentiation during the pregnancy period and thus inhibit milk component formation. These findings proved that PINC lncRNA regulates the lactogenic differentiation process through changes in its expression level during late pregnancy and early lactation.
In vitro models used to study the lactogenic differentiation
In vitro models are used to studyphysiological processes, diseases, and drug behaviour studies. The HC11 mouse MEC cell line is a popular in vitro model used to study lactogenic differentiation. The degree of lactogenic differentiation in the cultured HC11 cells was measured by morphological markers such as the formation of dome-shaped cell structures (mammospheres). Although there are many molecular markers to assess the degree of differentiation in MECs, phenotypic changes are a good measure of differentiation in the 2D culture system and milk proteins are accumulated in the domes due to the differentiation induced by lactogenic hormones dexamethasone, insulin, and prolactin [Citation164].
Gordon et al. developed the KIM-2 cell line, a conditionally immortal mammary epithelial cell line derived from a mid-pregnant mouse mammary gland [Citation165]. It carries transgene comprising the ovine β-lactoglobulin gene promoter that drives the expression of a temperature-sensitive variant of SV-40 large T antigen. This temperature – sensitive cell line can differentiate at semi-permissive 37 °C temperature upon the influence of lactogenic hormones and form polarized dome-like structures with tight junctions and express milk protein genes. Upon withdrawal of hormones, the cells undergo apoptosis as observed by nuclear morphology and flow cytometry. At permissive 33 °C temperature cells exhibit elongated spindle-like morphology and are transformed during prolonged culture. These cells can differentiate extensively in the absence of a basement membrane and can be used as a suitable cell model for differentiation and apoptosis study of MECs in response to physiological signals.
Jin et al. developed an in vitro model of human primary mammary epithelial cells isolated from normal breast tissue and having heterogenous morphologic features upon propagation [Citation166]. The cultured cells showed CK-18, desmoglein-3 and CK-19 positive luminal cells and vimentin, p63 and CK-14 positive myoepithelial cells, mimicking the in vivo heterogeneity of mammary gland tissue. Moreover, the cultured primary cells were found to retain the Er-α expression property and respond to oestrogen stimulation. The conditional reprogramming of normal MECs in two-dimensional (2D) and 3-dimensional (3D) culture systems maintained the cellular heterogeneity. More importantly, in the 3D condition, the CK-8 positive cells enclosed a lumen with ability to produce. More importantly, in the 3D condition, the CK-8 positive cells enclosed a lumen with the ability to produce the milk components upon lactogenic stimulation.
MAC-T cells have been widely used as an in vitro model to study the lactogenic differentiation in bovine MECs by the hormones such as dexamethasone, insulin, and prolactin. However, many other factors also have a role in lactogenesis. A study by Johnson et al. on the effect of GH on lactogenesis revealed that GH induced the production of α-s1 casein and α-lactalbumin [Citation167]. Moreover, GH also altered the lipid composition by affecting the proportion of triacylglycerol and sphingomyelin.
A study on the effect of STAT5 activation on the genetically engineered mouse model with breast cancers and the human breast cancer cell line revealed that precancerous MECs become lactogenic with suppression of EMT and reduction of the metastatic potential of the cancerous cells [Citation168].
Future perspectives
Study on the molecular events occurring during lactogenic differentiation holds tremendous potential in exploring many new proteins and their role in regulating the differentiation process and lactation. Protein signatures of lactation will facilitate developing the strategies for persistency and avoidance of truncated lactation in farm animals. Manipulation of the crucial proteins and signalling pathways that regulate the milk synthesis and maintenance of lactation would positively affect the augmentation of milk yield and the socio-economic upliftment of dairy farmers. Understanding the molecular dynamics will aid in developing in vitro models that suitably represent the mammary gland features during lactation. The disease of the mammary glands such as mastitis that occurs during lactation, has caused a substantial economic loss to the dairy industry and has affected the milk quality. Consumption of cow milk from infected animals will cause human disease with antibiotic residues in milk. Antibiotic therapy is the widely used treatment strategy so far. Understanding the molecular physiology of lactation will pave the way for alternative therapeutic strategies that can aid in regaining the milk-synthesizing potential of the mammary glands in subsequent lactations.
Conclusion
Mammary gland is a dynamic organ during pregnancy and lactation, during which the gland undergoes massive molecular and physiological changes to synthesize and secrete milk. The cellular processes like proliferation and branching morphogenesis during pregnancy, lactogenic differentiation, polarization of MECs and milk secretion during lactation, and apoptosis during involution follow a cyclic pattern in each pregnancy-lactation-involution cycle. Hormones, growth factors, cytokines, miRNAs, and many other regulatory proteins regulate the cyclic pattern. In-depth study on the molecular dynamics of mammary gland function and the signalling pathways involved, will pave the way for manipulation of genes to enhance the milk yield in dairy animals. The present review is focused on the involvement of several proteins during lactogenic differentiation and identifying the protein signatures of lactation. Various studies have been done on lactogenic differentiation of MECs and functional mammary gland; however, many proteins already identified so far, but their role during lactation needs further study. The present study will be helpful to the researchers working in this area to design their research work better with updated information on lactogenesis.
Author contributions
Conceptualization: Manoj Kumar Jena; Chrismawan Ardianto.
Funding acquisition: Khang Wen Goh, Long Chiau Ming, Chrismawan Ardianto.
Methodology: Farheen Badrealam Khan, Khang Wen Goh, Long Chiau Ming, Chrismawan Ardianto.
Project administration: Farheen Badrealam Khan, Khang Wen Goh, Long Chiau Ming, Chrismawan Ardianto.
Resources: Jalal Taneera, Ashok Kumar Mohanty, Abdullah Abdullah, Jalal Taneera, Sudarshan Kumar.
Software: Syed Azmal Ali.
Supervision: Ashok Kumar Mohanty, Sudarshan Kumar, Vikas Yadav, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu.
Validation: Syed Azmal Ali, Abdullah Abdullah.
Writing – original draft: Manoj Kumar Jena; Amarish Kumar Sharma, Vikas Yadav, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu, Anjan Kumar Sahoo, Prasana Kumar Rath, Khang Wen Goh, Long Chiau Ming, Chrismawan Ardianto.
Writing – review & editing: Amarish Kumar Sharma, Vikas Yadav, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu, Anjan Kumar Sahoo, Prasana Kumar Rath, Farheen Badrealam Khan, Abdullah Abdullah, Jalal Taneera, Sudarshan Kumar.
All authors have read and agreed to the submission of the manuscript.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The data that support the findings of this review article are available in PubMed Central [PMC] at [https://pubmed.ncbi.nlm.nih.gov/].
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment. Development. 2020;147(22):dev169862. doi:10.1242/dev.169862.
- Grewal SS, Edgar BA. Controlling cell division in yeast and animals: Does size matter? J Biol. 2003;2(1):5. doi:10.1186/1475-4924-2-5.
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135(6):995–1003. doi:10.1242/dev.005439.
- Inman JL, Robertson C, Mott JD, et al. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142(6):1028–1042. doi:10.1242/dev.087643.
- Bach K, Pensa S, Grzelak M, et al. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat Commun. 2017;8(1):2128. doi:10.1038/s41467-017-02001-5.
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–475. doi:10.1016/s1534-5807(01)00064-8.
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–725. doi:10.1038/nrm1714.
- Zhao D, Ma G, Zhang X, et al. Zinc finger homeodomain factor Zfhx3 is essential for mammary lactogenic differentiation by maintaining prolactin signaling activity. J Biol Chem. 2016;291(24):12809–12820. doi:10.1074/jbc.M116.719377.
- Brisken C, Ataca D. Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdiscip Rev Dev Biol. 2015;4(3):181–195. doi:10.1002/wdev.172.
- Melo AI, González-Mariscal G. Communication by olfactory signals in rabbits: its role in reproduction. Vitam Horm. 2010;83:351–371.
- Murphrey MB, Safadi AO, Histology VT, et al. StatPearls. Treasure Island (FL): Stat Pearls Publishing; 2023; [cited 2022 Oct 10]. Available from https://www.ncbi.nlm.nih.gov/books/NBK482199/
- Biswas SK, Banerjee S, Baker GW, et al. The mammary gland: basic structure and molecular signaling during development. Int J Mol Sci. 2022;23(7):3883. doi:10.3390/ijms23073883.
- Rios AC, Fu NY, Jamieson PR, et al. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat Commun. 2016;7(1):11400. doi:10.1038/ncomms11400.
- Romagnoli M, Bresson L, Di-Cicco A, et al. Laminin-binding integrins are essential for the maintenance of functional mammary secretory epithelium in lactation. Development. 2020;147(4):dev181552. doi:10.1242/dev.181552.
- Raymond K, Faraldo MM, Deugnier MA, et al. Integrins in mammary development. Semin Cell Dev Biol. 2012;23(5):599–605. doi:10.1016/j.semcdb.2012.03.008.
- Chammas R, Taverna D, Cella N, et al. Laminin and tenascin assembly and expression regulate HC11 mouse mammary cell differentiation. J Cell Sci. 1994;107(Pt 4):1031–1040. doi:10.1242/jcs.107.4.1031.
- Wirl G, Hermann M, Ekblom P, et al. Mammary epithelial cell differentiation in vitro is regulated by an interplay of EGF action and tenascin-C downregulation. J Cell Sci. 1995;108(Pt 6):2445–2456. doi:10.1242/jcs.108.6.2445.
- Morrison B, Cutler ML. The contribution of adhesion signaling to lactogenesis. J Cell Commun Signal. 2010;4(3):131–139. doi:10.1007/s12079-010-0099-6.
- Wang W, Morrison B, Galbaugh T, et al. Glucocorticoid-induced expression of connective tissue growth factor contributes to lactogenic differentiation of mouse mammary epithelial cells. J Cell Physiol. 2008;214(1):38–46. doi:10.1002/jcp.21159.
- Itahana Y, Piens M, Sumida T, et al. Regulation of clusterin expression in mammary epithelial cells. Exp Cell Res. 2007;313(5):943–951. doi:10.1016/j.yexcr.2006.12.010.
- Bruce A, Johnson A, Lewis J, et al. Integrins. Molecular biology of the cell, 4th ed. New York: Garland Science; 2002.
- Streuli CH, Bailey N, Bissell MJ, et al. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115(5):1383–1395. doi:10.1083/jcb.115.5.1383.
- Taddei I, Faraldo MM, Teulière J, et al. Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia. 2003;8(4):383–394. doi:10.1023/B:JOMG.0000017426.74915.b9.
- Li N, Zhang Y, Naylor MJ, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24(11):1942–1953. doi:10.1038/sj.emboj.7600674.
- Naylor MJ, Li N, Cheung J, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171(4):717–728. doi:10.1083/jcb.200503144.
- Romagnoli M, Cagnet S, Chiche A, et al. Deciphering the mammary stem cell niche: a role for Laminin-Binding integrins. Stem Cell Reports. 2019;12(4):831–844. doi:10.1016/j.stemcr.2019.02.008.
- Leahy DJ, Hendrickson WA, Aukhil I, et al. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992;258(5084):987–991. doi:10.1126/science.1279805.
- Bristow J, Carey W, Egging D, et al. Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2005;139C(1):24–30. doi:10.1002/ajmg.c.30071.
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296(5570):1046–1049. doi:10.1126/science.1067431.
- Wiseman BS, Sternlicht MD, Lund LR, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162(6):1123–1133. doi:10.1083/jcb.200302090.
- Stelwagen K, McFadden HA, Demmer J, et al. Prolactin, alone or in combination with glucocorticoids, enhances tight junction formation and expression of the tight junction protein occludin in mammary cells. Mol Cell Endocrinol. 1999;156(1–2):55–61. doi:10.1016/s0303-7207(99)00145-8.
- Morrison BL, Jose CC, Cutler ML. Connective tissue growth factor (CTGF/CCN2) enhances lactogenic differentiation of mammary epithelial cells via integrin-mediated cell adhesion. BMC Cell Biol. 2010;11(1):35. doi:10.1186/1471-2121-11-35.
- Kozłowski M, Wilczak J, Motyl T, et al. Role of extracellular matrix and prolactin in functional differentiation of bovine BME-UV1 mammary epithelial cells. Pol J Vet Sci. 2011;14(3):433–442. doi:10.2478/v10181-011-0064-1.
- Wanyonyi SS, Kumar A, Du Preez R, et al. Transcriptome analysis of mammary epithelial cell gene expression reveals novel roles of the extracellular matrix. Biochem Biophys Rep. 2017;12:120–128. doi:10.1016/j.bbrep.2017.08.013.
- Lee YJ, Hsu TC, Du JY, et al. Extracellular matrix controls insulin signaling in mammary epithelial cells through the RhoA/Rok pathway. J Cell Physiol. 2009;220(2):476–484. doi:10.1002/jcp.21793.
- Barcellos-Hoff MH, Aggeler J, Ram TG, et al. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105(2):223–235. doi:10.1242/dev.105.2.223.
- Aggeler J, Ward J, Blackie LM, et al. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99(Pt 2):407–417. doi:10.1242/jcs.99.2.407.
- Darcy KM, Zangani D, Shea-Eaton W, et al. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2000;36(9):578–592. doi:10.1007/BF02577526.
- Xu R, Spencer VA, Bissell MJ, et al. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem. 2007;282(20):14992–14999. doi:10.1074/jbc.M610316200.
- Knobil E, Neill JD, editors. The physiology of reproduction, 3rd ed. Amsterdam: Elsevier; 2006.
- Freeman ME, Kanyicska B. Prolactin: structure, function, and regulation of secretion. In: Fink JW, editor. Encyclopedia of stress, Vol. 3. San Diego: Academic Press; 2001. p. 71–78. doi:10.1016/B0-12-227555-6/00241-2.
- Boon WC, Chow JDY. The complex relationship between prolactin and the metabolic syndrome. J Endocrinol. 2017;232(1):R1–R15.
- Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7(3):225–252. doi:10.1023/a:1022896515287.
- Sánchez J, Priego T, Palou A. The hypothalamus-pituitary-adipose tissue axis: What is the relevance of its sexual dimorphism in metabolic regulation? Ann NY Acad Sci. 2008;1143:112–128.
- Ali SA. Phosphoproteomics of mammary gland during different stages of development and its role in lactation [Doctoral dissertation]. National Dairy Research Institute; 2021.
- Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7(1):49–66. doi:10.1023/a:1015770423167.
- Stiening CM, Hoying JB, Abdallah MB, et al. The effects of endocrine and mechanical stimulation on stage I lactogenesis in bovine mammary epithelial cells. J Dairy Sci. 2008;91(3):1053–1066. doi:10.3168/jds.2007-0161.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12(4):211–221. doi:10.1007/s10911-007-9054-4.
- Mida K, Shamay A, Argov-Argaman N. Elongation and desaturation pathways in mammary gland epithelial cells are associated with modulation of fat and membrane composition. J Agric Food Chem. 2012;60(42):10657–10665. doi:10.1021/jf302757j.
- Shao Y, Wall EH, McFadden TB, et al. Lactogenic hormones stimulate expression of lipogenic genes but not glucose transporters in bovine mammary gland. Domest Anim Endocrinol. 2013;44(2):57–69. doi:10.1016/j.domaniend.2012.09.001.
- Cella N, Cornejo-Uribe RR, Montes GS, et al. The lysosomal-associated membrane protein LAMP-1 is a novel differentiation marker for HC11 mouse mammary epithelial cells. Differentiation. 1996;61(2):113–120. doi:10.1046/j.1432-0436.1996.6120113.x.
- Li C, Wang M, Zhang T, et al. Insulin-induced gene 1 and 2 isoforms synergistically regulate triacylglycerol accumulation, lipid droplet formation, and lipogenic gene expression in goat mammary epithelial cells. J Dairy Sci. 2019;102(2):1736–1746. doi:10.3168/jds.2018-15492.
- Watt AP, Lefevre C, Wong CS, et al. Insulin regulates human mammosphere development and function. Cell Tissue Res. 2021;384(2):333–352. doi:10.1007/s00441-020-03360-0.
- Gallego MI, Binart N, Robinson GW, et al. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229(1):163–175. doi:10.1006/dbio.2000.9961.
- Mukhina S, Liu D, Guo K, et al. Autocrine growth hormone prevents lactogenic differentiation of mouse mammary epithelial cells. Endocrinology. 2006;147(4):1819–1829. doi:10.1210/en.2005-1082.
- Darcy KM, Shoemaker SF, Lee PP, et al. Hydrocortisone and progesterone regulation of the proliferation, morphogenesis, and functional differentiation of normal rat mammary epithelial cells in three-dimensional primary culture. J Cell Physiol. 1995;163(2):365–379. doi:10.1002/jcp.1041630217.
- Bomfim GF, Merighe GKF, de Oliveira SA, et al. Acute and chronic effects of cortisol on milk yield, the expression of key receptors, and apoptosis of mammary epithelial cells in Saanen goats. J Dairy Sci. 2022;105(1):818–830. doi:10.3168/jds.2021-20364.
- Baratta M, Grolli S, Poletti A, et al. Role of androgens in proliferation and differentiation of mouse mammary epithelial cell line HC11. J Endocrinol. 2000;167(1):53–60. doi:10.1677/joe.0.1670053.
- Lee PP, Darcy KM, Shudo K, et al. Interaction of retinoids with steroid and peptide hormones in modulating morphological and functional differentiation of normal rat mammary epithelial cells. Endocrinology. 1995;136(4):1718–1730. doi:10.1210/endo.136.4.7895683.
- Hernandez LL, Gregerson KA, Horseman ND. Mammary gland serotonin regulates parathyroid hormone-related protein and other bone-related signals. Am J Physiol Endocrinol Metab. 2012;302(8):E1009–E1015. doi:10.1152/ajpendo.00666.2011.
- VanHouten JN, Dann P, Stewart AF, et al. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112(9):1429–1436. doi:10.1172/JCI200319504.
- Sheftel CM, Hernandez LL. Serotonin stimulated parathyroid hormone related protein induction in the mammary epithelia by transglutaminase-dependent serotonylation. PLoS One. 2020;15(10):e0241192. doi:10.1371/journal.pone.0241192.
- Goffin V, Binart N, Touraine P, et al. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2019;81:47–67. doi:10.1146/annurev-physiol-020518-114632.
- Giepmans BN, van der Doelen AA, Sterk LM, et al. The RAS signaling pathway in GH and PRL secreting pituitary cells. Front Endocrinol. 2011;2:74. doi:10.3389/fendo.2011.00074.
- Clevenger CV, Furth PA, Hankinson SE, et al. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24(1):1–27. doi:10.1210/er.2001-0036.
- Liu J, Ding Z, Li Y, et al. Crosstalk between PI3K/AKT and PRL signaling pathways in breast cancer. Front Oncol. 2021;11:625695.
- Muraoka-Cook RS, Sandahl MA, Hunter D, et al. Prolactin and ErbB4/HER4 signaling interact via janus kinase 2 to induce mammary epithelial cell gene expression differentiation. Mol Endocrinol. 2008;22(10):2307–2321. doi:10.1210/me.2008-0055.
- Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008;13(1):93–103. doi:10.1007/s10911-008-9062-z.
- Wagner KU, Schmidt JW. The two faces of Janus kinases and their respective STATs in mammary gland development and cancer. J Carcinog. 2011;10(1):32. doi:10.4103/1477-3163.90677.
- Stern DF. ErbBs in mammary development. Exp Cell Res. 2003;284(1):89–98. doi:10.1016/s0014-4827(02)00103-9.
- Jones FE, Golding JP, Gassmann M. ErbB4 signaling during breast and neural development: novel genetic models reveal unique ErbB4 activities. Cell Cycle. 2003;2(6):554–558. doi:10.4161/cc.2.6.598.
- Muraoka-Cook RS, Sandahl MA, Strunk KE, et al. The intracellular domain of ErbB4 induces differentiation of mammary epithelial cells. Mol Biol Cell. 2006;17(9):4118–4129. doi:10.1091/mbc.e06-02-0101.
- Long W, Wagner KU, Lloyd KC, et al. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130(21):5257–5268. doi:10.1242/dev.00715.
- Jones FE, Welte T, Fu XY, et al. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J Cell Biol. 1999;147(1):77–88. doi:10.1083/jcb.147.1.77.
- Tidcombe H, Jackson-Fisher A, Mathers K, et al. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100(14):8281–8286. doi:10.1073/pnas.1436402100.
- Muraoka-Cook RS, Sandahl MA, Strunk KE, et al. ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Mol Cell Biol. 2009;29(18):4935–4948. doi:10.1128/MCB.01705-08.
- Williams MM, Vaught DB, Joly MM, et al. ErbB3 drives mammary epithelial survival and differentiation during pregnancy and lactation. Breast Cancer Res. 2017;19(1):105. doi:10.1186/s13058-017-0893-7.
- Shams A, Binothman N, Boudreault J, et al. Prolactin receptor-driven combined luminal and epithelial differentiation in breast cancer restricts plasticity, stemness, tumorigenesis and metastasis. Oncogenesis. 2021;10(1):10. doi:10.1038/s41389-020-00297-5.
- Shan L, Zhang R, Zhang W, et al. Image-based evaluation of the molecular events underlying HC11 mammary epithelial cell differentiation. Anal Biochem. 2008;382(2):122–128. doi:10.1016/j.ab.2008.08.004.
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi:10.1016/j.cell.2007.06.009.
- Maroulakou IG, Oemler W, Naber SP, et al. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol. 2008;217(2):468–477. doi:10.1002/jcp.21518.
- Cerrito MG, Galbaugh T, Wang W, et al. Dominant negative Ras enhances lactogenic hormone-induced differentiation by blocking activation of the Raf-Mek-Erk signal transduction pathway. J Cell Physiol. 2004;201(2):244–258. doi:10.1002/jcp.20077.
- Galbaugh T, Cerrito MG, Jose CC, et al. EGF-induced activation of Akt results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol. 2006;7(1):34. doi:10.1186/1471-2121-7-34.
- Jankiewicz M, Groner B, Desrivières S. Mammalian target of rapamycin regulates the growth of mammary epithelial cells through the inhibitor of deoxyribonucleic acid binding Id1 and their functional differentiation through Id2. Mol Endocrinol. 2006;20(10):2369–2381. doi:10.1210/me.2006-0071.
- Shan L, Yu M, Qiu C, et al. Id4 regulates mammary epithelial cell growth and differentiation and is overexpressed in rat mammary gland carcinomas. Am J Pathol. 2003;163(6):2495–2502. doi:10.1016/S0002-9440(10)63604-8.
- Desprez PY, Hara E, Bissell MJ, et al. Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol Cell Biol. 1995;15(6):3398–3404. doi:10.1128/MCB.15.6.3398.
- Wang B, Shi L, Men J, et al. Controlled synchronization of prolactin/STAT5 and AKT1/mTOR in bovine mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2020;56(3):243–252. doi:10.1007/s11626-020-00432-x.
- Jeon SW, Conejos JRV, Lee JS, et al. D-Methionine and 2-hydroxy-4-methylthiobutanoic acid alter beta-casein, proteins and metabolites linked in milk protein synthesis in bovine mammary epithelial cells. J Anim Sci Technol. 2022;64(3):481–499. doi:10.5187/jast.2022.e37.
- Park DS, Lee H, Riedel C, et al. Prolactin negatively regulates caveolin-1 gene expression in the mammary gland during lactation, via a Ras-dependent mechanism. J Biol Chem. 2001;276(51):48389–48397. doi:10.1074/jbc.M108210200.
- Howe LR, Watanabe O, Leonard J, et al. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63(8):1906–1913.
- Itoh S, Itoh F, Goumans MJ, et al. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000;267(24):6954–6967. doi:10.1046/j.1432-1327.2000.01828.x.
- Perotti C, Karayazi O, Moffat S, et al. The bone morphogenetic protein receptor-1A pathway is required for lactogenic differentiation of mammary epithelial cells in vitro. In Vitro Cell Dev Biol Anim. 2012;48(6):377–384. doi:10.1007/s11626-012-9522-z.
- Kirn-Safran CB, Julian J, Fongemie JE, et al. Changes in the cytologic distribution of heparin/heparan sulfate interacting protein/ribosomal protein L29 (HIP/RPL29) during in vivo and in vitro mouse mammary epithelial cell expression and differentiation. Dev Dyn. 2002;223(1):70–84. doi:10.1002/dvdy.1226.
- Strizzi L, Mancino M, Bianco C, et al. Netrin-1 can affect morphogenesis and differentiation of the mouse mammary gland. J Cell Physiol. 2008;216(3):824–834. doi:10.1002/jcp.21462.
- Srinivasan K, Strickland P, Valdes A, et al. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4(3):371–382. doi:10.1016/s1534-5807(03)00054-6.
- Strizzi L, Bianco C, Raafat A, et al. Netrin-1 regulates invasion and migration of mouse mammary epithelial cells overexpressing Cripto-1 in vitro and in vivo. J Cell Sci. 2005;118(Pt 20):4633–4643. doi:10.1242/jcs.02574.
- Talhouk RS, Elble RC, Bassam R, et al. Developmental expression patterns and regulation of connexins in the mouse mammary gland: expression of connexin30 in lactogenesis. Cell Tissue Res. 2005;319(1):49–59. doi:10.1007/s00441-004-0915-5.
- Zhao Y, Johansson C, Tran T, et al. Identification of a basic helix-loop-helix transcription factor expressed in mammary gland alveolar cells and required for maintenance of the differentiated state. Mol Endocrinol. 2006;20(9):2187–2198. doi:10.1210/me.2005-0214.
- Suzuki Y, Haga S, Katoh D, et al. Chemerin is a novel regulator of lactogenesis in bovine mammary epithelial cells. Biochem Biophys Res Commun. 2015;466(3):283–288. doi:10.1016/j.bbrc.2015.08.105.
- Xu HF, Luo J, Zhao WS, et al. Overexpression of SREBP1 (sterol regulatory element-binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J Dairy Sci. 2016;99(1):783–795. doi:10.3168/jds.2015-9736.
- Xu HF, Luo J, Zhang XY, et al. Activation of liver X receptor promotes fatty acid synthesis in goat mammary epithelial cells via modulation of SREBP1 expression. J Dairy Sci. 2019;102(4):3544–3555. doi:10.3168/jds.2018-15538.
- Kang Y, Hengbo S, Jun L, et al. PPARG modulated lipid accumulation in dairy GMEC via regulation of ADRP gene. J Cell Biochem. 2015;116(1):192–201. doi:10.1002/jcb.24958.
- Shi HB, Yu K, Luo J, et al. Adipocyte differentiation-related protein promotes lipid accumulation in goat mammary epithelial cells. J Dairy Sci. 2015;98(10):6954–6964. doi:10.3168/jds.2015-9452.
- Wittlin S, Sutherland KD, Visvader JE, et al. Identification of Taxreb107 as a lactogenic hormone responsive gene in mammary epithelial cells. Biochim Biophys Acta. 2003;1642(3):139–147. doi:10.1016/s0167-4889(03)00121-6.
- Dwivedi A, Padala C, Kumari A, et al. Hematopoietic PBX-interacting protein is a novel regulator of mammary epithelial cell differentiation. Febs J. 2022;289(6):1575–1590. doi:10.1111/febs.16242.
- Lochrie JD, Phillips K, Tonner E, et al. Insulin-like growth factor binding protein (IGFBP)-5 is upregulated during both differentiation and apoptosis in primary cultures of mouse mammary epithelial cells. J Cell Physiol. 2006;207(2):471–479. doi:10.1002/jcp.20587.
- Sapi E, Flick MB, Rodoy S, et al. Expression of CSF-I and CSF-I receptor by normal lactating mammary epithelial cells. J Soc Gynecol Investig. 1998;5(2):94–101. doi:10.1177/107155769800500208.
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi:10.1038/nrm2199.
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101(5):451–454. doi:10.1016/s0092-8674(00)80855-7.
- Yonekura S, Tsuchiya M, Tokutake Y, et al. The unfolded protein response is involved in both differentiation and apoptosis of bovine mammary epithelial cells. J Dairy Sci. 2018;101(4):3568–3578. doi:10.3168/jds.2017-13718.
- Tsuchiya M, Koizumi Y, Hayashi S, et al. The role of unfolded protein response in differentiation of mammary epithelial cells. Biochem Biophys Res Commun. 2017;484(4):903–908. doi:10.1016/j.bbrc.2017.02.042.
- Zinszner H, Kuroda X, Wang N, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. doi:10.1101/gad.12.7.982.
- Liu W, Capuco AV, Romagnolo DF. Expression of cytosolic NADP+-dependent isocitrate dehydrogenase in bovine mammary epithelium: modulation by regulators of differentiation and metabolic effectors. Exp Biol Med. 2006;231(5):599–610. doi:10.1177/153537020623100515.
- Seagroves TN, Krnacik S, Raught B, et al. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12(12):1917–1928. doi:10.1101/gad.12.12.1917.
- Anand V, Jaswal S, Singh S, et al. Functional characterization of mammary gland protein-40, a chitinase-like glycoprotein expressed during mammary gland apoptosis. Apoptosis. 2016;21(2):209–224. doi:10.1007/s10495-015-1196-z.
- Marte BM, Jeschke M, Graus-Porta D, et al. Neu differentiation factor/heregulin modulates growth and differentiation of HC11 mammary epithelial cells. Mol Endocrinol. 1995;9:14–23.
- Hynes NE, Taverna D, Harwerth IM, et al. Epidermal growth factor receptor, but not c-erbB-2, activation prevents lactogenic hormone induction of the β-casein gene in mouse mammary epithelial cells. Mol Cell Biol. 1990;10(8):4027–4034. doi:10.1128/mcb.10.8.4027-4034.1990.
- Happ B, Hynes NE, Groner B. Ha-ras and v-raf oncogenes, but not int-2 and c-myc, interfere with the lactogenic hormone dependent activation of the mammary gland specific transcription factor. Cell Growth Differ. 1993;4(1):9–15.
- Horsch K, Schaller MD, Hynes NE. The protein tyrosine phosphatase-PEST is implicated in the negative regulation of epidermal growth factor on PRL signaling in mammary epithelial cells. Mol Endocrinol. 2001;15(12):2182–2196. doi:10.1210/mend.15.12.0743.
- Jäger R, Pappas L, Schorle H. Lactogenic differentiation of HC11 cells is not accompanied by downregulation of AP-2 transcription factor genes. BMC Res Notes. 2011;4(1):345. doi:10.1186/1756-0500-4-345.
- Hardie DG. AMP-activated protein kinase – an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi:10.1101/gad.17420111.
- Huang J, Guesthier MA, Burgos SA. AMP-activated protein kinase controls lipid and lactose synthesis in bovine mammary epithelial cells. J Dairy Sci. 2020;103(1):340–351. doi:10.3168/jds.2019-16343.
- Burgos SA, Kim JJ, Dai M, et al. Energy depletion of bovine mammary epithelial cells activates AMPK and suppresses protein synthesis through inhibition of mTORC1 signaling. Horm Metab Res. 2013;45:183–189.
- Cai J, Zhao FQ, Liu JX, et al. Local mammary glucose supply regulates availability and intracellular metabolic pathways of glucose in the mammary gland of lactating dairy goats under malnutrition of energy. Front Physiol. 2018;9:1467. doi:10.3389/fphys.2018.01467.
- Shi H, Jiang N, Wei L, et al. AMPK-ChREBP axis mediates de novo milk fatty acid synthesis promoted by glucose in the mammary gland of lactating goats. Anim Nutr. 2022;10:234–242. doi:10.1016/j.aninu.2022.05.003.
- Zong Y, Zhang CS, Li M, et al. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 2019;29(6):460–473. doi:10.1038/s41422-019-0163-6.
- Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for chrebp: established roles and future goals. Cell Metab. 2017;26(2):324e41–324341. doi:10.1016/j.cmet.2017.07.004.
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, et al. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10(12):858–870. doi:10.1038/nrc2967.
- Tornillo G, Elia AR, Castellano I, et al. p130Cas alters the differentiation potential of mammary luminal progenitors by deregulating c-Kit activity. Stem Cells. 2013;31(7):1422–1433. doi:10.1002/stem.1403.
- Li M, Li Q, Gao X. Expression and function of leptin and its receptor in dairy goat mammary gland. J Dairy Res. 2010;77(2):213–219. doi:10.1017/S0022029910000063.
- Yonekura S, Sakamoto K, Komatsu T, et al. Growth hormone and lactogenic hormones can reduce the leptin mRNA expression in bovine mammary epithelial cells. Domest Anim Endocrinol. 2006;31(1):88–96. doi:10.1016/j.domaniend.2005.09.002.
- Gratacós FM, Brewer G. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip Rev RNA. 2010;1(3):457–473. doi:10.1002/wrna.26.
- Nagaoka K, Tanaka T, Imakawa K, et al. Involvement of RNA binding proteins AUF1 in mammary gland differentiation. Exp Cell Res. 2007;313(13):2937–2945. doi:10.1016/j.yexcr.2007.04.017.
- Desrivières S, Prinz T, Castro-Palomino LN, et al. Comparative proteomic analysis of proliferating and functionally differentiated mammary epithelial cells. Mol Cell Proteomics. 2003;2(10):1039–1054. doi:10.1074/mcp.M300032-MCP200.
- Jaswal S, Anand V, Ali SA, et al. TMT based deep proteome analysis of buffalo mammary epithelial cells and identification of novel protein signatures during lactogenic differentiation. Faseb J. 2021;35(6):e21621.
- Sornapudi TR, Nayak R, Guthikonda PK, et al. Comprehensive profiling of transcriptional networks specific for lactogenic differentiation of HC11 mammary epithelial stem-like cells. Sci Rep. 2018;8(1):11777. doi:10.1038/s41598-018-30122-4.
- Wang W, Jose C, Kenney N, et al. Global expression profiling reveals regulation of CTGF/CCN2 during lactogenic differentiation. J Cell Commun Signal. 2009;3(1):43–55. doi:10.1007/s12079-009-0047-5.
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi:10.1038/nrm2728.
- Goud B, Liu S, Storrie B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases. 2018;9(1–2):66–75. doi:10.1080/21541248.2017.1384087.
- Cayre S, Faraldo MM, Bardin S, et al. RAB6 GTPase regulates mammary secretory function by controlling the activation of STAT5. Development. 2020;147(19):190744. doi:10.1242/dev.190744.
- Kitayama M, Mizutani K, Maruoka M, et al. A novel nectin-mediated cell adhesion apparatus that is implicated in prolactin receptor signaling for mammary gland development. J Biol Chem. 2016;291(11):5817–5831. doi:10.1074/jbc.M115.685917.
- Shan L, Rouhani SA, Schut HA. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) modulates lactogenic hormone-mediated differentiation and gene expression in HC11 mouse mammary epithelial cells. Cell Growth Differ. 2001;12(12):649–656.
- Zhang Z, Wei Q, Zeng Y, et al. Effect of Hordei Fructus Germinatus on differential gene expression in the prolactin signaling pathway in the mammary gland of lactating rats. J Ethnopharmacol. 2021;268:113589. doi:10.1016/j.jep.2020.113589.
- Abate-Shen C. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat Rev Cancer. 2002;2(10):777–785. doi:10.1038/nrc907.
- Lewis MT. Homeobox genes in mammary gland development and neoplasia. Breast Cancer Res. 2000;2(3):158–169. doi:10.1186/bcr49.
- Chen H, Sukumar S. Role of homeobox genes in normal mammary gland development and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8(2):159–175. doi:10.1023/a:1025996707117.
- Yasuda H, Mizuno A, Tamaoki T, et al. ATBF1, a multiple-homeodomain zinc finger protein, selectively down-regulates at-rich elements of the human alpha-fetoprotein gene. Mol Cell Biol. 1994;14(2):1395–1401. doi:10.1128/MCB.14.2.1395.
- Li M, Fu X, Ma G, et al. Atbf1 regulates pubertal mammary gland development likely by inhibiting the pro-proliferative function of estrogen-ER signaling. PLoS One. 2012;7(12):e51283. doi:10.1371/journal.pone.0051283.
- Dong XY, Guo P, Sun X, et al. Estrogen up-regulates ATBF1 transcription but causes its protein degradation in estrogen receptor-positive breast cancer cells. J Biol Chem. 2011;286(16):13879–13890. doi:10.1074/jbc.M110.187849.
- Li M, Zhao D, Ma G, et al. Upregulation of ATBF1 by progesterone-PR signaling and its functional implication in mammary epithelial cells. Biochem Biophys Res Commun. 2013;430(1):358–363. doi:10.1016/j.bbrc.2012.11.009.
- Casey T, Suarez-Trujillo A, Cummings S, et al. Core circadian clock transcription factor BMAL1 regulates mammary epithelial cell growth, differentiation, and milk component synthesis. PLoS One. 2021;16(8):e0248199. doi:10.1371/journal.pone.0248199.
- Choi YS, Chakrabarti R, Escamilla-Hernandez R, et al. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol. 2009;329(2):227–241. doi:10.1016/j.ydbio.2009.02.032.
- Lee HY, Heo YT, Lee SE, et al. Short communication: retinoic acid plus prolactin to synergistically increase specific casein gene expression in MAC-T cells. J Dairy Sci. 2013;96(6):3835–3839. doi:10.3168/jds.2012-5945.
- Muroya S, Hagi T, Kimura A, et al. Lactogenic hormones alter cellular and extracellular microRNA expression in bovine mammary epithelial cell culture. J Anim Sci Biotechnol. 2016;7:8.
- Nagaoka K, Zhang H, Watanabe G, et al. Epithelial cell differentiation regulated by MicroRNA-200a in mammary glands. PLoS One. 2013;8(6):e65127. doi:10.1371/journal.pone.0065127.
- Chen K, Hou J, Song Y, et al. Chi-miR-3031 regulates beta-casein via the PI3K/AKT-mTOR signaling pathway in goat mammary epithelial cells (GMECs). BMC Vet Res. 2018;14(1):369. doi:10.1186/s12917-018-1695-6.
- Lin XZ, Luo J, Zhang LP, et al. MiR-27a suppresses triglyceride accumulation and affects gene mRNA expression associated with fat metabolism in dairy goat mammary gland epithelial cells. Gene. 2013a;521(1):15–23. doi:10.1016/j.gene.2013.03.050.
- Lin X, Luo J, Zhang L, et al. MiR-103 controls milk fat accumulation in goat (Capra hircus) mammary gland during lactation. PLoS One. 2013b;8(11):e79258. doi:10.1371/journal.pone.0079258.
- Wang H, Luo J, Chen Z, et al. MicroRNA-24 can control triacylglycerol synthesis in goat mammary epithelial cells by targeting the fatty acid synthase gene. J Dairy Sci. 2015;98(12):9001–9014. doi:10.3168/jds.2015-9418.
- Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome. 2008;19(7-8):454–492. doi:10.1007/s00335-008-9136-7.
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361. doi:10.1016/j.tcb.2011.04.001.
- Huarte M, Rinn JL. Large non-coding RNAs: Missing links in cancer? Hum Mol Genet. 2010;19(R2):R152–R161. doi:10.1093/hmg/ddq353.
- Shore AN, Kabotyanski EB, Roarty K, et al. Pregnancy-induced noncoding RNA (PINC) associates with polycombrepressive complex 2 and regulates mammary epithelial differentiation. PLoS Genet. 2012;8(7):e1002840. doi:10.1371/journal.pgen.1002840.
- Morrison B, Cutler ML. Mouse mammary epithelial cells form mammospheres during lactogenic differentiation. J Vis Exp. 2009;32:1265.
- Gordon KE, Binas B, Chapman RS, et al. A novel cell culture model for studying differentiation and apoptosis in the mouse mammary gland. Breast Cancer Res. 2000;2(3):222–235. doi:10.1186/bcr57.
- Jin L, Qu Y, Gomez LJ, et al. Characterization of primary human mammary epithelial cells isolated and propagated by conditional reprogrammed cell culture. Oncotarget. 2018;9(14):11503–11514. doi:10.18632/oncotarget.23817.
- Johnson TL, Fujimoto BA, Jiménez-Flores R, et al. Growth hormone alters lipid composition and increases the abundance of casein and lactalbumin mRNA in the MAC-T cell line. J Dairy Res. 2010;77(2):199–204. doi:10.1017/S0022029910000087.
- Lin M, Ku AT, Dong J, et al. STAT5 confers lactogenic properties in breast tumorigenesis and restricts metastatic potential. Oncogene. 2022;41(48):5214–5222. doi:10.1038/s41388-022-02500-w.
