Abstract
Neurological disorders such as neurodegenerative diseases and nervous system tumours affect more than one billion people throughout the globe. The physiological sensitivity of the nervous tissue limits the application of invasive therapies and leads to poor treatment and prognosis. One promising solution that has generated attention is Photodynamic therapy (PDT), which can potentially revolutionise the treatment landscape for neurological disorders. PDT attracted substantial recognition for anticancer efficacy and drug conjugation for targeted drug delivery. This review thoroughly explained the basic principles of PDT, scientific interventions and advances in PDT, and their complicated mechanism in treating brain-related pathologies. Furthermore, the merits and demerits of PDT in the context of neurological disorders offer a well-rounded perspective on its feasibility and challenges. In conclusion, this review encapsulates the significant potential of PDT in transforming the treatment landscape for neurological disorders, emphasising its role as a non-invasive, targeted therapeutic approach with multifaceted applications.
HIGHLIGHT POINTS
Photodynamic therapy is a promising tool to revolutionise the treatment landscape for neurological disorders.
The nexus between photodynamic therapy and biological drug conjugation is best suited for non-invasive neurological disorder treatment.
Introduction
Natural light has been used for three thousand years in treating ailments [Citation1,Citation2]. For instance, ancient Egyptians and the Chinese used sunlight to treat psoriasis, rickets, and vitiligo [Citation3]. Niels Finsen formally introduced phototherapy at the end of the nineteenth century and found that red-light exposure masked the formation of smallpox pustules. Later, he discovered that the sun’s ultraviolet light was also helpful in treating cutaneous tuberculosis. In 1903, he was awarded the Nobel Prize for these discoveries [Citation4,Citation5]. A German scientist, Friedrich Meyer–Belz, later in 1913, tested the effects of hematoporphyrin on his skin and experienced swelling and pain when body parts were exposed to light [Citation6].
In the 1960s, the primary mechanisms of photodynamic reactions attained much scientific attention. Photodynamic therapy (PDT) is now a fast-evolving technique for the detection and treatment of a broad variety of pathologies, from precancerous and cancerous lesions, including brain tumours, to various other neural disorders, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). It can treat meningeal lymphatic vessels and is becoming more prevalent due to its effective antimicrobial properties [Citation1,Citation6]. Currently, most cases of PDT applications rely on the use of photosensitizers (PS). PS are physiochemical agents capable of absorbing light of a specific wavelength, converting [Citation6], and transferring it as valuable energy to an oxygen molecule with the formation of reactive oxygen species (ROS), including oxygen-based free radicals, non-radical forms, and electronically excited singlet oxygen [Citation7]. The PDT is advantageous due to its efficiency, fewer side effects, and minimal invasiveness compared to chemotherapy and radiotherapy. PDT also has the key property of exploiting the integrity of the brain’s blood-brain barrier (BBB) for biomedical applications and is now considered a powerful method that targets malignant cells and other brain anomalies [Citation7].
Pathology of neurodegenerative disorders
Neurodegenerative disorders are characterised by neuronal loss and cognitive dysfunction [Citation8]. Besides, the misfolded proteins deposited and later spread, ultimately challenging the innate immune response and causing oxidative stress and mitochondrial dysfunction [Citation9]. Aggregated misfolded proteins are usually specific for each neurodegenerative disease [Citation2,Citation3]. However, some aggregated proteins are unrelated to a particular disease (). Like, α-synuclein aggregation is evident in dementia and multiple system atrophy [Citation6], while tau aggregates appear in AD and tauopathies, including primary age-related tauopathy and progressive supranuclear palsy (PSP). Huntington’s disease is an inherited disorder that gradually breaks down neurons in the nervous system [Citation10]. Chorea, a hallmark of Huntington’s disease, is a progressive neurological condition inherited in an autosomal dominant pattern [Citation11]. Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, affects the nervous system’s upper and lower motor neurons that control voluntary muscle movement [Citation12]. Patients with ALS may struggle to get therapeutically effective doses of drugs using standard drug delivery methods [Citation13].
Table 1. Characteristic features of the most common neurodegenerative disorders.
Mechanism of PDT
For PDT to function correctly, three things must be present: 1) a photosensitising substance (photosensitiser, PS), 2) a source of visible light (including oxygen), and 3) a gas capable of oxidising the PS. Singlet oxygen, produced in the molecules of protein and the lipids of the molecule, absorbs light; it moves from the cytoplasmic membrane and intracellular organelles when exposed to a quantum of light and is recognised to perform the primary role in PDT [Citation26]. When the PS molecule absorbs light, it moves from the ground to its excited state. At the same time, activated light molecules or quantum emission of fluorescence enter Type I or Type II photochemical processes [Citation27]. In Type I, PS molecules engage directly with malignant cell compoanents, creating interim reactive byproducts that combine with oxygen, producing a variety of highly active substances, predominantly active forms of oxygen, which participate in subsequent oxidation reactions [Citation28]. In this case, oxidant radicals, superoxide anion, and hydroxyl radicals are produced, oxidative stress is triggered, and cellular membranes are compromised in contradiction of their roles (). In Type I photodynamic reaction, the stimulated sensitiser molecule travels from an initial state to the singlet excited state and then to the triplet excited state [Citation29]. In contrast, in Type II, PS molecules interact with oxygen, transforming it into a highly active singlet state. It causes cellular necrosis and apoptosis by interacting with proteins, nucleic acids, and cell membrane lipids [Citation30,Citation31]. Fluorescence treatments are used to evaluate the dispersion of these dyes in both healthy and pathological tissue. The mortality of Paramecia in an atmosphere with low quantities of dyes like acridine, eosin, and fluorescing was previously recorded, leading to the invention of a contemporary method for studying PS and their effects on biological specimens [Citation13,Citation14]. Fluorescent dye-photosensitiser deposited in the tissues is activated locally by visible light due to the amount of oxygen in the tissues, triggering a cascade of events that subsequently results in the death of the targeted cells [Citation1].
Figure 1. General mechanism of action in PDT to PS and different reactions leading to cell death. PS is exposed to light, which causes it to transform into an excited singlet state. Fluorescence emissions or non-radiative decay may be used to partially return to the ground state or undergo intersystem crossing to the excited triplet state. Two different kinds of chemical reactions then work to promote the production of ROS: type I reactions produce free radicals and radical ions through electron transfer reactions, and type II reactions involve the response of PS with molecular oxygen, where the energy is transferred to the triplet ground state of the molecular oxygen to produce singlet oxygen. ROS causes photodynamic effects on pathogenic bacteria or tumour cells (used for PDT) [Citation32].
![Figure 1. General mechanism of action in PDT to PS and different reactions leading to cell death. PS is exposed to light, which causes it to transform into an excited singlet state. Fluorescence emissions or non-radiative decay may be used to partially return to the ground state or undergo intersystem crossing to the excited triplet state. Two different kinds of chemical reactions then work to promote the production of ROS: type I reactions produce free radicals and radical ions through electron transfer reactions, and type II reactions involve the response of PS with molecular oxygen, where the energy is transferred to the triplet ground state of the molecular oxygen to produce singlet oxygen. ROS causes photodynamic effects on pathogenic bacteria or tumour cells (used for PDT) [Citation32].](/cms/asset/e9297eea-82ed-409d-9eb3-ffbaced0acb8/ianb_a_2304814_f0001_c.jpg)
Photosensitizers (PS)
PS are chemical substances capable of being enthused by illumination at specific wavelengths of light [Citation33]. They have tumour-specificity or selective accumulation in tumour sites, restricted dark toxic effects, a higher adsorption peak between 600 and 800 nm wavelengths, a high quantum yield in creating singlet oxygen, and high cytotoxicity that depends on light [Citation34]. Thousands of recognised PS are available, including biological and synthetic in origin (such as chlorophyll, phycobilin, porphyrins, intermediate products of their synthesis, some antibiotics, quinine, riboflavin, and several other drugs) [Citation35]. The following attributes should be present in such PS: chemical purity and uniformity of composition; absence of dark toxicity; high capacity for accumulation in the target tissue; quick removal from the patient’s body; high photochemical activity as evidenced by a high quantum yield of singlet oxygen; and absorption of light in the long-wave region of the spectrum (600–800 nm), where biological tissues are most transparent, with a high coefficient of extinction [Citation14,Citation18].
The first improved therapeutic PS, the ‘hematoporphyrin derivative’ or HpD, did not have delineated chemical properties. Instead, it was a combination of several porphyrins, including hematoporphyrin, protoporphyrin, and deuteroporphyrin, as well as their derivatives, monomers, dimers, oligomers, and esters [Citation36]. The photodynamic characteristics of hematoporphyrin, initially discovered and described in 1911, have become the basis for the initial generation of therapeutic PS. Meyer–Betz was the first person to notice the effects of hematoporphyrin on the human body in 1912 [Citation37]. He did this by injecting himself with intravenous hematoporphyrin, which caused inflammation and discolouration under the impact of light from the sun that lasted for two months. It was the first time these effects had been observed [Citation38].
The accumulation of hematoporphyrin within the tumour cell can be used in therapeutic applications and optical imaging of malignant tumours. In the 1970s, PDT was first time used [Citation39]. The study detailed the effectiveness of hematoporphyrin derivative in PDT for treating squamous cell carcinoma of the skin, breast carcinomas, and metastatic tumours [Citation40]. Such medicine should be able to dependably stimulate different therapeutic rays in the laboratory, be hydrophilic for facile systemic delivery, and not be toxic to activated light. After the procedure, the drug should have the most efficient source of light to trigger the PS, which is also essential to the practical application of PDT [Citation41]. The light source must facilitate light penetration to the desired depth into the tissues, give complete and consistent lighting of the needed zone, and have a wavelength corresponding to the active product’s maximum absorbance. In this regard, the light source selection depends on the choice of medications and the target tissues’ depth, size, and features [Citation42]. The advancement of PDT technology can aid in the fight against various medical conditions, such as colon and bladder tumours, neurological and spinal cord tumours, and the creation of new skin treatment procedures in plastic surgery and cosmetology. Good outcomes of PDT for treating inflammation disorders demonstrate the method’s efficacy against aerobic, facultative, and obligate anaerobic bacteria and microscopic fungi [Citation43,Citation44].
Mechanism of PS
PS localisation is an essential determinant of the intracellular and tissue targets most heavily subjected to photosensitising effects and, thus, of the mechanism of photo-damage. Albumins, globulins, and low- and high-density lipoproteins in whey bind to PS in the circulation, forming aggregates from which only a trace of PS may escape [Citation45]. The PS’s polarity is influenced by its potential for binding to specific whey proteins. With a rise in PS’s hydrophilic nature, the prospect of dye binding to both high and low-density lipoproteins increases. The PS aggregates generated with proteins are consumed by endothelial cells in the vasculature, followed by the binding of dyes with the adventitia of the blood vessels, the arrival of PS in the extracellular matrix, and intracellular accumulation [Citation46]. During optical excitation and relaxation, the PS molecule returns to its initial state and can again participate in a chemical reaction. The entire cycle can be repeated after absorbing a new quantum of light energy. However, the cyclic utility of PS results in ‘burnout’ or their inability to participate in the photodynamic reaction [Citation47].
The liver, kidneys, spleen, and heart are the organs that have the highest concentration of PS following intravenous injection. These organs have a high blood supply and perforated capillaries, which increase the amount of light that can pass through them [Citation48]. It was reported that PS is moving from its usual location in the brain to various organs and tissues, including the lungs, intestines, stomach, and skin. Muscles show a minuscule accumulation of PS compared to other tissue types [Citation49]. The chemical makeup of the medication dictates the procedures that must be carried out to rid the patient’s body of the PS. Generally, the hydrophobic phospholipids are eliminated through the faeces and bile, while the hydrophilic phospholipids are eliminated through the urine. The accumulation of PS is more likely to occur in tumour tissues due to their greater propensity [Citation49].
The effectiveness of PS used for therapeutic purposes depends on the activated wavelengths close to those absorbed by tissue (the red area). Photosensitive substances (PSs) must keep a high triplet quantum yield when subjected to irradiation. It has been discovered that the PDT of most malignancies can benefit from applying several PSs. Different PSs have successfully induced cell death in various tumours, leading to new treatment options ().
Table 2. Distinct categories of PS and the malignancies to which they may be applied.
Nanoparticles in neurodegenerative disorders (NDs)
The BBB-related therapeutic inadequacies and drawbacks have created a dire need for innovative therapeutic strategies in treating NDs [Citation56]. Nanotechnology is a safe and promising CNS-specific drug or gene delivery platform among the various methods used. This method makes use of nanoscale materials (those with a size between 1 and 1000 nm) that can have a molecular level of interaction with biological systems. Nanoparticles (NPs) have been formulated using a wide range of materials, from natural polymers (proteins and polysaccharides) and synthetic polymers (PLGA and PCL) to inorganic minerals, e.g. gold, silver, and cerium (). It has been shown that nanocarriers are effective drug or gene carriers in the CNS [Citation57]. They are advantageous because of their high drug-loading capacity, low systemic toxicity, enhanced drug permeabilization, and strong physical and chemical stability. The various shapes and sizes of NPs have been produced for medication delivery to the brain. However, the ability of nanocarriers to cross the BBB is conditional on factors such as their size, surface chemistry, type, and polarity [Citation58]. Polysorbate coatings on surfaces can also aid in escaping transmembrane efflux systems such as P-glycoprotein pumps. Due to the simplicity of surface functionalization with ligands and cell-penetrating peptides (CPPs), lipid and polymeric NPs have been the most widely used for targeted brain delivery [Citation59].
Table 3. Commonly applied ligands for polymeric coating of NPs for targeting the brain and improving BBB’s permeability.
Types of NPs
Many types of NPs have different shapes and sizes (), and they are chemically and biologically synthesised and used in combination with PDT for various neurological disorders (). Ecofriendly NPs are alternatives to chemical and physical methods and are natural ways to synthesise NPs using natural biological systems, i.e. microorganisms, plants, or fungi. Generally, the NP’s mode of action may be either intracellular or extracellular, depending on the synthesis location.
Figure 2. Schematic illustration of organic and inorganic NPs used for nanotherapeutics or nano-drug products. (Illustration created with BioRender.com.).

Table 4. Different studies of NPs combining PDT for neurological disorders.
Inorganic NPs
Inorganic NPs typically include an inner inorganic core made up of either a metal or an oxide of a metal, as well as an exterior organic shell that maintains the particle in biological surroundings while also allowing it to transport photosynthetic medications. In addition, inorganic NPs can be modified by adding biomolecules such as ligands or antibodies, further improving the PDT PS tumour cell targeting [Citation94].
Metal-based NPs in PDT
PDT has become a viable option for cancer treatment because it does not involve surgery or other harmful side effects [Citation95]. Due to the antibacterial impact of the therapy, it can be used for a wide variety of medical purposes beyond just cancer treatment. It includes the healing process of serious infections, anti-acne therapy, psoriasis and herpes therapy, and therapeutic interventions for physical injury. As scientists progress towards improving illumination and synthesising novel active chemicals, PDT looks increasingly promising to diagnose and treat various disorders [Citation96]. In recent research, metal-based NPs have been proven to have utility as PS, delivery vehicles, and up-conversion instruments [Citation97,Citation98]. Metal NP dispersions, suspensions, and sols are particularly interesting because they can be used in various applications. NPs based on molybdenum oxide, titanium dioxide (TiO2), zinc oxide (ZnO), and tungsten oxide (WO3) are currently the subject of numerous studies looking into their potential use as PS in PDT [Citation99].
Gold-based NPs (GNPs)
In the field of medicine, GNPs are standard. Heating gold particles with IR laser radiation is a promising approach to cancer treatment [Citation100]. Reverberant absorbance of electrical radiation is a unique property of metal NPs when the NP size is substantially less than the wavelength. The surface plasmon resonance, or the aggregate oscillations of conduction electrons on the NP’s surface, is linked to this absorption. The plasmon resonance wavelength is often in the visible and shorter spectrum for metals. For instance, a gold nanostar in water has a surface plasmon resonance of about 520 nm [Citation37,Citation38].
Potential toxicity of GNPs
The possible toxicity of GNPs is the subject of many research efforts. The surface’s size, shape, chemical composition, and surface charge of GNPs significantly impact their cytotoxicity [Citation101]. Smaller particles (10–50 nm) were shown to be more harmful than bigger particles (100–200 nm) in in vivo toxicity investigations of intravenous colloid GNPs in mice [Citation102]. The toxicity of GNPs of triphenylphosphine derivatives, which are water soluble, was investigated in previous studies using four different cells or tissues (including connective tissue fibroblasts, epithelial cells, macrophages, and melanoma cells). The IC50 value within a cell line shifts from 30 to 56 M based on the combination of 1.4 nm GNPs and the cell line, suggesting that these cell lines are particularly susceptible to GNPs of this size. Concentrations of Tauredon (gold thiomalate) below 60 or 100 times are harmless when applied to GNPs with a length of 15 nm. Treatment with 110 M Au NPs in healthy and necrotic cells (1.4 nm diameter). These findings show that gold is a potential material for further PDT research and development [Citation40,Citation41].
Silver-based NPs (SNPs)
Silver is one of the most potent natural antibiotics, and for many years, people have been employing its killing power against a wide range of microbes. A product known as a colloidal nanosilver comprises microscopic SNPs suspended in water that have been demineralised and deionised [Citation103]. The size of SNPs typically ranges from 20 to 25 nm. They have an extraordinarily high specific surface area, which raises the amount of silver that comes into touch with bacteria or viruses and considerably boosts the effectiveness of silver as a bactericide. So, using silver in the form of NPs makes it possible to reduce the concentration of silver by hundreds of times while keeping all its bactericidal capabilities [Citation104].
Potential toxicity of SNPs
Previous studies discovered that SNPs toxicity is proportional to particle size. They also found that oxidative stress significantly mediates SNPs toxicity [Citation105]. Cell viability was tested systematically using three different SNPs sizes (15, 30, and 55 nm). The probable functions of alveolar macrophages in the onset of oxidative stress led to their selection as the subjects of this study. The toxicity was evaluated using mitochondrial function, cell membrane activity, and ROS [Citation50,Citation51]. The activity index dropped dramatically after 24 h of contact with Ag 15 nm and Ag 30 nm NPs (10–75 g/mL). The number of ROS increased by more than a factor of 10 when Ag 15 nm was present at 50 g/mL, suggesting that oxidative stress may play a role in Ag 15 nm’s cytotoxicity. Hence, SNPs are a safe and effective way to boost photodynamic action on microbes, and they can also be used in PDT of malignancies without harming mammalian cells [Citation106].
Copper-based NPs (CNPs)
Copper functions in the body as a trace element. Copper consumption should be around 900 micrograms per day, as suggested. Immunodeficiency develops when copper shortage reduces granulocyte phagocytic activity and immunoglobulin production [Citation107]. Copper plays a crucial physiologic role in cellular proliferation and differentiation. In immunodeficiency, copper gluconate (Cu2+) has been shown to boost IgG levels, cease the development of cancerous cells, and strengthen defense against the disease. NPs of copper sulphide are also commonly employed in PDT procedures. Recent studies have examined the photodynamic activity of CNPs and their drug-delivery capability in the intended tissue. It has been demonstrated that the Cu2-xS nanocrystal is an integral part of the PDT process and that only under the circumstance of near-infrared light irradiation can the ROS drive the biological reaction [Citation52–54].
Potential toxicity of CNPs
An examination of histology revealed that CNPs had severe toxicological effects and could cause significant harm to mice’s kidneys, livers, and spleens. The cytotoxicity of copper NPs has been comprehensively reported by researchers in the past [Citation108]. CNPs were tested for their cytotoxicity by the researchers by adding different sizes of CNPs (25, 50, 78, and 100 nm) as well as a micron grade of copper particles (500 nm) to cell lines derived from fish (PLHC-1 and RTH-149) and mammalian (H4IIE and HepG2), respectively [Citation103,Citation109]. According to the findings, their size and shape would significantly impact the toxicity of CNPs and the ions they discharge [Citation56,Citation57].
Cerium oxide NPs (CONPs)
CONPs are well-known for their ability to neutralise ROS associated with neuronal cell death and NDs. The exceptional oxidative capacity of these NPs can be traced back to the change in oxidised state from Ce+3 to Ce+4 [Citation110]. When paired with PEG coverings or metal chelators, these NPs have been shown to reduce Aβ-aggregation and, in turn, the lethal effect of AD in neuronal cells through modifying the brain-derived neurotrophic factor signalling pathway [Citation111]. In ischaemic stroke models, CONPs have been shown to scavenge peroxynitrite ROS successfully. In MS and ALS mice models, they have been shown to recover distal motor control [Citation112]. Many NP compositions have recently been described as having regenerative capability. Researchers investigated how well cerium dioxide (CeO2) NPs stimulated neurogenesis. They stated that a single dose of CeO2 NPs administered to the hippocampus region was sufficient to kick off the process of neuroplasticity. Cerium oxide’s taken to repair, and anti-inflammatory properties are to blame [Citation61,Citation62].
Magnetic NPs
Magnetic NPs are of considerable interest for study, and their applications in biology and medicine have tremendous potential. Despite harmful effects on healthy cells, these NPs can damage specific protein targets within the body [Citation113]. Compared to metal NPs, the cytotoxicity of magnetic NPs is moderate; as a result, magnetic NPs are used as drug carriers in therapeutic applications when warmed by laser or radiofrequency to the temperature at which diseased tissue is destroyed. Under magnetic NPs, metal-coated particles of various sizes are typically employed [Citation63,Citation64]. Due to their spherical shape and small size distribution, NPs with a shell of magnetite Fe3O4 are widely applied in diagnosing and treating many disorders. The magnetic mesoporous nanoclusters of gold have also shown reduced mouse tumour development and metastasis [Citation114].
Metal-organic frameworks in PDT
Metal-organic frameworks, often known as MOFs, are hybrid materials composed of organic and inorganic components. MOFs are formed when organic ligands and metallic clusters self-assemble into the structure via strong interactions connected to intramolecular pores [Citation115]. The organic ligands and metallic groups are arranged in such a way as to exhibit precise directivity, which can result in the formation of various adsorption capacities, optical absorption, and magnetic properties. The MOFs have a variety of benefits, including high porosity, low density, regular channel, large specific surface area, changeable aperture, and various morphology and customisation [Citation116]. MOFs have seen increased application in PDT over the past few years. Because of their structural and chemical diversity, high molecular loading capacities, and intrinsic good biocompatibility, nanoscale metal-organic frameworks, also known as NMOFs, have been demonstrated to have significant applications in biomedicine. The MOFs have already established themselves as a new category of representative NPs based on metal that can be used in PDT applications [Citation116].
Organic NPs
Due to their higher biomedical applications when contrasted to inorganic materials, naturally produced molecules, such as lipids and other organic compounds, can be utilised as delivery vehicles for nanomedicine. Lipid nanocarriers are more successful than free-drug delivery in preserving the curative component from deterioration, lowering cytotoxicity, and enhancing cytocompatibility [Citation117].
Liposomes
Liposomes are the most widely studied lipid carrier for brain-specific delivery. To improve transport throughout the BBB and target Aβ aggregates with solid potential, liposomes with dual purposes with mApoE and phosphatidic acid were created [Citation118]. A fibril may be destabilised by this liposomal formulation in vitro. Mannose was employed as the targeting ligand in combination with a CPP (penetratin and rabies virus glycoprotein peptide, RVG) to improve brain targeting and cellular absorption. Similarly, brain endothelial cells, astrocytes, and neurons absorbed RVG- and transferrin-modified liposomes at higher rates than unmodified liposomes [Citation70,Citation71]. According to a different study, a single intravenous injection of liposomes functionalised with transferrin and a CPP increased brain mobility in mice. All these investigations have linked surface modification to drug brain deposition. Similarly, VGF (VGF nerve growth factor inducible) was successfully delivered across both in vitro BBB models and in vivo mouse models when optimisation brain targets specific liposomes fused with mannuronic and either RVG, penetratin, rabies-derived peptide (RDP) or CGNHPHLAKYNGT (CGN) peptide were used. The transfection rate in functionalised-liposome-treated mice was 1.5–2.0 times (p < 0.05) more remarkable than in the control group. In addition, the synthesised liposome NPs showed well in vivo and in vitro biocompatibility [Citation119].
Nanomicelle
Polymeric nanomicelle has become an intriguing vehicle for delivering a wide range of therapeutic medicines [Citation120]. Polymers that self-assemble into micelles have a small interior diameter and are suitable for carrying cargo. Based on their hydrophilic and hydrophobic feature, they can do so at relatively low quantities [Citation121]. Recent research has shown that nanostructures of chitosan nanomicelles can transfect brain cells at therapeutically relevant doses. The benefits of chitosan nanomicelles include their adaptability to surface modification, nontoxicity at the application concentration, and biodegradability [Citation74]. Because of these properties, chitosan nanomicelles are promising vehicles for transporting therapeutic agents, such as proteins, DNA, and antibodies, to the brain. A recent study found that conjugated chitosan NPs significantly reduced α-syn aggregation in vitro and had substantial neuroprotective benefits in models of PD. Conjugation with other polymers, such as chitosan, can also improve transport across the BBB [Citation122].
Routes of exposure to NPs
The link between exposure, dose, and reaction is the fundamental concept behind NPs toxicology. So, it is reasonable to assume that if NPs reach the body, toxicological responses may be anticipated as a possible outcome. Although NPs are used in various biomedical and industrial applications, little is known about the potential toxicological consequences they could have on the pathology of biological systems. NPs can be ingested, inhaled, absorbed via the skin, or injected [Citation123]. These are the primary ways that people are exposed to them. Once within the body, NPs can travel through the circulatory system and eventually arrive at their target cells or sites [Citation124]. In addition, based on their properties, such as their size, shape, and high reactivity, they may be able to cross the BBB, or they may be able to reach the brain tissue via neurotransmission along the olfactory bulb. Both routes are possible. Because the exposure could be accidental, it is essential to have information on the potential toxicity of these NPs on the various organ systems and the correlation between the exposure method and the effects of the NPs [Citation77,Citation78].
Biophysical aspect of PDT in brain disorders
The behaviour of NPs in biological systems and that of NPs in the environment are inextricably linked, and frequently associated partnerships are required for such multidisciplinary study [Citation125]. Knowledge of material science, particularly chemical engineering, facilitates our understanding of the synthesis and physical properties of engineered NPs. Knowledge of organic and biochemistry allows us to describe engineered NPs composition, reaction, kinetics, and functionalization. Biophysics and biological chemistry knowledge in living systems emphasises the energetics, assembly, and interaction between NPs and solvent molecules, biomolecules, organelles, cells, and organisms, which is complementary to molecular cell biology information [Citation126]. Because of their site-directed target delivery and capacity to cross through the BBB into the central nervous system, NPs have emerged as a breakthrough treatment for neurodegenerative illnesses such as Parkinson’s disease (PD) [Citation127]. For example, NPs surface functionalised with peptidomimetic antibodies have been reported as molecular Trojan horses for transporting bulky molecules such as medicines and genes over the BBB [Citation128,Citation129]. However, at 20 nM concentration, biocompatible gold (Au) NPs have been shown to produce substantial -synuclein aggregation. Graphene is one of many NPs [Citation130,Citation131]. SPIONs (superparamagnetic iron-oxide NPs) have also been shown to block the fibrillation process during NDs [Citation132,Citation133]. Cerium oxide (CeO2) NPs have been shown to have neuroprotective potential due to their antioxidant and anti-apoptotic properties [Citation134]. Recently, biological network representations and biochemical mathematical models have been utilised to describe interactions (such as activating or inhibiting biological molecular activities and routes between entities) and disclose pharmacokinetic mechanisms in specific systems [Citation135].
Brain disorders, including cancer, are challenging for therapists because of their fragile and sensitive organs. Different approaches are followed for this purpose, including surgical removal of the tumours, laser therapy, and chemotherapy. No method effectively achieves the required target rather than causing other pathological consequences [Citation136]. PDT is an under-progressed method of treating the brain neoplasm. It is a specific and targeted way to treat inter-cranial tumours [Citation137]. It is accepted worldwide now and followed for the treatment of cancers. The PS is delivered to the target site and excited with source light. After excitation, the PS initiates a physiochemical reaction through which the cancer cell degenerates or dies [Citation138]. PS performance is based on its chemical structure. Organic and inorganic PS perform differently as anticancer agents [Citation139].
Almost all forms of PDT require oxygen for their mood of action. Although clear evidence is lacking, Type I responses may be at work in some circumstances. The dominating primary reactions are type 1, often known as photodynamic singlet oxygen (1O2) reactions [Citation140]. In D2O,1O2 has about 20 times longer lifespan (65 µs) than in H2O (3 µs) [Citation141]. Cholesterol combines with singlet oxygen to produce a variety of oxidative metabolites. Because of its selectivity, cholesterol is a valuable signal of singlet oxygen oxidation in situ when other detection techniques are challenging. The therapeutically approved sensitisers bind to membranes because they are lipophilic. PDT-induced cell death is frequently caused by membrane damage [Citation142].
Light, oxygen, and a photo-reactive substance are the three essential components of PDT. The hybrid semiconductor particles absorb light energy, which is transferred to molecular oxygen, forming deadly ROS [Citation143]. While brain tumours cannot be directly exposed to light, even the most inaccessible brain tumours may become accessible during surgery. Light-based treatments may serve as an ideal intraoperative adjuvant therapy. The benefits of nanoscale photosensitisation over ‘traditional’ PDT result from a synergistic combination of superior inorganic material physical characteristics [Citation144]. Additionally, NPs can potentially cross biological barriers such as the BBB [Citation145]. Although nanomaterials tend to aggregate passively in tumours due to the so-called improved ‘permeability and retention effect’ and are frequently used as ‘nanocarriers’ for chemotherapeutics, this passive technique has drawbacks due to its random delivery mechanism [Citation146,Citation147].
Biophysical behaviour of NPs in different neurodegenerative disorder therapy because of its role in tumour biology, the interleukin-13R2 receptor domain (IL13R2R) has received much attention. It binds to interleukin-13 (IL13), a crucial signalling molecule in cancer and inflammation, causing the ligand-receptor complex to be internalised into the tumour cell. On the surface of various malignancies, including GBM, IL-13R2R is shown to be selectively overexpressed [Citation148]. For two reasons, PDT is tumour specific. The laser beam is directed towards the cancer, and the PS is tumour specific. The tumour selectivity of PS has been extensively studied elsewhere [Citation149]. Systemically given sensitisers selectively localise in tumours because malignancies include numerous macrophages that store aggregated sensitisers and likely monomerize them. Since tumour vasculature is frequently leaky, PS aggregated or attached to macromolecules diffuse more easily into tumours than normal tissues, as shown in [Citation150]. Several sensitisers protonate and become more lipophilic when the pH of the extracellular compartment of tumours is decreased. The lymphatic system of many cancers is inadequate, allowing for longer retention of sensitiser molecules in tumours than in normal tissue [Citation150]. The excited PS initiates the production of ROS, leading to a reaction chain transferring the energy to macromolecules [Citation27]. The macromolecules’ energy affects the cell organelles like lysosomes, mitochondria, endoplasmic reticulum, and membrane. It loses its function once the energy affects the membrane and cell organelles. Ultimately, cell death occurs in several ways, such as necrosis, apoptosis, and immunological response [Citation151].
Figure 3. Nano PS activity mechanism on the brain cancer cell. After the delivery of NP to the target site, it acts in two ways. It causes the aggregation of lymphocytes, which leads to the destruction of cancer cells via T-cells. The PS NPs can also aggregate at the target site where the light source induces their excitation and causes the cytoplasmic organelles to degenerate, so the cancer cells deactivate. (Illustration created with BioRender.com.).
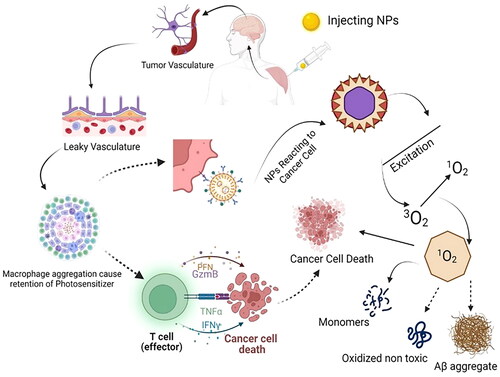
Glioma is one of the most common and aggressive types of brain tumour, with a high fatality rate and a poor prognosis. It is treated with surgery, radiotherapy, and chemotherapy [Citation152]. Due to gliomas’ specific pathological conditions and invasive growth patterns, chemotherapy remains the most convenient method for glioma treatment. Regulated drug release in response to endogenous stimulation, real-time monitoring of medication distribution in tissues, and synergy with other medicines to effectively limit tumour development or kill tumour cells are all possible [Citation153].
Different inorganic metal NPs with a high specific surface area and customisable material characteristics are employed as nanomedicine delivery vehicles for complete disease diagnostics and therapy [Citation154]. Different sizes of inorganic nano-metal carriers, for example, have been utilised to treat and diagnose brain gliomas. Ruthenium NPs have strong biocompatibility and surface functional modification; ruthenium NPs have potential usefulness as nano-carriers for anticancer medication delivery [Citation155].
NPs and PDT in neurodegeneration
The small NPs have unique physiological properties like crossing the BBB and reaching the cells, which may facilitate nerve regeneration [Citation156]. Evidence shows NPs can modulate electrical processes and improve neural cell differentiation, rejuvenation, and survival [Citation157]. Metallic NPs may affect neuronal electrical activity due to their conductivity and potential to react with neural cells [Citation158]. Several types of NPs have been developed: gold, zinc oxide, Mn-ferrite, and carbon nanotubes, which manipulate neuronal activity () [Citation159]. Certain NPs, like silver and iron oxide, are toxic to neurons and cannot be used to modulate their activities [Citation160]. Amplification of axon and dendritic outgrowth is a side effect of metal NPs, making them helpful for boosting neurite outgrowth. GNPs are used in biophotonics because of their surface plasmon resonance capabilities [Citation161].
Figure 4. Transportation mechanism of NPs to the target site across the BBB. The neurovascular unit is a sophisticated network that controls the metabolic demands of brain cells by adjusting cerebral blood flow and solute traffic across the BBB in response to central nervous system inputs, both physiological and pathological. (Illustration created with BioRender.com.).
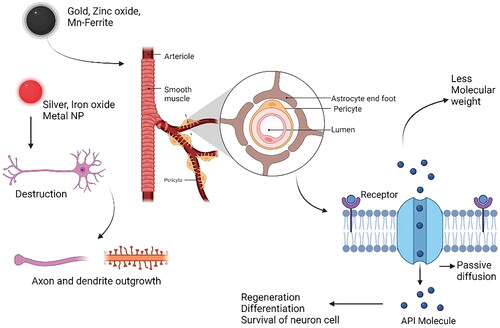
NPs treating Alzheimer’s disease
AD is a progressive neurodegenerative disorder that affects over 24 million people worldwide and has a massive medical and socio-economic impact [Citation162]. While there is a wide range of active pharmaceutical ingredients (API) available for the prevention and possible therapy of AD, their application is restricted by the selective nature of the BBB as well as their significant peripheral side effects [Citation163].
Chitosan NPs are the best option since they can be produced quickly and efficiently, and the material and production costs are inexpensive [Citation164]. On the other hand, the poor water solubility of most chitosan forms can impede the creation of nano-pharmaceuticals. Moreover, chitosan is soluble in a dilute aqueous acidic environment (pH 6.5), allowing it to be engineered into NPs using several processes such as emulsification, reverse micellization, ionic gelation, and desolation [Citation165].
GNPs with specific physical and chemical benefits also perform effectively in AD diseases [Citation166]. Sensors, catalysis, enzyme detection, medical imaging, antibody detection, immunoassays, DNA absorption and release, protease fluorescence detection, and neurochemical quantification are the applications of GNPs that can be achieved while treating AD [Citation167].
Numerous studies on chitosan-based NPs demonstrate potential nanocarriers for targeted nasal-brain medication delivery in treating nervous system illnesses [Citation168]. These particles improved penetration by causing mucosal adhesion and encouraging contact between the positive amino groups of chitosan and those of damaging mucosal sialic acid or other negative groups. However, further research is needed, such as toxicity testing of these NPs for long-term usage [Citation169,Citation170]. The most widely used NPs are porphyrin, phenothiazine, and cyanine [Citation171]. Recently, a few types of APIs, like cholinesterase and phosphodiesterase inhibitors, have been suggested as a treatment for the symptoms of AD [Citation172]. These parameters are used to treat AD tau hyperphosphorylation, non-steroidal anti-inflammatory medications (NSAIDs), and antioxidants (e.g. GSK3 serine-threonine kinase inhibitors), stem cells, neurotrophins (brain-derived neurotrophic factor), vitamins, metal chelators, and methylene blue, an inhibitor of intracellular NFTs [Citation173].
NPs treating Parkinson’s disease
While treating PD, nanometer-targeting drugs, magnetic NPs are more efficient than other nanocarriers. These particles have a small particle size and large surface area and exhibit superparamagnetic behaviours [Citation174]. These properties can cope with traditional administration problems [Citation168]. In the case of PD, the long-chain oleic acid (OA) polymer and its salts are examined, which prevents the aggregation of iron oxide NPs and for efficient medication administration [Citation175]. On the other hand, it is a nanopolymer material with a low critical solution temperature (LCST), thermo-responsiveness, and pH sensitivity. These properties of NIPAm allow for targeted and controlled release. Moreover, research has shown that nerve growth factor (NGF) and its associated receptor tyrosine kinases are essential for neuronal survival and development [Citation176]. Prussian blue NPs, metal oxide NPs, quantum dots, and several other types of NPs have also been reported to treat PD CuxO NP clusters (NCs) due to their biocompatibility and mimicking behaviour with proteins CAT, GPx, and SOD, CuxO NCs because of having the antioxidant potential in treating PD [Citation177]. In an in vitro study, the variability of CuxO NCs was examined in different cell cultures. Using NIH-3T3 cells, the protective role played by CuxO NCs against UVA-induced cell death was evaluated. In UVA-treated NIH-3T3 cells, CuxO NCs significantly improved cell viability and inhibited apoptosis [Citation178]. Recent research has demonstrated that neurotrophin receptor (Trk) internalisation and trafficking pare vitalin neurotrophin-mediated signalling. Growing evidence from PD research has revealed the relevance of NGF in promoting cancer cell uptake via NGF receptor-mediated endocytosis [Citation179].
Gene therapy linked with effective RNA interference is another option in PD treatment that incorporates strategies to successfully transfer genetic carriers to target cells without producing toxicity or adverse effects. In gene therapy, viral vectors are commonly utilised [Citation180]. Unfortunately, researchers have discovered that viral vectors used to treat many neurological illnesses have serious side effects: they appear to cause potent immunogenicity, mutagenesis, and other biohazards, limiting their application [Citation181]. NPs have a clear advantage for gene therapy in nervous system illnesses because they can directly target cells, prevent gene integration, and are less immunogenic. Nonetheless, magnetic NPs utilised as a gene delivery mechanism have been described rarely, notably in PD [Citation116,Citation176].
As a benefit of NPs, numerous nanocarriers were designed to carry medication to treat PD efficiently. Lactoferrin-modified polyamidoamine (PAMAM) and polyethyleneglycol (PEG) NPs containing a neurotrophic factor gene (hGDNF), a plasmid for the human glial cell line, were created [Citation182]. Pahuja et al. synthesised dopamine-loaded PLGA (DA-PLGA) NPs that traversed the BBB primarily in the substantia nigra and striatum (PD-altered areas) of 6-hydroxydopamine rats to efficiently transport dopamine to the brain via BBB efficiently [Citation183]. According to their findings, DA-PLGA NPs reduce the toxicity of bulk dopamine and offer a unique treatment option for PD. After that, other medications, such as ropinirole (RP) drug loaded into PLGA NPs, were produced to demonstrate drug transport to the brain for treating PD with notable outcomes [Citation183,Citation184].
PDT with NPs for stroke
A stroke, characterised by a reduction or blockage of blood supply to the brain, remains a significant concern for neurological disorders. NPs can deliver neuroprotective agents to treat stroke-induced neuronal tissue damage. For instance, a selective caspase-3 inhibitor (Z-DEVD-FMK)-loaded chitosan NPs linked with a transferrin receptor antibody demonstrated encouraging effects in stroke therapy. In a middle cerebral artery occlusion (MCAO) mouse model of stroke, these nanocomposites crossed the BBB, leading to a substantial reduction in infarction volume (by around 40%) and neurological impairments induced by ischaemia [Citation185].
Another promising neuroprotective agent is adenosine, known for its moderate toxicity and short half-life in circulation. To address these limitations, researchers have designed NPs by conjugating adenosine with squalene. The functionalised NPs reduced the infarction area while improving neurological deficiency scores [Citation186]. Mesenchymal stem cells (MSC) based on therapeutic techniques have received much interest due to their potential advantages. Still, their applications are restricted due to poor transport to injured tissues and insufficient release of neuroprotective proteins [Citation187]. To counter these challenges, magnetosome-like ferrimagnetic iron oxide nano-chains (MFIONs) establish non-viral, magnetic field-independent gene transfection methods offering a promising avenue for post-stroke recovery [Citation188]. These examples underscore the evolving role of NPs in treating stroke and their potential to enhance therapeutic outcomes for this devasting neurological condition.
Huntington’s disease
Selenium NPs are used in therapy to regulate HD-related cognitive and neurological disorders [Citation189] and prevent the fibrillation of proteins in the extracellular space, blocking the aggregation of polyglutamine-containing mutant huntingtin protein in model neuronal cells and suppressing mutant huntingtin aggregates in HD [Citation190]. The designed poly (trehalose) NPs are 1000–10,000 times more efficient than molecular trehalose [Citation191]. For effective brain targeting, entrance into neuronal cells, and suppression of mutant huntingtin aggregation, trehalose NPs with a zwitterionic surface charge and a trehalose multivalency (i.e. number of trehalose molecules per NP) of ∼80–200 are crucial [Citation192].
Amyotrophic lateral sclerosis (ALS)
Strategies based on nanotechnology make use of NPs that have shown impressive potential in delivering single or multiple therapeutic agents [Citation91,Citation193,Citation194]. Metallic copper NPs (Cu (II) ATSM) transport copper into neurons using destructive mitochondria [Citation195]. Different studies suggest using miRNA as a treatment for ALS [Citation196]. The miRNA delivery system may include viral vectors and natural materials, e.g. lipids and polymers [Citation25,Citation197]. Cerium oxide NPs (CeNPs) are effective against ROS and nitrogen oxides. It can protect molecular oxygen from forming ROS and anti-inflammation in a cell [Citation198]. The administration of (CeNPs) can reduce disease severity and improve survival [Citation199].
Advantages of PDT
PDT has various benefits compared to more standard methods of treating cancer. Skin becomes more photosensitive after being exposed to first-generation PS. When used appropriately, however, PDT has no lasting negative consequences. It requires less downtime than traditional operations and can be performed without hospitalisation. PDT can kill the tumour and its blood supply, a significant factor in tumour metastasis and invasion [Citation200]. PDT’s dual selectivity allows precise and direct application in the desired tissue. The selective nature of PDT is due to two prime primes: the ability of some PS to accumulate in tumour tissue preferentially and the ability of light to be irradiated only in the target tissue. Since PS is applied directly to the lesions to be treated, it is more likely to accumulate selectively in the tumour in the case of topical application. To be effective, PS administered intravenously must first reach and accumulate within the tumour [Citation37]. Yet, unlike radiation, PDT can be administered multiple times to the same spot. After recovery, there is minimal to no scarring. Finally, the cost is typically lower compared to other therapeutic modalities in cancer treatment. Many of PDT’s applications rely on advances in the physical, chemical, and pharmacological sciences, and the area that studies them is an interdisciplinary one spanning the physical and life sciences. The importance of finding novel PS or improving existing ones is high, as this may enhance the anticancer effects of PDT [Citation106].
Demerits of PDT with NPs in brain disorders
Around 20 PSs have been marketed or employed in clinical studies. However, conventional PDT suffers several significant obstacles that limit its widespread clinical application. To begin, tumours often exhibit atypical cell proliferation, apoptosis, deformed vasculatures, and related hypoxic conditions [Citation201]. In such situations, residual tumour cells are likely to withstand the effects of standard PDT, resulting in limited tumour suppression or tumour recurrence, according to the PDT processes mentioned above. Second, due to the potential adverse effects on normal tissues, patients were advised to avoid exposure to sunlight/indoor light for an extended period following PDT therapy [Citation202]. Moreover, the most recent available PDT regimens are only effective for surface therapy, making them ineffective for deep-seated cancers. In the face of these grave obstacles, researchers have made significant attempts to overcome them.
Numerous reviews have been published on activatable PS and nano-system-based strategies for enhancing PDT. However, the molecular design of existing small-molecule PSs for directly resolving the fundamental PDT problems, such as finite tumour suppression, inadequate tumour targeting, and restricted therapeutic depth, still needs further rigorous investigation [Citation203].
Conclusions
PDT shows superior results for treating cancer due to fewer side effects and high target specificity. Unlike radiotherapy and chemotherapy in PDT, the only area of affected tissue exposed to light is targeted and does not produce toxicant components. Efforts have been made to design effective PS for PDT and small-size NPs further due to their high surface areas, and biocompatibility is rigorously studied. Gold nanorods, for instance, have a higher PDT effect (at least 10 folds) than the PTT effect in killing tumour cells. NPs also have high extinction coefficients and utilise low light intensities and irradiation time in targeting deep tissue tumours. These properties of NPs help switch the dominant roles of PDT and PTT by changing the activation wavelength. The SNPs are dynamic and can be translated into highly tuneable optical features, which can be used for attractive applications in PDT. PDT also shows effectiveness for other major brain diseases, like caspase-3 inhibitor (Z-DEVD-FMK)-loaded chitosan NPs linked with a transferrin receptor antibody and DA-PLGA NPs demonstrated encouraging effects in stroke therapy. The Prussian blue NPs, metal oxide NPs, quantum dots, and several other NPs effectively reduce the disease mechanism of PD.
The significant progress in PDT, especially in synergy with nanotechnology, offers a promising glimpse into the future. Anticipated advancements include refining PS and NPs selection, optimising delivery methods, and ultimately enhancing the precision and effectiveness of PDT in treating neurological disorders. The integration of biophysical insights and nanotechnology promises a more personalised treatment approach. Future research should prioritise safety, long-term effects, and potential combination therapies to leverage PDT’s potential fully. The emergence of PDT as a potent, non-invasive therapeutic option for neurological disorders is on the horizon, instilling fresh hope for patients and healthcare providers. The outlook is optimistic, with PDT poised to play a pivotal role in addressing the complexities of neurological disorders.
Recently, the U.S. Food and Drug Administration (FDA) introduced the recommendations for low-level light therapy (LLLT) devices considering coder of federal regulations (CFR) 878. These guidelines aim for faster approval processes for novel treatments based on PDT. These recommendations can considerably reduce the time it takes for PDT-related research to get from preclinical research to clinical trials and, finally, to market. Changes in FDA rules may broaden the permitted indications for some photosensitizers or PDT procedures. This expansion of uses may open novel possibilities for studying PDT in treating neurological diseases. The FDA has used programs such as Fast Track, Breakthrough Therapy, and Accelerated Approval to speed the review of medications that address unmet medical needs. If these programs continue to evolve, they may provide a speedier regulatory pathway for PDT for neurological disorders. Additionally, the FDA Modernisation Act 2.0 eases the preclinical testing process for novel therapies by accepting cutting-edge microphysiological systems-based alternatives to animal testing. Hence, the approval process for PDT-based modalities will be swifter than before.
In conclusion, despite the controversy, reports show no harmful effects of laser treatments. Laser treatments show minimally invasive procedures and satisfactory results, requiring further investigation. Using different PS, PDT therapy targets malignant brain tumours, primarily gliomas. However, the process is generally still in the clinical trial stages and not ready to be used as a standard treatment strategy.
Authors’ contributions
Conceptualisation, A.N.; writing—original draft preparation, A.N., M.U., T.K., M.H., and M.K.; writing—review and editing, A.N., A.K, H.M.U.F, A.M.N., W.J. and Q.B.: supervision, H.M.U.F., and Q.B. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were analysed or created in this study.
Additional information
Funding
References
- Sun J, Kormakov S, Liu Y, et al. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules. 2018;23(7):1704. doi: 10.3390/molecules23071704.
- Ghorbani J, Rahban D, Aghamiri S, et al. Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther. 2018;27(4):293–302.
- Paszko E, Ehrhardt C, Senge MO, et al. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011;8(1):14–29. doi: 10.1016/j.pdpdt.2010.12.001.
- Mehraban N, Freeman HS. Developments in PDT sensitizers for increased selectivity and singlet oxygen production. Materials. 2015;8(7):4421–4456. doi: 10.3390/ma8074421.
- Zhang Y, Zhang Y, Mei Y, et al. Reactive oxygen species enlightened therapeutic strategy for oral and maxillofacial diseases—art of destruction and reconstruction. Biomedicines. 2022;10(11):2905. doi: 10.3390/biomedicines10112905.
- Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a.
- Monro S, Colón KL, Yin H, et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem Rev. 2018;119(2):797–828. doi: 10.1021/acs.chemrev.8b00211.
- Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9(7):a028035. doi: 10.1101/cshperspect.a028035.
- Singh A, Kukreti R, Saso L, et al. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583. doi: 10.3390/molecules24081583.
- Finkbeiner S. Huntington’s disease. Cold Spring Harb Perspect Biol. 2011;3(6):a007476–a007476. doi: 10.1101/cshperspect.a007476.
- Coppen EM, Roos RA. Current pharmacological approaches to reduce chorea in Huntington’s disease. Drugs. 2017;77(1):29–46. doi: 10.1007/s40265-016-0670-4.
- Al-Chalabi A, Hardiman O, Kiernan MC, et al. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016;15(11):1182–1194. doi: 10.1016/S1474-4422(16)30199-5.
- Mead RJ, Shan N, Reiser HJ, et al. Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov. 2023;22(3):185–212. doi: 10.1038/s41573-022-00612-2.
- Erkkinen MG, Kim M-O, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4):a033118. doi: 10.1101/cshperspect.a033118.
- Penke B, Bogár F, Paragi G, et al. Key peptides and proteins in Alzheimer’s disease. Curr Protein Pept Sci. 2019;20(6):577–599. doi: 10.2174/1389203720666190103123434.
- Martin J. Molecular pathobiology of neurodegenerative diseases. N Engl J Med. 1999;340(25):1970–1980. doi: 10.1056/NEJM199906243402507.
- Li M, Xu C, Ren J, et al. Photodegradation of β-sheet amyloid fibrils associated with Alzheimer’s disease by using polyoxometalates as photocatalysts. Chem Commun (Camb). 2013;49(97):11394–11396. doi: 10.1039/c3cc46772d.
- Li C, Wang J, Liu L. Alzheimer’s therapeutic strategy: photoactive platforms for suppressing the aggregation of amyloid β protein. Front Chem. 2020;8:509. doi: 10.3389/fchem.2020.00509.
- von O, und Halbach B, Schober A, et al. Genes, proteins, and neurotoxins involved in parkinson’s disease. Prog Neurobiol. 2004;73(3):151–177. doi: 10.1016/j.pneurobio.2004.05.002.
- Dubey T, Chinnathambi S. Photodynamic treatment modulates various GTPase and cellular signalling pathways in tauopathy. Small GTPases. 2022;13(1):183–195. doi: 10.1080/21541248.2021.1940722.
- Saberi S, Stauffer JE, Schulte DJ, et al. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin. 2015;33(4):855–876. doi: 10.1016/j.ncl.2015.07.012.
- Hayden E, Cone A, Ju S. Supersaturated proteins in ALS. Proc Natl Acad Sci U S A. 2017;114(20):5065–5066. doi: 10.1073/pnas.1704885114.
- Russ J. Systematic interaction mapping reveals novel modifiers of neurodegenerative disease processes [thesis]. zur Erlangung des akademischen Grades; 2012.
- Gironi M, Arnò C, Comi G, et al. Multiple sclerosis and neurodegenerative diseases. In: Immune rebalancing. Amsterdam: Elsevier; 2016. p. 63–84.
- Bahia CMCdS, Pereira JS. Obstructive sleep apnea and neurodegenerative diseases: a bidirectional relation. Dement Neuropsychol. 2015;9(1):9–15. doi: 10.1590/S1980-57642015DN91000003.
- Bacellar IO, Tsubone TM, Pavani C, et al. Photodynamic efficiency: from molecular photochemistry to cell death. Int J Mol Sci. 2015;16(9):20523–20559. doi: 10.3390/ijms160920523.
- Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96(1):1–8. doi: 10.1016/j.jphotobiol.2009.04.001.
- Maharjan PS, Bhattarai HK. Singlet oxygen, photodynamic therapy, and mechanisms of cancer cell death. J Oncol. 2022;2022:7211485–7211420. doi: 10.1155/2022/7211485.
- Uzdensky A. The biophysical aspects of photodynamic therapy. Biophysics. 2016;61(3):461–469. doi: 10.1134/S0006350916030192.
- Juzenas P, Chen W, Sun Y-P, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008;60(15):1600–1614. doi: 10.1016/j.addr.2008.08.004.
- Li C, Jia P-P, Xu Y-L, et al. Photoacoustic imaging-guided chemo-photothermal combinational therapy based on emissive Pt (II) metallacycle-loaded biomimic melanin dots. Sci. China Chem. 2021;64(1):134–142. doi: 10.1007/s11426-020-9856-7.
- Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115(4):1990–2042. doi: 10.1021/cr5004198.
- Berlanda J, Kiesslich T, Engelhardt V, et al. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J Photochem Photobiol B. 2010;100(3):173–180. doi: 10.1016/j.jphotobiol.2010.06.004.
- Drzewiecka-Matuszek A, Rutkowska-Zbik D. Application of TD-DFT theory to studying porphyrinoid-based photosensitizers for photodynamic therapy: a review. Molecules. 2021;26(23):7176. doi: 10.3390/molecules26237176.
- Rosa LP, Da Silva FC, Nader SA, et al. In vitro effectiveness of antimicrobial photodynamic therapy (APDT) using a 660 nm laser and malachite green dye in Staphylococcus aureus biofilms arranged on compact and cancellous bone specimens. Lasers Med Sci. 2014;29(6):1959–1965. doi: 10.1007/s10103-014-1613-5.
- O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85(5):1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x.
- Correia JH, Rodrigues JA, Pimenta S, et al. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332. doi: 10.3390/pharmaceutics13091332.
- Ackroyd R, Kelty C, Brown N, et al. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74(5):656–669. doi: 10.1562/0031-8655(2001)074<0656:THOPAP>2.0.CO;2.
- Pekkanen AM, DeWitt MR, Rylander MN. Nanoparticle enhanced optical imaging and phototherapy of cancer. J Biomed Nanotechnol. 2014;10(9):1677–1712. doi: 10.1166/jbn.2014.1988.
- Algorri JF, Ochoa M, Roldán-Varona P, et al. Photodynamic therapy: a compendium of latest reviews. Cancers. 2021;13(17):4447. doi: 10.3390/cancers13174447.
- Kinsella TJ, Colussi VC, Oleinick NL, et al. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2(6):917–927. doi: 10.1517/14656566.2.6.917.
- Grossman CE, Carter SL, Czupryna J, et al. Fluence rate differences in photodynamic therapy efficacy and activation of epidermal growth factor receptor after treatment of the tumor-involved murine thoracic cavity. Int J Mol Sci. 2016;17(1):101. doi: 10.3390/ijms17010101.
- Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4(3):283–293. doi: 10.1177/153303460500400308.
- Jia P-P, Xu L, Hu Y-X, et al. Orthogonal self-assembly of a two-step fluorescence-resonance energy transfer system with improved photosensitization efficiency and photooxidation activity. J Am Chem Soc. 2020;143(1):399–408. doi: 10.1021/jacs.0c11370.
- Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24(1):69–79. doi: 10.1038/cr.2013.161.
- Martinez J, Cunha LD, Park S, et al. RETRACTED ARTICLE: noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533(7601):115–119. doi: 10.1038/nature17950.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071.
- Richter AM, Cerruti-Sola S, Sternberg ED, et al. Biodistribution of tritiated benzoporphyrin derivative (3H-BPD-MA), a new potent photosensitizer, in normal and tumor-bearing mice. J Photochem Photobiol B. 1990;5(2):231–244. doi: 10.1016/1011-1344(90)80008-l.
- Irvine DJ, Hanson MC, Rakhra K, et al. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109–11146. doi: 10.1021/acs.chemrev.5b00109.
- Gavrina AI, Shirmanova MV, Aksenova NA, et al. Photodynamic therapy of mouse tumor model using chlorin e6-polyvinyl alcohol complex. J Photochem Photobiol B. 2018;178:614–622. doi: 10.1016/j.jphotobiol.2017.12.016.
- Gao Y-H, Lovreković V, Kussayeva A, et al. The photodynamic activities of dimethyl 131-[2-(guanidinyl) ethylamino] chlorin e6 photosensitizers in A549 tumor. Eur J Med Chem. 2019;177:144–152. doi: 10.1016/j.ejmech.2019.05.050.
- Mae Y, Kanda T, Sugihara T, et al. Verteporfin‑photodynamic therapy is effective on gastric cancer cells. Mol Clin Oncol. 2020;13(3):10. doi: 10.3892/mco.2020.2081.
- Banerjee S, MacRobert A, Mosse C, et al. Photodynamic therapy: inception to application in breast cancer. Breast. 2017;31:105–113. doi: 10.1016/j.breast.2016.09.016.
- Li Z, Wang Y, Wang J, et al. Evaluation of the efficacy of 5-aminolevulinic acid photodynamic therapy for the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn Ther. 2020;29:101596. doi: 10.1016/j.pdpdt.2019.101596.
- Hosokawa S, Takahashi G, Sugiyama K-I, et al. Porfimer sodium-mediated photodynamic therapy in patients with head and neck squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2020;29:101627. doi: 10.1016/j.pdpdt.2019.101627.
- Pino A, Fumagalli G, Bifari F, et al. New neurons in adult brain: distribution, molecular mechanisms and therapies. Biochem Pharmacol. 2017;141:4–22. doi: 10.1016/j.bcp.2017.07.003.
- Modi G, Pillay V, Choonara YE. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann N Y Acad Sci. 2010;1184(1):154–172. doi: 10.1111/j.1749-6632.2009.05108.x.
- Mignani S, Bryszewska M, Zablocka M, et al. Can dendrimer based nanoparticles fight neurodegenerative diseases? Current situation versus other established approaches. Prog Polym Sci. 2017;64:23–51. doi: 10.1016/j.progpolymsci.2016.09.006.
- Lamptey RN, Chaulagain B, Trivedi R, et al. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci. 2022;23(3):1851. doi: 10.3390/ijms23031851.
- Shojai S, Haeri Rohani S-A, Moosavi-Movahedi AA, et al. Human serum albumin in neurodegeneration. Rev Neurosci. 2022;33(7):803–817. doi: 10.1515/revneuro-2021-0165.
- Saraiva C, Praça C, Ferreira R, et al. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044.
- Giau VV, Bagyinszky E, An SSA, et al. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266.
- Lohr KM, Frost B, Scherzer C, et al. Biotin rescues mitochondrial dysfunction and neurotoxicity in a tauopathy model. Proc Natl Acad Sci U S A. 2020;117(52):33608–33618. doi: 10.1073/pnas.1922392117.
- Vassilakopoulou V, Karachaliou C-E, Evangelou A, et al. Peptide-based vaccines for neurodegenerative diseases: recent endeavors and future perspectives. Vaccines. 2021;9(11):1278. doi: 10.3390/vaccines9111278.
- Moustapha A. Neurodegenerative diseases: potential effect of glutathione. In: Glutathione system and oxidative stress in health and disease. London: IntechOpen; 2020.
- Shaughness M, Acs D, Brabazon F, et al. Role of insulin in neurotrauma and neurodegeneration: a review. Front Neurosci. 2020;14:547175. doi: 10.3389/fnins.2020.547175.
- Betuing S, Pikuleva IA, Castellano JM. Cholesterol and neurodegenerative diseases-pressing questions and how to address them. Front Aging Neurosci. 2022;14:948153. doi: 10.3389/fnagi.2022.948153.
- Zou X, Zhong L, Zhu C, et al. Role of leptin in mood disorder and neurodegenerative disease. Front Neurosci. 2019;13:378. doi: 10.3389/fnins.2019.00378.
- Kim DK, Seo MY, Lim SW, et al. Serum melanotransferrin, p97 as a biochemical marker of Alzheimer’s disease. Neuropsychopharmacology. 2001;25(1):84–90. doi: 10.1016/S0893-133X(00)00230-X.
- Rohn S, Suttkus A, Arendt T, et al. RVG peptide as transfection reagent for specific cdk4 gene silencing in vitro and in vivo. J Drug Target. 2012;20(4):381–388. doi: 10.3109/1061186X.2012.669526.
- Feng Z, Guo J, Liu X, et al. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials. 2020;255:120210. doi: 10.1016/j.biomaterials.2020.120210.
- Yang C, Fu Y, Huang C, et al. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials. 2020;255:120194. doi: 10.1016/j.biomaterials.2020.120194.
- Sheng S, Liu F, Lin L, et al. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J Control Release. 2020;328:631–639. doi: 10.1016/j.jconrel.2020.09.029.
- Cho MH, Li Y, Lo P-C, et al. Fucoidan-based theranostic nanogel for enhancing imaging and photodynamic therapy of cancer. Nanomicro Lett. 2020;12(1):47. doi: 10.1007/s40820-020-0384-8.
- Zhou S, Hu X, Xia R, et al. A paclitaxel prodrug activatable by irradiation in a hypoxic microenvironment. Angew Chem Int Ed Engl. 2020;59(51):23198–23205. doi: 10.1002/anie.202008732.
- Um W, Park J, Ko H, et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials. 2019;224:119494. doi: 10.1016/j.biomaterials.2019.119494.
- Chi Y, Qin J, Li Z, et al. Enhanced anti-tumor efficacy of 5-aminolevulinic acid-gold nanoparticles-mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz J Med Biol Res. 2020;53(5):e8457. doi: 10.1590/1414-431x20208457.
- Huang C, Chen F, Zhang L, et al. 99mTc radiolabeled HA/TPGS-based curcumin-loaded nanoparticle for breast cancer synergistic theranostics: design, in vitro and in vivo evaluation. Int J Nanomedicine. 2020;15:2987–2998. doi: 10.2147/IJN.S242490.
- Uthaman S, Pillarisetti S, Mathew AP, et al. Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy. Biomaterials. 2020;232:119702. doi: 10.1016/j.biomaterials.2019.119702.
- Kim Y, Uthaman S, Pillarisetti S, et al. Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy. Acta Biomater. 2020;108:273–284. doi: 10.1016/j.actbio.2020.03.027.
- Lu L, Zhao X, Fu T, et al. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials. 2020;230:119666. doi: 10.1016/j.biomaterials.2019.119666.
- Pan Q, Tian J, Zhu H, et al. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomater Sci Eng. 2020;6(4):2175–2185. doi: 10.1021/acsbiomaterials.0c00163.
- Ji C, Gao Q, Dong X, et al. A size‐reducible nanodrug with an aggregation‐enhanced photodynamic effect for deep chemo‐photodynamic therapy. Angew Chem Int Ed Engl. 2018;57(35):11384–11388. doi: 10.1002/anie.201807602.
- Brynskikh AM, Zhao Y, Mosley RL, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine. 2010;5(3):379–396. doi: 10.2217/nnm.10.7.
- Mukherjee A, Madamsetty VS, Paul MK, et al. Recent advancements of nanomedicine towards antiangiogenic therapy in cancer. Int J Mol Sci. 2020;21(2):455. doi: 10.3390/ijms21020455.
- Siddiqi KS, Husen A, Sohrab SS, et al. Recent status of nanomaterial fabrication and their potential applications in neurological disease management. Nanoscale Res Lett. 2018;13(1):231. doi: 10.1186/s11671-018-2638-7.
- de Mendoza AE-H, Préat V, Mollinedo F, et al. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J Control Release. 2011;156(3):421–426. doi: 10.1016/j.jconrel.2011.07.030.
- Mukhtar M, Bilal M, Rahdar A, et al. Nanomaterials for diagnosis and treatment of brain cancer: recent updates. Chemosensors. 2020;8(4):117. doi: 10.3390/chemosensors8040117.
- Caruso G, Raudino G, Caffo M. Patented nanomedicines for the treatment of brain tumors. Pharm Pat Anal. 2013;2(6):745–754. doi: 10.4155/ppa.13.56.
- Discher BM, Won YY, Ege DS, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143.
- Shevtsov M, Multhoff G. Recent developments of magnetic nanoparticles for theranostics of brain tumor. Curr Drug Metab. 2016;17(8):737–744. doi: 10.2174/1389200217666160607232540.
- DeCoteau W, Heckman KL, Estevez AY, et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine. 2016;12(8):2311–2320. doi: 10.1016/j.nano.2016.06.009.
- Asil SM, Ahlawat J, Barroso GG, et al. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater Sci. 2020;8(15):4109–4128. doi: 10.1039/d0bm00809e.
- Shchukin DG, Sukhorukov GB. Nanoparticle synthesis in engineered organic nanoscale reactors. Adv Mater. 2004;16(8):671–682. doi: 10.1002/adma.200306466.
- Chen Z, Zhang A, Wang X, et al. The advances of carbon nanotubes in cancer diagnostics and therapeutics. J Nanomater. 2017;2017:1–13. doi: 10.1155/2017/3418932.
- Chouikrat R, Seve A, Vanderesse R, et al. Non polymeric nanoparticles for photodynamic therapy applications: recent developments. Curr Med Chem. 2012;19(6):781–792. doi: 10.2174/092986712799034897.
- Nguyen KT, Menon JU, Jadeja PV, et al. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics. 2013;3:152.
- Huang B, Liu X, Yang G, et al. A near-infrared organoplatinum (II) metallacycle conjugated with heptamethine cyanine for trimodal cancer therapy. CCS Chem. 2022;4(6):2090–2101. doi: 10.31635/ccschem.021.202100950.
- Bagheri S, Muhd Julkapli N, Bee Abd Hamid S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci World J. 2014;2014:1–21. doi: 10.1155/2014/727496.
- Alkilany AM, Thompson LB, Boulos SP, et al. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64(2):190–199. doi: 10.1016/j.addr.2011.03.005.
- Re F, Gregori M, Masserini M. Nanotechnology for neurodegenerative disorders. Maturitas. 2012;73(1):45–51. doi: 10.1016/j.maturitas.2011.12.015.
- Sani A, Cao C, Cui D. Toxicity of gold nanoparticles (AuNPs): a review. Biochem Biophys Rep. 2021;26:100991. doi: 10.1016/j.bbrep.2021.100991.
- Suthar JK, Vaidya A, Ravindran S. Toxic implications of silver nanoparticles on the Central nervous system: a systematic literature review. J Appl Toxicol. 2023;43(1):4–21. doi: 10.1002/jat.4317.
- Migliore L, Uboldi C, Di Bucchianico S, et al. Nanomaterials and neurodegeneration. Environ Mol Mutagen. 2015;56(2):149–170. doi: 10.1002/em.21931.
- Elle RE, et al. Dietary exposure to silver nanoparticles in Sprague–Dawley rats: effects on oxidative stress and inflammation. Food Chem Toxicol. 2013;60:297–301.
- De Grandis RA, Santos PWdSD, Oliveira KMd, et al. Novel lawsone-containing ruthenium (II) complexes: synthesis, characterization and anticancer activity on 2D and 3D spheroid models of prostate cancer cells. Bioorg Chem. 2019;85:455–468. doi: 10.1016/j.bioorg.2019.02.010.
- Marin S, Vlasceanu GM, Tiplea RE, et al. Applications and toxicity of silver nanoparticles: a recent review. Curr Top Med Chem. 2015;15(16):1596–1604. doi: 10.2174/1568026615666150414142209.
- Palza H, Nuñez M, Bastías R, et al. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int J Antimicrob Agents. 2018;51(6):912–917. doi: 10.1016/j.ijantimicag.2018.02.007.
- Hasan KF, Wang H, Mahmud S, et al. Enhancing mechanical and antibacterial performances of organic cotton materials with greenly synthesized colored silver nanoparticles. IJCST. 2022;34(4):549–565. doi: 10.1108/IJCST-05-2021-0071.
- Cronholm P, Midander K, Karlsson HL, et al. Effect of sonication and serum proteins on copper release from copper nanoparticles and the toxicity towards lung epithelial cells. Nanotoxicology. 2011;5(2):269–281. doi: 10.3109/17435390.2010.536268.
- Song L, Connolly M, Fernández-Cruz ML, et al. Species-specific toxicity of copper nanoparticles among mammalian and piscine cell lines. Nanotoxicology. 2014;8(4):383–393. doi: 10.3109/17435390.2013.790997.
- Arya A, Gangwar A, Singh SK, et al. Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK–PKC–CBP signaling cascade. Int J Nanomedicine. 2016;11:1159–1173. doi: 10.2147/IJN.S102096.
- Zavvari F, Nahavandi A, Shahbazi A. Neuroprotective effects of cerium oxide nanoparticles on experimental stress-induced depression in male rats. J Chem Neuroanat. 2020;106:101799. doi: 10.1016/j.jchemneu.2020.101799.
- Liu X, Zhang H, Zhang T, et al. Magnetic nanomaterials-mediated cancer diagnosis and therapy. Prog Biomed Eng. 2021;4(1):012005. doi: 10.1088/2516-1091/ac3111.
- Choi K-H, Nam KC, Cho G, et al. Enhanced photodynamic anticancer activities of multifunctional magnetic nanoparticles (Fe3O4) conjugated with chlorin e6 and folic acid in prostate and breast cancer cells. Nanomaterials. 2018;8(9):722. doi: 10.3390/nano8090722.
- Lismont M, Dreesen L, Wuttke S. Metal‐organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv Funct Materials. 2017;27(14):1606314. doi: 10.1002/adfm.201606314.
- Chedid G, Yassin A. Recent trends in covalent and metal organic frameworks for biomedical applications. Nanomaterials. 2018;8(11):916. doi: 10.3390/nano8110916.
- Pinzón-Daza ML, Campia I, Kopecka J, et al. Nanoparticle-and liposome-carried drugs: new strategies for active targeting and drug delivery across blood-brain barrier. Curr Drug Metab. 2013;14(6):625–640. doi: 10.2174/1389200211314060001.
- Arora S, Singh J. In vitro and in vivo optimization of liposomal nanoparticles based brain targeted VGF gene therapy. Int J Pharm. 2021;608:121095. doi: 10.1016/j.ijpharm.2021.121095.
- Kurrikoff K, Vunk B, Langel Ü. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin Biol Ther. 2021;21(3):361–370. doi: 10.1080/14712598.2021.1823368.
- Kim S, Ohulchanskyy TY, Pudavar HE, et al. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J Am Chem Soc. 2007;129(9):2669–2675. doi: 10.1021/ja0680257.
- Sharma D, Singh J. Synthesis and characterization of fatty acid grafted chitosan polymer and their nanomicelles for nonviral gene delivery applications. Bioconjug Chem. 2017;28(11):2772–2783. doi: 10.1021/acs.bioconjchem.7b00505.
- Xie J, Gonzalez-Carter D, Tockary TA, et al. Dual-sensitive nanomicelles enhancing systemic delivery of therapeutically active antibodies specifically into the brain. ACS Nano. 2020;14(6):6729–6742. doi: 10.1021/acsnano.9b09991.
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–MR71. doi: 10.1116/1.2815690.
- Yang W, Thordarson P, Gooding JJ, et al. Carbon nanotubes for biological and biomedical applications. Nanotechnology. 2007;18(41):412001. doi: 10.1088/0957-4484/18/41/412001.
- Ke PC, Lamm MH. A biophysical perspective of understanding nanoparticles at large. Phys Chem Chem Phys. 2011;13(16):7273–7283. doi: 10.1039/c0cp02891f.
- Spuch C, Saida O, Navarro C. Advances in the treatment of neurodegenerative disorders employing nanoparticles. Recent Pat Drug Deliv Formul. 2012;6(1):2–18. doi: 10.2174/187221112799219125.
- Schlachetzki F, Zhang Y, Boado RJ, et al. Gene therapy of the brain: the trans-vascular approach. Neurology. 2004;62(8):1275–1281. doi: 10.1212/01.wnl.0000120551.38463.d9.
- Pardridge WM. Molecular trojan horses for blood–brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. doi: 10.1016/j.coph.2006.06.001.
- Mahmoudi M, Hosseinkhani H, Hosseinkhani M, et al. Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem Rev. 2011;111(2):253–280. doi: 10.1021/cr1001832.
- Padmanabhan P, Kumar A, Kumar S, et al. Nanoparticles in practice for molecular-imaging applications: an overview. Acta Biomater. 2016;41:1–16. doi: 10.1016/j.actbio.2016.06.003.
- Mirsadeghi S, Dinarvand R, Ghahremani MH, et al. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale. 2015;7(11):5004–5013. doi: 10.1039/c4nr06009a.
- Mahmoudi M, Akhavan O, Ghavami M, et al. Graphene oxide strongly inhibits amyloid beta fibrillation. Nanoscale. 2012;4(23):7322–7325. doi: 10.1039/c2nr31657a.
- Hegazy MAE, Maklad HM, Abd Elmonsif DA, et al. The possible role of cerium oxide (CeO2) nanoparticles in prevention of neurobehavioral and neurochemical changes in 6-hydroxydopamineinduced parkinsonian disease. Alexandria J Med. 2017;53(4):351–360. doi: 10.1016/j.ajme.2016.12.006.
- Kaushik AC, Sahi S. Boolean network model for GPR142 against type 2 diabetes and relative dynamic change ratio analysis using systems and biological circuits approach. Syst Synth Biol. 2015;9(1-2):45–54. doi: 10.1007/s11693-015-9163-0.
- de Paula LB, Primo FL, Tedesco AC. Nanomedicine associated with photodynamic therapy for glioblastoma treatment. Biophys Rev. 2017;9(5):761–773. doi: 10.1007/s12551-017-0293-3.
- Mishchenko TA, Turubanova VD, Mitroshina EV, et al. Effect of novel porphyrazine photosensitizers on normal and tumor brain cells. J Biophotonics. 2020;13(1):e201960077. doi: 10.1002/jbio.201960077.
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114.
- Verebová V, Beneš J, Staničová J. Biophysical characterization and anticancer activities of photosensitive phytoanthraquinones represented by hypericin and its model compounds. Molecules. 2020;25(23):5666. doi: 10.3390/molecules25235666.
- Deda DK, Araki K. Nanotechnology, light and chemical action: an effective combination to kill cancer cells. J Braz Chem Soc. 2015;26:2448–2470. doi: 10.5935/0103-5053.20150316.
- Gorman A, Rodgers M. Current perspectives of singlet oxygen detection in biological environments. J Photochem Photobiol B. 1992;14(3):159–176. doi: 10.1016/1011-1344(92)85095-c.
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1(1):1–21. doi: 10.1039/b108586g.
- Kawakami K, Takeshita F, Puri RK. Identification of distinct roles for a dileucine and a tyrosine internalization motif in the interleukin (IL)-13 binding component IL-13 receptor α2 chain. J Biol Chem. 2001;276(27):25114–25120. doi: 10.1074/jbc.M100936200.
- Rozhkova EA, Ulasov I, Lai B, et al. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 2009;9(9):3337–3342. doi: 10.1021/nl901610f.
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387.
- Nasir A, Khan A, Li J, et al. Nanotechnology, a tool for diagnostics and treatment of cancer. Curr Top Med Chem. 2021;21(15):1360–1376. doi: 10.2174/1568026621666210701144124.
- Wykosky J, Gibo D, Stanton C, et al. IL-13 receptor alpha-2, EphA2, and fra-1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14(1):199–208. doi: 10.1158/1078-0432.CCR-07-1990.
- Nelson JS, Liaw L-H, Orenstein A, et al. Mechanism of tumor destruction following photodynamic therapy with hematoporphyrin derivative, chlorin, and phthalocyanine. J Natl Cancer Inst. 1988;80(20):1599–1605. doi: 10.1093/jnci/80.20.1599.
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004;1(4):279–293. doi: 10.1016/S1572-1000(05)00007-4.
- Kaneko S, Fujimoto S, Yamaguchi H, et al. Photodynamic therapy of malignant gliomas. In: Intracranial gliomas part III-innovative treatment modalities. Vol. 32. Basel: Karger; 2018. p. 1–13.
- Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet. 2003;361(9354):323–331. doi: 10.1016/S0140-6736(03)12328-8.
- Van Tellingen O, Yetkin-Arik B, De Gooijer M, et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002.
- Montaseri H, Kruger CA, Abrahamse H. Inorganic nanoparticles applied for active targeted photodynamic therapy of breast cancer. Pharmaceutics. 2021;13(3):296. doi: 10.3390/pharmaceutics13030296.
- Dou Y-K, Shang Y, He X-W, et al. Preparation of a ruthenium-complex-functionalized two-photon-excited red fluorescence silicon nanoparticle composite for targeted fluorescence imaging and photodynamic therapy in vitro. ACS Appl Mater Interfaces. 2019;11(15):13954–13963. doi: 10.1021/acsami.9b00288.
- Zhang T-T, Li W, Meng G, et al. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci. 2016;4(2):219–229. doi: 10.1039/c5bm00383k.
- Paviolo C, Stoddart PR. Gold nanoparticles for modulating neuronal behavior. Nanomaterials. 2017;7(4):92. doi: 10.3390/nano7040092.
- Sawicki K, Czajka M, Matysiak-Kucharek M, et al. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol Rev. 2019;8(1):175–200. doi: 10.1515/ntrev-2019-0017.
- Aghaie T, Jazayeri MH, Manian M, et al. Gold nanoparticle and polyethylene glycol in neural regeneration in the treatment of neurodegenerative diseases. J Cell Biochem. 2019;120(3):2749–2755. doi: 10.1002/jcb.27415.
- Muheem A. Recent patents, regulatory issues, and toxicity of nanoparticles in neuronal disorders. CDM. 2021;22(4):263–279. doi: 10.2174/18755453MTEyjMzIn3.
- Gogurla N, Kundu SC, Ray SK. Gold nanoparticle-embedded silk protein-ZnO nanorod hybrids for flexible bio-photonic devices. Nanotechnology. 2017;28(14):145202. doi: 10.1088/1361-6528/aa6144.
- Mioshi E, Foxe D, Leslie F, et al. The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1):68–73. doi: 10.1097/WAD.0b013e318247a0bc.
- Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171.
- Manek E, Darvas F, Petroianu GA. Use of biodegradable, chitosan-based nanoparticles in the treatment of Alzheimer’s disease. Molecules. 2020;25(20):4866. doi: 10.3390/molecules25204866.
- Naskar S, Sharma S, Kuotsu K. Chitosan-based nanoparticles: an overview of biomedical applications and its preparation. J Drug Delivery Sci Technol. 2019;49:66–81. doi: 10.1016/j.jddst.2018.10.022.
- Dos Santos Tramontin N, da Silva S, Arruda R, et al. Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol Neurobiol. 2020;57(2):926–936. doi: 10.1007/s12035-019-01780-w.
- Hu K, Chen X, Chen W, et al. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomedicine. 2018;14(4):1123–1136. doi: 10.1016/j.nano.2018.01.020.
- Yu S, Xu X, Feng J, et al. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282–293. doi: 10.1016/j.ijpharm.2019.02.012.
- Elnaggar YS, Etman SM, Abdelmonsif DA, et al. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104(10):3544–3556. doi: 10.1002/jps.24557.
- Rabiee N, Ahmadi S, Afshari R, et al. Polymeric nanoparticles for nasal drug delivery to the brain: relevance to Alzheimer’s disease. Adv Ther. 2021;4(3):2000076. doi: 10.1002/adtp.202000076.
- Panza F, Seripa D, Solfrizzi V, et al. Tau aggregation inhibitors: the future of Alzheimer’s pharmacotherapy? Expert Opin Pharmacother. 2016;17(4):457–461. doi: 10.1517/14656566.2016.1146686.
- Adnet T, Groo A-C, Picard C, et al. Pharmacotechnical development of a nasal drug delivery composite nanosystem intended for Alzheimer’s disease treatment. Pharmaceutics. 2020;12(3):251. doi: 10.3390/pharmaceutics12030251.
- Villarejo-Galende A, González-Sánchez M, Blanco-Palmero VA, et al. Non-steroidal anti-inflammatory drugs as candidates for the prevention or treatment of Alzheimer’s disease: do they still have a role? Curr Alzheimer Res. 2020;17(11):1013–1022. doi: 10.2174/1567205017666201127163018.
- Upadhyay N, Tripathi M, Chaddha RK, et al. Development of sensitive magnetic nanoparticle assisted rapid sandwich assay (s-MARSA) to monitor Parkinson’s disease and schizophrenia pharmacotherapy. Anal Biochem. 2023;667:115082. doi: 10.1016/j.ab.2023.115082.
- Moayeri A, Darvishi M, Amraei M. Homing of super paramagnetic iron oxide nanoparticles (SPIONs) labeled adipose-derived stem cells by magnetic attraction in a rat model of Parkinson’s disease. Int J Nanomedicine. 2020;15:1297–1308. doi: 10.2147/IJN.S238266.
- Niu S, Zhang L-K, Zhang L, et al. Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics. 2017;7(2):344–356. doi: 10.7150/thno.16562.
- Ouyang Y, Fadeev M, Zhang P, et al. Aptamer-functionalized Ce4+-ion-modified C-dots: peroxidase mimicking aptananozymes for the oxidation of dopamine and cytotoxic effects toward cancer cells. ACS Appl Mater Interfaces. 2022;14(50):55365–55375. doi: 10.1021/acsami.2c16199.
- Hao C, Qu A, Xu L, et al. Chiral molecule-mediated porous Cu x O nanoparticle clusters with antioxidation activity for ameliorating Parkinson’s disease. J Am Chem Soc. 2018;141(2):1091–1099. doi: 10.1021/jacs.8b11856.
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33(2-3):199–227. doi: 10.1016/s0165-0173(00)00030-8.
- Axelsen TM, Woldbye DP. Gene therapy for Parkinson’s disease, an update. J Parkinsons Dis. 2018;8(2):195–215. doi: 10.3233/JPD-181331.
- Mandel RJ, Manfredsson FP, Foust KD, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13(3):463–483. doi: 10.1016/j.ymthe.2005.11.009.
- Wu H, Wang T, Bohn MC, et al. Novel magnetic hydroxyapatite nanoparticles as non‐viral vectors for the glial cell line‐derived neurotrophic factor gene. Adv Funct Materials. 2010;20(1):67–77. doi: 10.1002/adfm.200901108.
- Pahuja R, Seth K, Shukla A, et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano. 2015;9(5):4850–4871. doi: 10.1021/nn506408v.
- Jahansooz F, Hosseinzade BE, Zarmi AH, et al. Dopamine-loaded poly (butyl cyanoacrylate) nanoparticles reverse behavioral deficits in Parkinson’s animal models. Ther Deliv. 2020;11(6):387–399. doi: 10.4155/tde-2020-0026.
- Yemisci M, Caban S, Gursoy-Ozdemir Y, et al. Systemically administered brain-targeted nanoparticles transport peptides across the blood—brain barrier and provide neuroprotection. J Cereb Blood Flow Metab. 2015;35(3):469–475. doi: 10.1038/jcbfm.2014.220.
- Barcin C, Denktas AE, Lennon RJ, et al. Comparison of combination therapy of adenosine and nitroprusside with adenosine alone in the treatment of angiographic no‐reflow phenomenon. Catheter Cardiovasc Interv. 2004;61(4):484–491. doi: 10.1002/ccd.20010.
- Honmou O, Onodera R, Sasaki M, et al. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med. 2012;18(5):292–297. doi: 10.1016/j.molmed.2012.02.003.
- Zhang T, Li F, Xu Q, et al. Ferrimagnetic nanochains‐based mesenchymal stem cell engineering for highly efficient post‐stroke recovery. Adv Funct Materials. 2019;29(24):1900603. doi: 10.1002/adfm.201900603.
- Abdelfattah MS, Badr SEA, Lotfy SA, et al. Rutin and selenium co-administration reverse 3-nitropropionic acid-induced neurochemical and molecular impairments in a mouse model of Huntington’s disease. Neurotox Res. 2020;37(1):77–92. doi: 10.1007/s12640-019-00086-y.
- Debnath K, Pradhan N, Singh BK, et al. Poly (trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Appl Mater Interfaces. 2017;9(28):24126–24139. doi: 10.1021/acsami.7b06510.
- Liu B, Zhang L, Zhang Q, et al. Membrane stabilization of poly (ethylene glycol)-b-polypeptide-g-trehalose assists cryopreservation of red blood cells. ACS Appl Bio Mater. 2020;3(5):3294–3303. doi: 10.1021/acsabm.0c00247.
- Pradhan N, Jana NR, Jana NR. Inhibition of protein aggregation by iron oxide nanoparticles conjugated with glutamine-and proline-based osmolytes. ACS Appl Nano Mater. 2018;1(3):1094–1103. doi: 10.1021/acsanm.7b00245.
- Velasco-Aguirre C, Morales F, Gallardo-Toledo E, et al. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: preclinical approaches. Int J Nanomedicine. 2015;10:4919–4936. doi: 10.2147/IJN.S82310.
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. vol2016;1(1):10–29. doi: 10.1002/btm2.10003.
- McAllum EJ, Lim NK-H, Hickey JL, et al. Therapeutic effects of CuII (atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7–8):586–590. doi: 10.3109/21678421.2013.824000.
- Paez-Colasante X, Figueroa-Romero C, Sakowski SA, et al. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat Rev Neurol. 2015;11(5):266–279. doi: 10.1038/nrneurol.2015.57.
- Kim JY, Park J, Chang JY, et al. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. 2016;25(5):241–251. doi: 10.5607/en.2016.25.5.241.
- Dhall A, Self W. Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants. 2018;7(8):97. doi: 10.3390/antiox7080097.
- Park K, Park J, Lee H, et al. Toxicity and tissue distribution of cerium oxide nanoparticles in rats by two different routes: single intravenous injection and single oral administration. Arch Pharm Res. 2018;41(11):1108–1116. doi: 10.1007/s12272-018-1074-7.
- Calixto GMF, Bernegossi J, De Freitas LM, et al. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review. Molecules. 2016;21(3):342. doi: 10.3390/molecules21030342.
- Overchuk M, Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–237. doi: 10.1016/j.biomaterials.2017.10.024.
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143. doi: 10.1002/btm2.10143.
- Foggiato AA, Silva DF, Castro RCFR. Effect of photodynamic therapy on surface decontamination in clinical orthodontic instruments. Photodiagnosis Photodyn Ther. 2018;24:123–128. doi: 10.1016/j.pdpdt.2018.09.003.

